Timeline of chemistry
Encyclopedia
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
, defined as the scientific study of the composition of matter and of its interactions. The history of chemistry
History of chemistry
By 1000 BC, ancient civilizations used technologies that would eventually form the basis of the various branches of chemistry. Examples include extracting metals from ores, making pottery and glazes, fermenting beer and wine, making pigments for cosmetics and painting, extracting chemicals from...
in its modern form arguably began with the English scientist Robert Boyle
Robert Boyle
Robert Boyle FRS was a 17th century natural philosopher, chemist, physicist, and inventor, also noted for his writings in theology. He has been variously described as English, Irish, or Anglo-Irish, his father having come to Ireland from England during the time of the English plantations of...
, though its roots can be traced back to the earliest recorded history.
Early ideas that later became incorporated into the modern science of chemistry come from two main sources. Natural philosophers
Natural philosophy
Natural philosophy or the philosophy of nature , is a term applied to the study of nature and the physical universe that was dominant before the development of modern science...
(such as Aristotle
Aristotle
Aristotle was a Greek philosopher and polymath, a student of Plato and teacher of Alexander the Great. His writings cover many subjects, including physics, metaphysics, poetry, theater, music, logic, rhetoric, linguistics, politics, government, ethics, biology, and zoology...
and Democritus
Democritus
Democritus was an Ancient Greek philosopher born in Abdera, Thrace, Greece. He was an influential pre-Socratic philosopher and pupil of Leucippus, who formulated an atomic theory for the cosmos....
) used deductive reasoning
Deductive reasoning
Deductive reasoning, also called deductive logic, is reasoning which constructs or evaluates deductive arguments. Deductive arguments are attempts to show that a conclusion necessarily follows from a set of premises or hypothesis...
in an attempt to explain the behavior of the world around them. Alchemists
Alchemy
Alchemy is an influential philosophical tradition whose early practitioners’ claims to profound powers were known from antiquity. The defining objectives of alchemy are varied; these include the creation of the fabled philosopher's stone possessing powers including the capability of turning base...
(such as Geber
Geber
Abu Musa Jābir ibn Hayyān, often known simply as Geber, was a prominent polymath: a chemist and alchemist, astronomer and astrologer, engineer, geologist, philosopher, physicist, and pharmacist and physician. Born and educated in Tus, he later traveled to Kufa...
and Rhazes) were people who used experimental techniques in an attempt to extend the life or perform material conversions, such as turning base metals into gold.
In the 17th century, a synthesis of the ideas of these two disciplines, that is the deductive and the experimental, leads to the development of a process of thinking known as the scientific method
Scientific method
Scientific method refers to a body of techniques for investigating phenomena, acquiring new knowledge, or correcting and integrating previous knowledge. To be termed scientific, a method of inquiry must be based on gathering empirical and measurable evidence subject to specific principles of...
. With the introduction of the scientific method, the modern science of chemistry was born.
Known as "the central science
The central science
Chemistry is often called the central science because of its role in connecting the physical sciences, which include chemistry, with the life sciences and applied sciences such as medicine and engineering. The nature of this relationship is one of the main topics in the philosophy of chemistry and...
", the study of chemistry is strongly influenced by, and exerts a strong influence on, many other scientific and technological fields. Many events considered central to our modern understanding of chemistry are also considered key discoveries in such fields as physics, biology, astronomy, geology, and materials science to name a few.
Pre-17th century


Scientific method
Scientific method refers to a body of techniques for investigating phenomena, acquiring new knowledge, or correcting and integrating previous knowledge. To be termed scientific, a method of inquiry must be based on gathering empirical and measurable evidence subject to specific principles of...
and its application to the field of chemistry, it is somewhat controversial to consider many of the people listed below as "chemists" in the modern sense of the word. However, the ideas of certain great thinkers, either for their prescience, or for their wide and long-term acceptance, bear listing here.
c. 3000 BCE: Egyptians
Egyptians
Egyptians are nation an ethnic group made up of Mediterranean North Africans, the indigenous people of Egypt.Egyptian identity is closely tied to geography. The population of Egypt is concentrated in the lower Nile Valley, the small strip of cultivable land stretching from the First Cataract to...
formulate the theory of the Ogdoad
Ogdoad
In Egyptian mythology, the Ogdoad were eight deities worshipped in Hermopolis during what is called the Old Kingdom, the third through sixth dynasties, dated between 2686 to 2134 BC...
, or the “primordial forces”, from which all was formed. These were the elements of chaos
Chaos (cosmogony)
Chaos refers to the formless or void state preceding the creation of the universe or cosmos in the Greek creation myths, more specifically the initial "gap" created by the original separation of heaven and earth....
, numbered in eight, that existed before the creation of the sun.
c. 1900 BCE: Hermes Trismegistus
Hermes Trismegistus
Hermes Trismegistus is the eponymous author of the Hermetic Corpus, a sacred text belonging to the genre of divine revelation.-Origin and identity:...
, semi-mythical ancient Egypt
Ancient Egypt
Ancient Egypt was an ancient civilization of Northeastern Africa, concentrated along the lower reaches of the Nile River in what is now the modern country of Egypt. Egyptian civilization coalesced around 3150 BC with the political unification of Upper and Lower Egypt under the first pharaoh...
ian adept king, is thought to have founded the art of alchemy
Alchemy
Alchemy is an influential philosophical tradition whose early practitioners’ claims to profound powers were known from antiquity. The defining objectives of alchemy are varied; these include the creation of the fabled philosopher's stone possessing powers including the capability of turning base...
.
c. 1200 BCE: Tapputi-Belatikallim
Tapputi
Tapputi, also referred to as Tapputi-Belatekallim, is considered to be the world’s first chemist, a perfume-maker mentioned in a cuneiform tablet from the second millennium BC in Mesopotamia. She used flowers, oil, and calamus along with cyperus, myrrh, and balsam. She added water then distilled...
, a perfume-maker and early chemist, was mentioned in a cuneiform
Cuneiform
Cuneiform can refer to:*Cuneiform script, an ancient writing system originating in Mesopotamia in the 4th millennium BC*Cuneiform , three bones in the human foot*Cuneiform Records, a music record label...
tablet in Mesopotamia
Mesopotamia
Mesopotamia is a toponym for the area of the Tigris–Euphrates river system, largely corresponding to modern-day Iraq, northeastern Syria, southeastern Turkey and southwestern Iran.Widely considered to be the cradle of civilization, Bronze Age Mesopotamia included Sumer and the...
.
c. 450 BCE: Empedocles
Empedocles
Empedocles was a Greek pre-Socratic philosopher and a citizen of Agrigentum, a Greek city in Sicily. Empedocles' philosophy is best known for being the originator of the cosmogenic theory of the four Classical elements...
asserts that all things are composed of four primal elements
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
: earth, air, fire, and water, whereby two active and opposing force
Force
In physics, a force is any influence that causes an object to undergo a change in speed, a change in direction, or a change in shape. In other words, a force is that which can cause an object with mass to change its velocity , i.e., to accelerate, or which can cause a flexible object to deform...
s, love and hate, or affinity and antipathy, act upon these elements, combining and separating them into infinitely varied forms.
c. 440 BCE: Leucippus
Leucippus
Leucippus or Leukippos was one of the earliest Greeks to develop the theory of atomism — the idea that everything is composed entirely of various imperishable, indivisible elements called atoms — which was elaborated in greater detail by his pupil and successor, Democritus...
and Democritus
Democritus
Democritus was an Ancient Greek philosopher born in Abdera, Thrace, Greece. He was an influential pre-Socratic philosopher and pupil of Leucippus, who formulated an atomic theory for the cosmos....
propose the idea of the atom, an indivisible particle that all matter is made of. This idea is largely rejected by natural philosophers in favor of the Aristotlean view.
c. 360 BCE: Plato
Plato
Plato , was a Classical Greek philosopher, mathematician, student of Socrates, writer of philosophical dialogues, and founder of the Academy in Athens, the first institution of higher learning in the Western world. Along with his mentor, Socrates, and his student, Aristotle, Plato helped to lay the...
coins term ‘elements
Classical element
Many philosophies and worldviews have a set of classical elements believed to reflect the simplest essential parts and principles of which anything consists or upon which the constitution and fundamental powers of anything are based. Most frequently, classical elements refer to ancient beliefs...
’ (stoicheia) and in his dialogue Timaeus
Timaeus (dialogue)
Timaeus is one of Plato's dialogues, mostly in the form of a long monologue given by the title character, written circa 360 BC. The work puts forward speculation on the nature of the physical world and human beings. It is followed by the dialogue Critias.Speakers of the dialogue are Socrates,...
, which includes a discussion of the composition of inorganic and organic bodies and is a rudimentary treatise on chemistry, assumes that the minute particle of each element had a special geometric shape: tetrahedron
Tetrahedron
In geometry, a tetrahedron is a polyhedron composed of four triangular faces, three of which meet at each vertex. A regular tetrahedron is one in which the four triangles are regular, or "equilateral", and is one of the Platonic solids...
(fire), octahedron
Octahedron
In geometry, an octahedron is a polyhedron with eight faces. A regular octahedron is a Platonic solid composed of eight equilateral triangles, four of which meet at each vertex....
(air), icosahedron
Icosahedron
In geometry, an icosahedron is a regular polyhedron with 20 identical equilateral triangular faces, 30 edges and 12 vertices. It is one of the five Platonic solids....
(water), and cube
Cube
In geometry, a cube is a three-dimensional solid object bounded by six square faces, facets or sides, with three meeting at each vertex. The cube can also be called a regular hexahedron and is one of the five Platonic solids. It is a special kind of square prism, of rectangular parallelepiped and...
(earth).
c. 350 BCE: Aristotle
Aristotle
Aristotle was a Greek philosopher and polymath, a student of Plato and teacher of Alexander the Great. His writings cover many subjects, including physics, metaphysics, poetry, theater, music, logic, rhetoric, linguistics, politics, government, ethics, biology, and zoology...
, expanding on Empedocles, proposes idea of a substance as a combination of matter and form. Describes theory of the Five Elements
Five elements
Five elements may refer to: In philosophy: *Five elements *Mahabhuta*Pancha Tattva *Five elements In science:*Boron, element 5*Group 5 element*Period 5 element-See also:...
, fire, water, earth, air, and aether. This theory is largely accepted throughout the western world for over 1000 years.
c. 50 BCE: Lucretius
Lucretius
Titus Lucretius Carus was a Roman poet and philosopher. His only known work is an epic philosophical poem laying out the beliefs of Epicureanism, De rerum natura, translated into English as On the Nature of Things or "On the Nature of the Universe".Virtually no details have come down concerning...
publishes De Rerum Natura
On the Nature of Things
De rerum natura is a 1st century BC didactic poem by the Roman poet and philosopher Lucretius with the goal of explaining Epicurean philosophy to a Roman audience. The poem, written in some 7,400 dactylic hexameters, is divided into six untitled books, and explores Epicurean physics through richly...
, a poetic description of the ideas of Atomism
Atomism
Atomism is a natural philosophy that developed in several ancient traditions. The atomists theorized that the natural world consists of two fundamental parts: indivisible atoms and empty void.According to Aristotle, atoms are indestructible and immutable and there are an infinite variety of shapes...
.
c. 300: Zosimos of Panopolis
Zosimos of Panopolis
Zosimos of Panopolis was an Egyptian or Greek alchemist and Gnostic mystic from the end of the 3rd and beginning of the 4th century AD. He was born in Panopolis, present day Akhmim in the south of Egypt, ca. 300. He wrote the oldest known books on alchemy, of which quotations in the Greek language...
writes some of the oldest known books on alchemy
Alchemy
Alchemy is an influential philosophical tradition whose early practitioners’ claims to profound powers were known from antiquity. The defining objectives of alchemy are varied; these include the creation of the fabled philosopher's stone possessing powers including the capability of turning base...
, which he defines as the study of the composition of waters, movement, growth, embodying and disembodying, drawing the spirits from bodies and bonding the spirits within bodies.
c. 770: Abu Musa Jabir ibn Hayyan
Geber
Abu Musa Jābir ibn Hayyān, often known simply as Geber, was a prominent polymath: a chemist and alchemist, astronomer and astrologer, engineer, geologist, philosopher, physicist, and pharmacist and physician. Born and educated in Tus, he later traveled to Kufa...
(aka Geber), an Arab/Persian alchemist who is "considered by many to be the father of chemistry", develops an early experimental method
Scientific method
Scientific method refers to a body of techniques for investigating phenomena, acquiring new knowledge, or correcting and integrating previous knowledge. To be termed scientific, a method of inquiry must be based on gathering empirical and measurable evidence subject to specific principles of...
for chemistry, and isolates numerous acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
s, including hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
, nitric acid
Nitric acid
Nitric acid , also known as aqua fortis and spirit of nitre, is a highly corrosive and toxic strong acid.Colorless when pure, older samples tend to acquire a yellow cast due to the accumulation of oxides of nitrogen. If the solution contains more than 86% nitric acid, it is referred to as fuming...
, citric acid
Citric acid
Citric acid is a weak organic acid. It is a natural preservative/conservative and is also used to add an acidic, or sour, taste to foods and soft drinks...
, acetic acid
Acetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
, tartaric acid
Tartaric acid
Tartaric acid is a white crystalline diprotic organic acid. It occurs naturally in many plants, particularly grapes, bananas, and tamarinds; is commonly combined with baking soda to function as a leavening agent in recipes, and is one of the main acids found in wine. It is added to other foods to...
, and aqua regia
Aqua regia
Aqua regia or aqua regis is a highly corrosive mixture of acids, fuming yellow or red solution, also called nitro-hydrochloric acid. The mixture is formed by freshly mixing concentrated nitric acid and hydrochloric acid, usually in a volume ratio of 1:3, respectively...
.
c. 1000: Abū al-Rayhān al-Bīrūnī and Avicenna
Avicenna
Abū ʿAlī al-Ḥusayn ibn ʿAbd Allāh ibn Sīnā , commonly known as Ibn Sīnā or by his Latinized name Avicenna, was a Persian polymath, who wrote almost 450 treatises on a wide range of subjects, of which around 240 have survived...
, both Persian chemists, refute the practice of alchemy
Alchemy
Alchemy is an influential philosophical tradition whose early practitioners’ claims to profound powers were known from antiquity. The defining objectives of alchemy are varied; these include the creation of the fabled philosopher's stone possessing powers including the capability of turning base...
and the theory of the transmutation of metals
Philosopher's stone
The philosopher's stone is a legendary alchemical substance said to be capable of turning base metals into gold or silver. It was also sometimes believed to be an elixir of life, useful for rejuvenation and possibly for achieving immortality. For many centuries, it was the most sought-after goal...
.
c. 1167: Alchemists in the School of Salerno make the first references to the distillation of wine.
c. 1220: Robert Grosseteste
Robert Grosseteste
Robert Grosseteste or Grossetete was an English statesman, scholastic philosopher, theologian and Bishop of Lincoln. He was born of humble parents at Stradbroke in Suffolk. A.C...
publishes several Aristotelian commentaries where he lays out an early framework for the scientific method
Scientific method
Scientific method refers to a body of techniques for investigating phenomena, acquiring new knowledge, or correcting and integrating previous knowledge. To be termed scientific, a method of inquiry must be based on gathering empirical and measurable evidence subject to specific principles of...
.
c 1250:Tadeo Alderotti develops Fractional distillation
Fractional distillation
Fractional distillation is the separation of a mixture into its component parts, or fractions, such as in separating chemical compounds by their boiling point by heating them to a temperature at which several fractions of the compound will evaporate. It is a special type of distillation...
, which is much more effective than its predecessors.
c 1260: St Albertus Magnus
Albertus Magnus
Albertus Magnus, O.P. , also known as Albert the Great and Albert of Cologne, is a Catholic saint. He was a German Dominican friar and a bishop, who achieved fame for his comprehensive knowledge of and advocacy for the peaceful coexistence of science and religion. Those such as James A. Weisheipl...
discovers Arsenic
Arsenic
Arsenic is a chemical element with the symbol As, atomic number 33 and relative atomic mass 74.92. Arsenic occurs in many minerals, usually in conjunction with sulfur and metals, and also as a pure elemental crystal. It was first documented by Albertus Magnus in 1250.Arsenic is a metalloid...
and Silver nitrate
Silver nitrate
Silver nitrate is an inorganic compound with chemical formula . This compound is a versatile precursor to many other silver compounds, such as those used in photography. It is far less sensitive to light than the halides...
. He also made one of the first references to sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
.
c. 1267: Roger Bacon
Roger Bacon
Roger Bacon, O.F.M. , also known as Doctor Mirabilis , was an English philosopher and Franciscan friar who placed considerable emphasis on the study of nature through empirical methods...
publishes Opus Maius, which among other things, proposes an early form of the scientific method, and contains results of his experiments with gunpowder
Gunpowder
Gunpowder, also known since in the late 19th century as black powder, was the first chemical explosive and the only one known until the mid 1800s. It is a mixture of sulfur, charcoal, and potassium nitrate - with the sulfur and charcoal acting as fuels, while the saltpeter works as an oxidizer...
.
c. 1310: Pseudo-Geber
Pseudo-Geber
Pseudo-Geber is the name assigned by modern scholars to an anonymous European alchemist born in the 13th century, sometimes identified with Paul of Taranto, who wrote books on alchemy and metallurgy, in Latin, under the pen name of "Geber". "Geber" is the shortened and Latinised form of the name...
, an anonymous Spanish alchemist who wrote under the name of Geber, publishes several books that establish the long-held theory that all metals were composed of various proportions of sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
and mercury
Mercury (element)
Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum...
. He is one of the first to describe nitric acid
Nitric acid
Nitric acid , also known as aqua fortis and spirit of nitre, is a highly corrosive and toxic strong acid.Colorless when pure, older samples tend to acquire a yellow cast due to the accumulation of oxides of nitrogen. If the solution contains more than 86% nitric acid, it is referred to as fuming...
, aqua regia
Aqua regia
Aqua regia or aqua regis is a highly corrosive mixture of acids, fuming yellow or red solution, also called nitro-hydrochloric acid. The mixture is formed by freshly mixing concentrated nitric acid and hydrochloric acid, usually in a volume ratio of 1:3, respectively...
, and aqua fortis
Aqua fortis
Aqua fortis, or "strong water," in alchemy, is a solution of nitric acid in water. Being highly corrosive, the solution was used in alchemy for dissolving silver and most other metals with the notable exception of gold, which can only be dissolved using aqua regia...
.
c. 1530: Paracelsus
Paracelsus
Paracelsus was a German-Swiss Renaissance physician, botanist, alchemist, astrologer, and general occultist....
develops the study of iatrochemistry
Iatrochemistry
Iatrochemistry is a branch of both chemistry and medicine. Having its roots in alchemy, iatrochemistry seeks to provide chemical solutions to diseases and medical ailments....
, a subdiscipline of alchemy dedicated to extending the life, thus being the roots of the modern pharmaceutical industry. It is also claimed that he is the first to use the word "chemistry".
1597: Andreas Libavius
Andreas Libavius
Andreas Libavius was a German doctor and chemist.-Life:Libavius was born in Halle, Germany, as Andreas Libau. In Halle he attended the gymnasium and studied from the year 1576 in University of Wittenberg. From 1577 on he studied in the University of Jena in the faculties of philosophy and history...
publishes Alchemia, a prototype chemistry
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
textbook.
17th and 18th centuries
1605:Sir Francis Bacon publishes The Proficience and Advancement of Learning, which contains a description of what would later be known as the scientific methodScientific method
Scientific method refers to a body of techniques for investigating phenomena, acquiring new knowledge, or correcting and integrating previous knowledge. To be termed scientific, a method of inquiry must be based on gathering empirical and measurable evidence subject to specific principles of...
.
1605:Michal Sedziwój
Michal Sedziwój
Michał Sędziwój of Ostoja coat of arms was a Polish alchemist, philosopher, and medical doctor....
publishes the alchemical treatise A New Light of Alchemy which proposed the existence of the "food of life" within air, much later recognized as oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
.
1615:Jean Beguin
Jean Beguin
Jean Beguin was an iatrochemist noted for his 1610 Tyrocinium Chymicum , which many consider to be one of the first chemistry textbooks. In the 1615 edition of his textbook, Beguin made the first-ever chemical equation or rudimentary reaction diagrams, showing the results of reactions in which...
publishes the Tyrocinium Chymicum
Tyrocinium Chymicum
Tyrocinium Chymicum was a published set of chemistry lecture notes started by Jean Beguin in 1610 in Paris, France. It has been suggested that it was the first chemistry text book...
, an early chemistry textbook, and in it draws the first-ever chemical equation
Chemical equation
A chemical equation is the symbolic representation of a chemical reaction where the reactant entities are given on the left hand side and the product entities on the right hand side. The coefficients next to the symbols and formulae of entities are the absolute values of the stoichiometric numbers...
.
1637:René Descartes
René Descartes
René Descartes ; was a French philosopher and writer who spent most of his adult life in the Dutch Republic. He has been dubbed the 'Father of Modern Philosophy', and much subsequent Western philosophy is a response to his writings, which are studied closely to this day...
publishes Discours de la méthode, which contains an outline of the scientific method.
1648:Posthumous publication of the book Ortus medicinae by Jan Baptist van Helmont
Jan Baptist van Helmont
Jan Baptist van Helmont was an early modern period Flemish chemist, physiologist, and physician. He worked during the years just after Paracelsus and iatrochemistry, and is sometimes considered to be "the founder of pneumatic chemistry"...
, which is cited by some as a major transitional work between alchemy and chemistry, and as an important influence on Robert Boyle. The book contains the results of numerous experiments and establishes an early version of the Law of conservation of mass.

Robert Boyle
Robert Boyle FRS was a 17th century natural philosopher, chemist, physicist, and inventor, also noted for his writings in theology. He has been variously described as English, Irish, or Anglo-Irish, his father having come to Ireland from England during the time of the English plantations of...
publishes The Sceptical Chymist
The Sceptical Chymist
The Sceptical Chymist: or Chymico-Physical Doubts & Paradoxes is the title of Robert Boyle's masterpiece of scientific literature, published in London in 1661. In the form of a dialogue, the Sceptical Chymist presented Boyle's hypothesis that matter consisted of atoms and clusters of atoms in...
, a treatise on the distinction between chemistry and alchemy
Alchemy
Alchemy is an influential philosophical tradition whose early practitioners’ claims to profound powers were known from antiquity. The defining objectives of alchemy are varied; these include the creation of the fabled philosopher's stone possessing powers including the capability of turning base...
. It contains some of the earliest modern ideas of atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
s, molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
s, and chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
, and marks the beginning of the history of modern chemistry.
1662:Robert Boyle proposes Boyle's Law
Boyle's law
Boyle's law is one of many gas laws and a special case of the ideal gas law. Boyle's law describes the inversely proportional relationship between the absolute pressure and volume of a gas, if the temperature is kept constant within a closed system...
, an experimentally based description of the behavior of gas
Gas
Gas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
es, specifically the relationship between pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
and volume
Volume
Volume is the quantity of three-dimensional space enclosed by some closed boundary, for example, the space that a substance or shape occupies or contains....
.
1735:Swedish chemist Georg Brandt
Georg Brandt
-External links:** by Uno Boklund in: Charles C. Gillispie, ed., Dictionary of Scientific Biography , vol. 2, pages 421-422....
analyzes a dark blue pigment found in copper ore. Brandt demonstrated that the pigment contained a new element, later named cobalt
Cobalt
Cobalt is a chemical element with symbol Co and atomic number 27. It is found naturally only in chemically combined form. The free element, produced by reductive smelting, is a hard, lustrous, silver-gray metal....
.
1754:Joseph Black
Joseph Black
Joseph Black FRSE FRCPE FPSG was a Scottish physician and chemist, known for his discoveries of latent heat, specific heat, and carbon dioxide. He was professor of Medicine at University of Glasgow . James Watt, who was appointed as philosophical instrument maker at the same university...
isolates carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
, which he called "fixed air".
1757:Louis Claude Cadet de Gassicourt
Louis Claude Cadet de Gassicourt
Louis Claude Cadet de Gassicourt was a French chemist who synthesised the first organometalic compound.He obtained a red liquid by the reaction of potassium acetate with arsenic trioxide...
, while investigating arsenic compounds, creates Cadet's fuming liquid
Cadet's fuming liquid
Cadet's fuming liquid was the first organometallic compound to be synthesized. In 1760, the French chemist Louis Claude Cadet de Gassicourt synthesized a red liquid by the reaction of potassium acetate with arsenic trioxide....
, later discovered to be Cacodyl oxide
Cacodyl oxide
Cacodyl oxide is a chemical compound of the formula [2As]2O. This organoarsenic compound is primarily of historical significance as it is sometimes considered to be the first organometallic compound synthesized in relatively pure form....
, considered to be the first synthetic organometallic compound.
1758:Joseph Black formulates the concept of latent heat
Latent heat
Latent heat is the heat released or absorbed by a chemical substance or a thermodynamic system during a process that occurs without a change in temperature. A typical example is a change of state of matter, meaning a phase transition such as the melting of ice or the boiling of water. The term was...
to explain the thermochemistry
Thermochemistry
Thermochemistry is the study of the energy and heat associated with chemical reactions and/or physical transformations. A reaction may release or absorb energy, and a phase change may do the same, such as in melting and boiling. Thermochemistry focuses on these energy changes, particularly on the...
of phase changes.
1766:Henry Cavendish
Henry Cavendish
Henry Cavendish FRS was a British scientist noted for his discovery of hydrogen or what he called "inflammable air". He described the density of inflammable air, which formed water on combustion, in a 1766 paper "On Factitious Airs". Antoine Lavoisier later reproduced Cavendish's experiment and...
discovers hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
as a colorless, odourless gas that burns and can form an explosive mixture with air.
1773–1774: Carl Wilhelm Scheele
Carl Wilhelm Scheele
Carl Wilhelm Scheele was a German-Swedish pharmaceutical chemist. Isaac Asimov called him "hard-luck Scheele" because he made a number of chemical discoveries before others who are generally given the credit...
and Joseph Priestly independently isolate oxygen, called by Priestly "dephlogisticated air" and Scheele "fire air".

Antoine Lavoisier
Antoine-Laurent de Lavoisier , the "father of modern chemistry", was a French nobleman prominent in the histories of chemistry and biology...
, considered "The father of modern chemistry", recognizes and names oxygen, and recognizes its importance and role in combustion.
1787:Antoine Lavoisier publishes Méthode de nomenclature chimique, the first modern system of chemical nomenclature.
1787:Jacques Charles
Jacques Charles
Jacques Alexandre César Charles was a French inventor, scientist, mathematician, and balloonist.Charles and the Robert brothers launched the world's first hydrogen-filled balloon in August 1783, then in December 1783, Charles and his co-pilot Nicolas-Louis Robert ascended to a height of about...
proposes Charles's Law
Charles's law
Charles' law is an experimental gas law which describes how gases tend to expand when heated. It was first published by French natural philosopher Joseph Louis Gay-Lussac in 1802, although he credited the discovery to unpublished work from the 1780s by Jacques Charles...
, a corollary of Boyle's Law, describes relationship between temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
and volume of a gas.
1789:Antoine Lavoisier publishes Traité Élémentaire de Chimie
Traité Élémentaire de Chimie
Traité élémentaire de chimie is an influential textbook written by Antoine Lavoisier published in 1789 and translated into English by Robert Kerr in 1790.The book is considered to be the first modern chemical textbook...
, the first modern chemistry textbook. It is a complete survey of (at that time) modern chemistry, including the first concise definition of the law of conservation of mass, and thus also represents the founding of the discipline of stoichiometry
Stoichiometry
Stoichiometry is a branch of chemistry that deals with the relative quantities of reactants and products in chemical reactions. In a balanced chemical reaction, the relations among quantities of reactants and products typically form a ratio of whole numbers...
or quantitative chemical analysis.
1797:Joseph Proust
Joseph Proust
Joseph Louis Proust was a French chemist.-Life:Joseph L. Proust was born on September 26, 1754 in Angers, France. His father served as an apothecary in Angers. Joseph studied chemistry in his father’s shop and later came to Paris where he gained the appointment of apothecary in chief to the...
proposes the law of definite proportions
Law of definite proportions
In chemistry, the law of definite proportions, sometimes called Proust's Law, states that a chemical compound always contains exactly the same proportion of elements by mass. An equivalent statement is the law of constant composition, which states that all samples of a given chemical compound have...
, which states that elements always combine in small, whole number ratios to form compounds.
1800:Alessandro Volta
Alessandro Volta
Count Alessandro Giuseppe Antonio Anastasio Gerolamo Umberto Volta was a Lombard physicist known especially for the invention of the battery in 1800.-Early life and works:...
devises the first chemical battery
Voltaic pile
A voltaic pile is a set of individual Galvanic cells placed in series. The voltaic pile, invented by Alessandro Volta in 1800, was the first electric battery...
, thereby founding the discipline of electrochemistry
Electrochemistry
Electrochemistry is a branch of chemistry that studies chemical reactions which take place in a solution at the interface of an electron conductor and an ionic conductor , and which involve electron transfer between the electrode and the electrolyte or species in solution.If a chemical reaction is...
.
19th century

John Dalton
John Dalton FRS was an English chemist, meteorologist and physicist. He is best known for his pioneering work in the development of modern atomic theory, and his research into colour blindness .-Early life:John Dalton was born into a Quaker family at Eaglesfield, near Cockermouth, Cumberland,...
proposes Dalton's Law
Dalton's law
In chemistry and physics, Dalton's law states that the total pressure exerted by a gaseous mixture is equal to the sum of the partial pressures of each individual component in a gas mixture...
, which describes relationship between the components in a mixture of gases and the relative pressure each contributes to that of the overall mixture.
1805:Joseph Louis Gay-Lussac
Joseph Louis Gay-Lussac
- External links :* from the American Chemical Society* from the Encyclopædia Britannica, 10th Edition * , Paris...
discovers that water is composed of two parts hydrogen and one part oxygen by volume.
1808:Joseph Louis Gay-Lussac collects and discovers several chemical and physical properties of air and of other gases, including experimental proofs of Boyle's and Charles's laws, and of relationships between density and composition of gases.
1808:John Dalton publishes New System of Chemical Philosophy, which contains first modern scientific description of the atomic theory
Atomic theory
In chemistry and physics, atomic theory is a theory of the nature of matter, which states that matter is composed of discrete units called atoms, as opposed to the obsolete notion that matter could be divided into any arbitrarily small quantity...
, and clear description of the law of multiple proportions
Law of multiple proportions
In chemistry, the law of multiple proportions is one of the basic laws of stoichiometry, alongside the law of definite proportions. It is sometimes called Dalton's Law after its discoverer, the English chemist John Dalton.The statement of the law is:...
.
1808:Jöns Jakob Berzelius
Jöns Jakob Berzelius
Jöns Jacob Berzelius was a Swedish chemist. He worked out the modern technique of chemical formula notation, and is together with John Dalton, Antoine Lavoisier, and Robert Boyle considered a father of modern chemistry...
publishes Lärbok i Kemien in which he proposes modern chemical symbols and notation, and of the concept of relative atomic weight
Atomic weight
Atomic weight is a dimensionless physical quantity, the ratio of the average mass of atoms of an element to 1/12 of the mass of an atom of carbon-12...
.
1811:Amedeo Avogadro
Amedeo Avogadro
Lorenzo Romano Amedeo Carlo Avogadro di Quaregna e di Cerreto, Count of Quaregna and Cerreto was an Italian savant. He is most noted for his contributions to molecular theory, including what is known as Avogadro's law...
proposes Avogadro's law
Avogadro's law
Avogadro's law is a gas law named after Amedeo Avogadro who, in 1811, hypothesized that two given samples of an ideal gas, at the same temperature, pressure and volume, contain the same number of molecules...
, that equal volumes of gases under constant temperature and pressure contain equal number of molecules.
Friedrich Wöhler
Friedrich Wöhler was a German chemist, best known for his synthesis of urea, but also the first to isolate several chemical elements.-Biography:He was born in Eschersheim, which belonged to aau...
and Justus von Liebig
Justus von Liebig
Justus von Liebig was a German chemist who made major contributions to agricultural and biological chemistry, and worked on the organization of organic chemistry. As a professor, he devised the modern laboratory-oriented teaching method, and for such innovations, he is regarded as one of the...
perform the first confirmed discovery and explanation of isomers, earlier named by Berzelius. Working with cyanic acid and fulminic acid, they correctly deduce that isomerism was caused by differing arrangements of atoms within a molecular structure.
1827:William Prout classifies biomolecules into their modern groupings: carbohydrates, proteins and lipids.
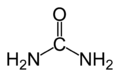
1828:Friedrich Wöhler synthesizes urea
Urea
Urea or carbamide is an organic compound with the chemical formula CO2. The molecule has two —NH2 groups joined by a carbonyl functional group....
, thereby establishing that organic compounds could be produced from inorganic starting materials, disproving the theory of vitalism
Vitalism
Vitalism, as defined by the Merriam-Webster dictionary, is#a doctrine that the functions of a living organism are due to a vital principle distinct from biochemical reactions...
.
1832:Friedrich Wöhler and Justus von Liebig discover and explain functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
s and radicals
Radical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
in relation to organic chemistry.
1840:Germain Hess proposes Hess's Law
Hess's law
Hess' law is a relationship in physical chemistry named for Germain Hess, a Swiss-born Russian chemist and physician.The law states that the enthalpy change for a reaction that is carried out in a series of steps is equal to the sum of the enthalpy changes for the individual steps.The law is an...
, an early statement of the Law of conservation of energy, which establishes that energy changes in a chemical process depend only on the states of the starting and product materials and not on the specific pathway taken between the two states.
1847:Hermann Kolbe obtains acetic acid
Acetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
from completely inorganic sources, further disproving vitalism.
1848:Lord Kelvin establishes concept of absolute zero
Absolute zero
Absolute zero is the theoretical temperature at which entropy reaches its minimum value. The laws of thermodynamics state that absolute zero cannot be reached using only thermodynamic means....
, the temperature at which all molecular motion ceases.
1849:Louis Pasteur
Louis Pasteur
Louis Pasteur was a French chemist and microbiologist born in Dole. He is remembered for his remarkable breakthroughs in the causes and preventions of diseases. His discoveries reduced mortality from puerperal fever, and he created the first vaccine for rabies and anthrax. His experiments...
discovers that the racemic
Racemic
In chemistry, a racemic mixture, or racemate , is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule. The first known racemic mixture was "racemic acid", which Louis Pasteur found to be a mixture of the two enantiomeric isomers of tartaric acid.- Nomenclature :A...
form of tartaric acid
Tartaric acid
Tartaric acid is a white crystalline diprotic organic acid. It occurs naturally in many plants, particularly grapes, bananas, and tamarinds; is commonly combined with baking soda to function as a leavening agent in recipes, and is one of the main acids found in wine. It is added to other foods to...
is a mixture of the levorotatory and dextrotatory forms, thus clarifying the nature of optical rotation
Optical rotation
Optical rotation is the turning of the plane of linearly polarized light about the direction of motion as the light travels through certain materials. It occurs in solutions of chiral molecules such as sucrose , solids with rotated crystal planes such as quartz, and spin-polarized gases of atoms...
and advancing the field of stereochemistry
Stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms within molecules. An important branch of stereochemistry is the study of chiral molecules....
.
1852:August Beer
August Beer
August Beer was a German physicist and mathematician. Beer was born in Trier, where he studied mathematics and natural sciences. He worked for Julius Plücker in Bonn afterwards, where he earned a Ph.D. in 1848 and became a lecturer in 1850. In 1854, Beer published his book Einleitung in die höhere...
proposes Beer's law, which explains the relationship between the composition of a mixture and the amount of light it will absorb. Based partly on earlier work by Pierre Bouguer
Pierre Bouguer
Pierre Bouguer was a French mathematician, geophysicist, geodesist, and astronomer. He is also known as "the father of naval architecture"....
and Johann Heinrich Lambert
Johann Heinrich Lambert
Johann Heinrich Lambert was a Swiss mathematician, physicist, philosopher and astronomer.Asteroid 187 Lamberta was named in his honour.-Biography:...
, it establishes the analytical
Analytical chemistry
Analytical chemistry is the study of the separation, identification, and quantification of the chemical components of natural and artificial materials. Qualitative analysis gives an indication of the identity of the chemical species in the sample and quantitative analysis determines the amount of...
technique known as spectrophotometry
Spectrophotometry
In chemistry, spectrophotometry is the quantitative measurement of the reflection or transmission properties of a material as a function of wavelength...
.
1855:Benjamin Silliman, Jr. pioneers methods of petroleum cracking, which makes the entire modern petrochemical
Petrochemical
Petrochemicals are chemical products derived from petroleum. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as corn or sugar cane....
industry possible.
1856:William Henry Perkin synthesizes Perkin's mauve, the first synthetic dye. Created as an accidental byproduct of an attempt to create quinine
Quinine
Quinine is a natural white crystalline alkaloid having antipyretic , antimalarial, analgesic , anti-inflammatory properties and a bitter taste. It is a stereoisomer of quinidine which, unlike quinine, is an anti-arrhythmic...
from coal tar
Coal tar
Coal tar is a brown or black liquid of extremely high viscosity, which smells of naphthalene and aromatic hydrocarbons. Coal tar is among the by-products when coal iscarbonized to make coke or gasified to make coal gas...
. This discovery is the foundation of the dye synthesis industry, one of the earliest successful chemical industries.
1857:Friedrich August Kekulé von Stradonitz
Friedrich August Kekulé von Stradonitz
Friedrich August Kekule von Stradonitz was a German organic chemist. From the 1850s until his death, Kekule was one of the most prominent chemists in Europe, especially in theoretical chemistry...
proposes that carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
is tetravalent, or forms exactly four chemical bonds.
1859–1860: Gustav Kirchhoff
Gustav Kirchhoff
Gustav Robert Kirchhoff was a German physicist who contributed to the fundamental understanding of electrical circuits, spectroscopy, and the emission of black-body radiation by heated objects...
and Robert Bunsen
Robert Bunsen
Robert Wilhelm Eberhard Bunsen was a German chemist. He investigated emission spectra of heated elements, and discovered caesium and rubidium with Gustav Kirchhoff. Bunsen developed several gas-analytical methods, was a pioneer in photochemistry, and did early work in the field of organoarsenic...
lay the foundations of spectroscopy
Spectroscopy
Spectroscopy is the study of the interaction between matter and radiated energy. Historically, spectroscopy originated through the study of visible light dispersed according to its wavelength, e.g., by a prism. Later the concept was expanded greatly to comprise any interaction with radiative...
as a means of chemical analysis, which lead them to the discovery of caesium
Caesium
Caesium or cesium is the chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-gold alkali metal with a melting point of 28 °C , which makes it one of only five elemental metals that are liquid at room temperature...
and rubidium
Rubidium
Rubidium is a chemical element with the symbol Rb and atomic number 37. Rubidium is a soft, silvery-white metallic element of the alkali metal group. Its atomic mass is 85.4678. Elemental rubidium is highly reactive, with properties similar to those of other elements in group 1, such as very rapid...
. Other workers soon used the same technique to discover indium
Indium
Indium is a chemical element with the symbol In and atomic number 49. This rare, very soft, malleable and easily fusible post-transition metal is chemically similar to gallium and thallium, and shows the intermediate properties between these two...
, thalium, and helium
Helium
Helium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table...
.
1860:Stanislao Cannizzaro
Stanislao Cannizzaro
Stanislao Cannizzaro, FRS was an Italian chemist. He is remembered today largely for the Cannizzaro reaction and for his influential role in the atomic-weight deliberations of the Karlsruhe Congress in 1860.-Biography:...
, resurrecting Avogadro's ideas regarding diatomic molecules, compiles a table of atomic weight
Atomic weight
Atomic weight is a dimensionless physical quantity, the ratio of the average mass of atoms of an element to 1/12 of the mass of an atom of carbon-12...
s and presents it at the 1860 Karlsruhe Congress
Karlsruhe Congress
The Karlsruhe Congress was an international meeting of chemists held in Karlsruhe, Germany from 3 to 5 September, 1860. It was the first international conference of chemistry worldwide.- The meeting :...
, ending decades of conflicting atomic weights and molecular formulas, and leading to Mendeleev's discovery of the periodic law.
1862:Alexander Parkes
Alexander Parkes
Alexander Parkes was a metallurgist and inventor from Birmingham, England. He created Parkesine, the first man-made plastic.-Biography:...
exhibits Parkesine
Parkesine
Parkesine is the trademark for the first man-made plastic. It was patented by Alexander Parkes in 1856. In 1866 Parkes formed the Parkesine Company to mass produce the material. The company, however, failed due to poor product quality as Parkes tried to reduce costs...
, one of the earliest synthetic polymer
Synthetic polymer
Synthetic polymers are often referred to as "plastics", such as the well-known polyethylene and nylon. However, most of them can be classified in at least three main categories: thermoplastics, thermosets and elastomers....
s, at the International Exhibition in London. This discovery formed the foundation of the modern plastics industry.
1862:Alexandre-Emile Béguyer de Chancourtois
Alexandre-Emile Béguyer de Chancourtois
Alexandre-Emile Béguyer de Chancourtois was a French geologist and mineralogist who was the first to arrange the chemical elements in order of atomic weights, doing so in 1862. De Chancourtois only published his paper, but did not publish his actual graph with the proposed arrangement...
publishes the telluric helix, an early, three-dimensional version of the Periodic Table of the Elements
Periodic table
The periodic table of the chemical elements is a tabular display of the 118 known chemical elements organized by selected properties of their atomic structures. Elements are presented by increasing atomic number, the number of protons in an atom's atomic nucleus...
.
1864:John Newlands
John Alexander Reina Newlands
John Alexander Reina Newlands was an English chemist who invented the Periodic Table.Newlands was born in London and was the son of a scottish Presbyterian minister and his Italian wife....
proposes the law of octaves, a precursor to the Periodic Law.
1864:Lothar Meyer develops an early version of the periodic table, with 28 elements organized by valence
Valence (chemistry)
In chemistry, valence, also known as valency or valence number, is a measure of the number of bonds formed by an atom of a given element. "Valence" can be defined as the number of valence bonds...
.
1864:Cato Maximilian Guldberg
Cato Maximilian Guldberg
Cato Maximilian Guldberg was a Norwegian mathematician and chemist.-Career:Guldberg worked at the Royal Frederick University. Together with his brother-in-law, Peter Waage, he proposed the law of mass action...
and Peter Waage
Peter Waage
Peter Waage , the son of a ship's captain, was a significant Norwegian chemist and professor at the Royal Frederick University. Along with his brother-in-law Cato Maximilian Guldberg, he co-discovered and developed the law of mass action between 1864 and 1879.He grew up in Hidra...
, building on Claude Louis Berthollet’s ideas, proposed the Law of Mass Action.
1865:Johann Josef Loschmidt
Johann Josef Loschmidt
Jan or Johann Josef Loschmidt , who referred to himself mostly as 'Josef' , was a notable Austrian scientist who performed groundbreaking work in chemistry, physics , and crystal forms.Born in Carlsbad, a town located in the Austrian Empire , Loschmidt...
determines exact number of molecules in a mole
Mole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
, later named Avogadro's Number
Avogadro's number
In chemistry and physics, the Avogadro constant is defined as the ratio of the number of constituent particles N in a sample to the amount of substance n through the relationship NA = N/n. Thus, it is the proportionality factor that relates the molar mass of an entity, i.e...
.
1865:Friedrich August Kekulé von Stradonitz, based partially on the work of Loschmidt and others, establishes structure of benzene as a six carbon ring with alternating single and double bonds.
1865:Adolf von Baeyer
Adolf von Baeyer
Johann Friedrich Wilhelm Adolf von Baeyer was a German chemist who synthesized indigo, and was the 1905 recipient of the Nobel Prize in Chemistry. Born in Berlin, he initially studied mathematics and physics at Berlin University before moving to Heidelberg to study chemistry with Robert Bunsen...
begins work on indigo dye
Indigo dye
Indigo dye is an organic compound with a distinctive blue color . Historically, indigo was a natural dye extracted from plants, and this process was important economically because blue dyes were once rare. Nearly all indigo dye produced today — several thousand tons each year — is synthetic...
, a milestone in modern industrial organic chemistry which revolutionizes the dye industry.
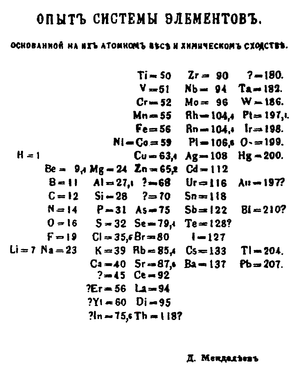
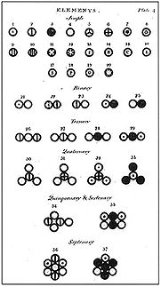
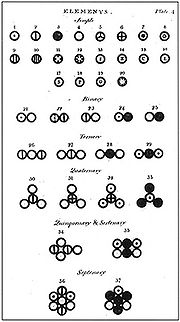
Dmitri Mendeleev
Dmitri Ivanovich Mendeleev , was a Russian chemist and inventor. He is credited as being the creator of the first version of the periodic table of elements...
publishes the first modern periodic table, with the 66 known elements organized by atomic weights. The strength of his table was its ability to accurately predict the properties of as-yet unknown elements.
1873:Jacobus Henricus van 't Hoff
Jacobus Henricus van 't Hoff
Jacobus Henricus van 't Hoff, Jr. was a Dutch physical and organic chemist and the first winner of the Nobel Prize in chemistry. He is best known for his discoveries in chemical kinetics, chemical equilibrium, osmotic pressure, and stereochemistry...
and Joseph Achille Le Bel
Joseph Achille Le Bel
Joseph Achille Le Bel was a French chemist. He is best known for his work in stereochemistry. Le Bel was educated at the École Polytechnique in Paris. In 1874 he announced his theory outlining the relationship between molecular structure and optical activity...
, working independently, develop a model of chemical bonding that explains the chirality experiments of Pasteur and provides a physical cause for optical activity in chiral compounds.
1876:Josiah Willard Gibbs
Josiah Willard Gibbs
Josiah Willard Gibbs was an American theoretical physicist, chemist, and mathematician. He devised much of the theoretical foundation for chemical thermodynamics as well as physical chemistry. As a mathematician, he invented vector analysis . Yale University awarded Gibbs the first American Ph.D...
publishes On the Equilibrium of Heterogeneous Substances
On the Equilibrium of Heterogeneous Substances
In the history of thermodynamics, On the Equilibrium of Heterogeneous Substances is a 300-page paper written by American mathematical-engineer Willard Gibbs...
, a compilation of his work on thermodynamics and physical chemistry
Physical chemistry
Physical chemistry is the study of macroscopic, atomic, subatomic, and particulate phenomena in chemical systems in terms of physical laws and concepts...
which lays out the concept of free energy
Thermodynamic free energy
The thermodynamic free energy is the amount of work that a thermodynamic system can perform. The concept is useful in the thermodynamics of chemical or thermal processes in engineering and science. The free energy is the internal energy of a system less the amount of energy that cannot be used to...
to explain the physical basis of chemical equilibria.
1877:Ludwig Boltzmann
Ludwig Boltzmann
Ludwig Eduard Boltzmann was an Austrian physicist famous for his founding contributions in the fields of statistical mechanics and statistical thermodynamics...
establishes statistical derivations of many important physical and chemical concepts, including entropy
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
, and distributions of molecular velocities in the gas phase.
1883:Svante Arrhenius
Svante Arrhenius
Svante August Arrhenius was a Swedish scientist, originally a physicist, but often referred to as a chemist, and one of the founders of the science of physical chemistry...
develops ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
theory to explain conductivity in electrolyte
Electrolyte
In chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
s.
1884:Jacobus Henricus van 't Hoff publishes Études de Dynamique chimique, a seminal study on chemical kinetics
Chemical kinetics
Chemical kinetics, also known as reaction kinetics, is the study of rates of chemical processes. Chemical kinetics includes investigations of how different experimental conditions can influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition...
.
1884:Hermann Emil Fischer
Hermann Emil Fischer
Hermann Emil Fischer, Emil Fischer was a German chemist and 1902 recipient of the Nobel Prize in Chemistry. He discovered the Fischer esterification. He developed the Fischer projection, a symbolic way of drawing asymmetric carbon atoms.-Early years:Fischer was born in Euskirchen, near Cologne,...
proposes structure of purine
Purine
A purine is a heterocyclic aromatic organic compound, consisting of a pyrimidine ring fused to an imidazole ring. Purines, including substituted purines and their tautomers, are the most widely distributed kind of nitrogen-containing heterocycle in nature....
, a key structure in many biomolecules, which he later synthesized in 1898. Also begins work on the chemistry of glucose
Glucose
Glucose is a simple sugar and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate...
and related sugar
Sugar
Sugar is a class of edible crystalline carbohydrates, mainly sucrose, lactose, and fructose, characterized by a sweet flavor.Sucrose in its refined form primarily comes from sugar cane and sugar beet...
s.
1884:Henry Louis Le Chatelier develops Le Chatelier's principle
Le Châtelier's principle
In chemistry, Le Chatelier's principle, also called the Chatelier's principle, can be used to predict the effect of a change in conditions on a chemical equilibrium. The principle is named after Henry Louis Le Chatelier and sometimes Karl Ferdinand Braun who discovered it independently...
, which explains the response of dynamic chemical equilibria
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
to external stresses.
1885:Eugene Goldstein names the cathode ray
Cathode ray
Cathode rays are streams of electrons observed in vacuum tubes. If an evacuated glass tube is equipped with two electrodes and a voltage is applied, the glass opposite of the negative electrode is observed to glow, due to electrons emitted from and travelling perpendicular to the cathode Cathode...
, later discovered to be composed of electrons, and the canal ray, later discovered to be positive hydrogen ions that had been stripped of their electrons in a cathode ray tube
Cathode ray tube
The cathode ray tube is a vacuum tube containing an electron gun and a fluorescent screen used to view images. It has a means to accelerate and deflect the electron beam onto the fluorescent screen to create the images. The image may represent electrical waveforms , pictures , radar targets and...
. These would later be named proton
Proton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
s.
1893:Alfred Werner
Alfred Werner
Alfred Werner was a Swiss chemist who was a student at ETH Zurich and a professor at the University of Zurich. He won the Nobel Prize in Chemistry in 1913 for proposing the octahedral configuration of transition metal complexes. Werner developed the basis for modern coordination chemistry...
discovers the octahedral structure of cobalt complexes, thus establishing the field of coordination chemistry.
1894–1898: William Ramsay
William Ramsay
Sir William Ramsay was a Scottish chemist who discovered the noble gases and received the Nobel Prize in Chemistry in 1904 "in recognition of his services in the discovery of the inert gaseous elements in air" .-Early years:Ramsay was born in Glasgow on 2...
discovers the noble gases, which fill a large and unexpected gap in the periodic table and led to models of chemical bonding.
1897:J. J. Thomson
J. J. Thomson
Sir Joseph John "J. J." Thomson, OM, FRS was a British physicist and Nobel laureate. He is credited for the discovery of the electron and of isotopes, and the invention of the mass spectrometer...
discovers the electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
using the cathode ray tube
Cathode ray tube
The cathode ray tube is a vacuum tube containing an electron gun and a fluorescent screen used to view images. It has a means to accelerate and deflect the electron beam onto the fluorescent screen to create the images. The image may represent electrical waveforms , pictures , radar targets and...
.
1898:Wilhelm Wien
Wilhelm Wien
Wilhelm Carl Werner Otto Fritz Franz Wien was a German physicist who, in 1893, used theories about heat and electromagnetism to deduce Wien's displacement law, which calculates the emission of a blackbody at any temperature from the emission at any one reference temperature.He also formulated an...
demonstrates that canal rays (streams of positive ions) can be deflected by magnetic fields, and that the amount of deflection is proportional to the mass-to-charge ratio
Mass-to-charge ratio
The mass-to-charge ratio ratio is a physical quantity that is widely used in the electrodynamics of charged particles, e.g. in electron optics and ion optics. It appears in the scientific fields of lithography, electron microscopy, cathode ray tubes, accelerator physics, nuclear physics, Auger...
. This discovery would lead to the analytical
Analytical chemistry
Analytical chemistry is the study of the separation, identification, and quantification of the chemical components of natural and artificial materials. Qualitative analysis gives an indication of the identity of the chemical species in the sample and quantitative analysis determines the amount of...
technique known as mass spectrometry
Mass spectrometry
Mass spectrometry is an analytical technique that measures the mass-to-charge ratio of charged particles.It is used for determining masses of particles, for determining the elemental composition of a sample or molecule, and for elucidating the chemical structures of molecules, such as peptides and...
.
1898:Maria Sklodowska-Curie and Pierre Curie
Pierre Curie
Pierre Curie was a French physicist, a pioneer in crystallography, magnetism, piezoelectricity and radioactivity, and Nobel laureate. He was the son of Dr. Eugène Curie and Sophie-Claire Depouilly Curie ...
isolate radium
Radium
Radium is a chemical element with atomic number 88, represented by the symbol Ra. Radium is an almost pure-white alkaline earth metal, but it readily oxidizes on exposure to air, becoming black in color. All isotopes of radium are highly radioactive, with the most stable isotope being radium-226,...
and polonium
Polonium
Polonium is a chemical element with the symbol Po and atomic number 84, discovered in 1898 by Marie Skłodowska-Curie and Pierre Curie. A rare and highly radioactive element, polonium is chemically similar to bismuth and tellurium, and it occurs in uranium ores. Polonium has been studied for...
from pitchblende.
c. 1900: Ernest Rutherford
Ernest Rutherford
Ernest Rutherford, 1st Baron Rutherford of Nelson OM, FRS was a New Zealand-born British chemist and physicist who became known as the father of nuclear physics...
discovers the source of radioactivity as decaying atoms; coins terms for various types of radiation.
20th century
1903:Mikhail Semyonovich Tsvet invents chromatographyChromatography
Chromatography is the collective term for a set of laboratory techniques for the separation of mixtures....
, an important analytic technique.
1904:Hantaro Nagaoka proposes an early nuclear model
Bohr model
In atomic physics, the Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with electrostatic forces providing attraction,...
of the atom, where electrons orbit a dense massive nucleus.
1905:Fritz Haber
Fritz Haber
Fritz Haber was a German chemist, who received the Nobel Prize in Chemistry in 1918 for his development for synthesizing ammonia, important for fertilizers and explosives. Haber, along with Max Born, proposed the Born–Haber cycle as a method for evaluating the lattice energy of an ionic solid...
and Carl Bosch
Carl Bosch
Carl Bosch was a German chemist and engineer and Nobel laureate in chemistry. He was a pioneer in the field of high-pressure industrial chemistry and founder of IG Farben, at one point the world's largest chemical company....
develop the Haber process
Haber process
The Haber process, also called the Haber–Bosch process, is the nitrogen fixation reaction of nitrogen gas and hydrogen gas, over an enriched iron or ruthenium catalyst, which is used to industrially produce ammonia....
for making ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
from its elements, a milestone in industrial chemistry with deep consequences in agriculture.
1905:Albert Einstein
Albert Einstein
Albert Einstein was a German-born theoretical physicist who developed the theory of general relativity, effecting a revolution in physics. For this achievement, Einstein is often regarded as the father of modern physics and one of the most prolific intellects in human history...
explains Brownian motion
Brownian motion
Brownian motion or pedesis is the presumably random drifting of particles suspended in a fluid or the mathematical model used to describe such random movements, which is often called a particle theory.The mathematical model of Brownian motion has several real-world applications...
in a way that definitively proves atomic theory.
1907:Leo Hendrik Baekeland invents bakelite, one of the first commercially successful plastic
Plastic
A plastic material is any of a wide range of synthetic or semi-synthetic organic solids used in the manufacture of industrial products. Plastics are typically polymers of high molecular mass, and may contain other substances to improve performance and/or reduce production costs...
s.

Robert Millikan
Robert A. Millikan was an American experimental physicist, and Nobel laureate in physics for his measurement of the charge on the electron and for his work on the photoelectric effect. He served as president of Caltech from 1921 to 1945...
measures the charge of individual electrons with unprecedented accuracy through the oil drop experiment, confirming that all electrons have the same charge and mass.
1909:S. P. L. Sørensen
S. P. L. Sørensen
Søren Peder Lauritz Sørensen was a Danish chemist, famous for the introduction of the concept of pH, a scale for measuring acidity and basicity. He was born in Havrebjerg, Denmark....
invents the pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
concept and develops methods for measuring acidity.
1911:Antonius Van den Broek
Antonius Van den Broek
Antonius Johannes van den Broek was a Dutch amateur physicist notable for being the first who realized that the number of an element in the periodic table corresponds to the charge of its atomic nucleus....
proposes the idea that the elements on the periodic table are more properly organized by positive nuclear charge rather than atomic weight.
1911:The first Solvay Conference
Solvay Conference
The International Solvay Institutes for Physics and Chemistry, located in Brussels, were founded by the Belgian industrialist Ernest Solvay in 1912, following the historic invitation-only 1911 Conseil Solvay, the turning point in world physics...
is held in Brussels
Brussels
Brussels , officially the Brussels Region or Brussels-Capital Region , is the capital of Belgium and the de facto capital of the European Union...
, bringing together most of the most prominent scientists of the day. Conferences in physics and chemistry continue to be held periodically to this day.
1911:Ernest Rutherford, Hans Geiger, and Ernest Marsden
Ernest Marsden
Sir Ernest Marsden was an English-New Zealand physicist. He was born in East Lancashire, living in Rishton and educated at Queen Elizabeth's Grammar School, Blackburn, where an inter-house trophy rewarding academic excellence bears his name.He met Ernest Rutherford at the University of Manchester...
perform the Gold foil experiment
Geiger-Marsden experiment
The Geiger–Marsden experiment was an experiment to probe the structure of the atom performed by Hans Geiger and Ernest Marsden in 1909, under the direction of Ernest Rutherford at the Physical Laboratories of the University of Manchester...
, which proves the nuclear model of the atom, with a small, dense, positive nucleus surrounded by a diffuse electron cloud.
1912:William Henry Bragg
William Henry Bragg
Sir William Henry Bragg OM, KBE, PRS was a British physicist, chemist, mathematician and active sportsman who uniquely shared a Nobel Prize with his son William Lawrence Bragg - the 1915 Nobel Prize in Physics...
and William Lawrence Bragg
William Lawrence Bragg
Sir William Lawrence Bragg CH OBE MC FRS was an Australian-born British physicist and X-ray crystallographer, discoverer of the Bragg law of X-ray diffraction, which is basic for the determination of crystal structure. He was joint winner of the Nobel Prize for Physics in 1915. He was knighted...
propose Bragg's law
Bragg's law
In physics, Bragg's law gives the angles for coherent and incoherent scattering from a crystal lattice. When X-rays are incident on an atom, they make the electronic cloud move as does any electromagnetic wave...
and establish the field of X-ray crystallography
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
, an important tool for elucidating the crystal structure of substances.
1912:Peter Debye
Peter Debye
Peter Joseph William Debye FRS was a Dutch physicist and physical chemist, and Nobel laureate in Chemistry.-Early life:...
develops the concept of molecular dipole
Dipole
In physics, there are several kinds of dipoles:*An electric dipole is a separation of positive and negative charges. The simplest example of this is a pair of electric charges of equal magnitude but opposite sign, separated by some distance. A permanent electric dipole is called an electret.*A...
to describe asymmetric charge distribution in some molecules.
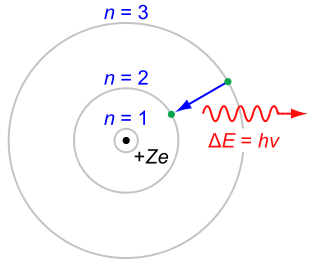
Niels Bohr
Niels Henrik David Bohr was a Danish physicist who made foundational contributions to understanding atomic structure and quantum mechanics, for which he received the Nobel Prize in Physics in 1922. Bohr mentored and collaborated with many of the top physicists of the century at his institute in...
introduces concepts of quantum mechanics
Quantum mechanics
Quantum mechanics, also known as quantum physics or quantum theory, is a branch of physics providing a mathematical description of much of the dual particle-like and wave-like behavior and interactions of energy and matter. It departs from classical mechanics primarily at the atomic and subatomic...
to atomic structure by proposing what is now known as the Bohr model
Bohr model
In atomic physics, the Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with electrostatic forces providing attraction,...
of the atom, where electrons exist only in strictly defined orbitals
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus...
.
1913:Henry Moseley
Henry Moseley
Henry Gwyn Jeffreys Moseley was an English physicist. Moseley's outstanding contribution to the science of physics was the justification from physical laws of the previous empirical and chemical concept of the atomic number. This stemmed from his development of Moseley's law in X-ray spectra...
, working from Van den Broek's earlier idea, introduces concept of atomic number
Atomic number
In chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element...
to fix inadequacies of Mendeleev's periodic table, which had been based on atomic weight,
1913:Frederick Soddy
Frederick Soddy
Frederick Soddy was an English radiochemist who explained, with Ernest Rutherford, that radioactivity is due to the transmutation of elements, now known to involve nuclear reactions. He also proved the existence of isotopes of certain radioactive elements...
proposes the concept of isotope
Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
s, that elements with the same chemical properties may have differing atomic weights.
1913:J. J. Thomson
J. J. Thomson
Sir Joseph John "J. J." Thomson, OM, FRS was a British physicist and Nobel laureate. He is credited for the discovery of the electron and of isotopes, and the invention of the mass spectrometer...
expanding on the work of Wien, shows that charged subatomic particles can be separated by their mass-to-charge ratio, a technique known as mass spectrometry
Mass spectrometry
Mass spectrometry is an analytical technique that measures the mass-to-charge ratio of charged particles.It is used for determining masses of particles, for determining the elemental composition of a sample or molecule, and for elucidating the chemical structures of molecules, such as peptides and...
.
1916:Gilbert N. Lewis
Gilbert N. Lewis
Gilbert Newton Lewis was an American physical chemist known for the discovery of the covalent bond , his purification of heavy water, his reformulation of chemical thermodynamics in a mathematically rigorous manner accessible to ordinary chemists, his theory of Lewis acids and...
publishes "The Atom and the Molecule", the foundation of valence bond theory
Valence bond theory
In chemistry, valence bond theory is one of two basic theories, along with molecular orbital theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of the dissociated atoms combine to give individual chemical bonds...
.
1921:Otto Stern
Otto Stern
Otto Stern was a German physicist and Nobel laureate in physics.-Biography:Stern was born in Sohrau, now Żory in the German Empire's Kingdom of Prussia and studied at Breslau, now Wrocław in Lower Silesia....
and Walther Gerlach establish concept of quantum mechanical spin
Spin (physics)
In quantum mechanics and particle physics, spin is a fundamental characteristic property of elementary particles, composite particles , and atomic nuclei.It is worth noting that the intrinsic property of subatomic particles called spin and discussed in this article, is related in some small ways,...
in subatomic particles.
1923:Gilbert N. Lewis and Merle Randall
Merle Randall
Merle Randall was an American physical chemist famous for his work, over the period of 25 years, in measuring free energy calculations of compounds with Gilbert N. Lewis...
publish Thermodynamics and the Free Energy of Chemical Substances, first modern treatise on chemical thermodynamics
Chemical thermodynamics
Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics...
.
1923:Gilbert N. Lewis develops the electron pair theory of acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
/base
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
reactions.
1924:Louis de Broglie introduces the wave-model of atomic structure, based on the ideas of wave-particle duality.
1925:Wolfgang Pauli
Wolfgang Pauli
Wolfgang Ernst Pauli was an Austrian theoretical physicist and one of the pioneers of quantum physics. In 1945, after being nominated by Albert Einstein, he received the Nobel Prize in Physics for his "decisive contribution through his discovery of a new law of Nature, the exclusion principle or...
develops the exclusion principle
Pauli exclusion principle
The Pauli exclusion principle is the quantum mechanical principle that no two identical fermions may occupy the same quantum state simultaneously. A more rigorous statement is that the total wave function for two identical fermions is anti-symmetric with respect to exchange of the particles...
, which states that no two electrons around a single nucleus may have the same quantum state, as described by four quantum numbers.
1926:Erwin Schrödinger
Erwin Schrödinger
Erwin Rudolf Josef Alexander Schrödinger was an Austrian physicist and theoretical biologist who was one of the fathers of quantum mechanics, and is famed for a number of important contributions to physics, especially the Schrödinger equation, for which he received the Nobel Prize in Physics in 1933...
proposes the Schrödinger equation
Schrödinger equation
The Schrödinger equation was formulated in 1926 by Austrian physicist Erwin Schrödinger. Used in physics , it is an equation that describes how the quantum state of a physical system changes in time....
, which provides a mathematical basis for the wave model of atomic structure.
1927:Werner Heisenberg
Werner Heisenberg
Werner Karl Heisenberg was a German theoretical physicist who made foundational contributions to quantum mechanics and is best known for asserting the uncertainty principle of quantum theory...
develops the uncertainty principle
Uncertainty principle
In quantum mechanics, the Heisenberg uncertainty principle states a fundamental limit on the accuracy with which certain pairs of physical properties of a particle, such as position and momentum, can be simultaneously known...
which, among other things, explains the mechanics of electron motion around the nucleus.
1927:Fritz London
Fritz London
Fritz Wolfgang London was a German theoretical physicist. His fundamental contributions to the theories of chemical bonding and of intermolecular forces are today considered classic and are discussed in standard textbooks of physical chemistry.With his brother Heinz, he made a significant...
and Walter Heitler
Walter Heitler
Walter Heinrich Heitler was a German physicist who made contributions to quantum electrodynamics and quantum field theory...
apply quantum mechanics to explain covalent bonding in the hydrogen molecule, which marked the birth of quantum chemistry
Quantum chemistry
Quantum chemistry is a branch of chemistry whose primary focus is the application of quantum mechanics in physical models and experiments of chemical systems...
.
c. 1930: Linus Pauling
Linus Pauling
Linus Carl Pauling was an American chemist, biochemist, peace activist, author, and educator. He was one of the most influential chemists in history and ranks among the most important scientists of the 20th century...
proposes Pauling's rules
Pauling's rules
Pauling's rules are five rules published by Linus Pauling in 1929 for determining the crystal structures of complex ionic crystals.-First rule:...
, which are key principles for the use of X-ray crystallography
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
to deduce molecular structure.
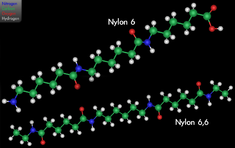
Wallace Carothers
Wallace Hume Carothers was an American chemist, inventor and the leader of organic chemistry at DuPont, credited with the invention of nylon....
leads a team of chemists at DuPont
DuPont
E. I. du Pont de Nemours and Company , commonly referred to as DuPont, is an American chemical company that was founded in July 1802 as a gunpowder mill by Eleuthère Irénée du Pont. DuPont was the world's third largest chemical company based on market capitalization and ninth based on revenue in 2009...
who invent nylon
Nylon
Nylon is a generic designation for a family of synthetic polymers known generically as polyamides, first produced on February 28, 1935, by Wallace Carothers at DuPont's research facility at the DuPont Experimental Station...
, one of the most commercially successful synthetic polymers in history.
1931:Erich Hückel
Erich Hückel
Erich Armand Arthur Joseph Hückel was a German physicist and physical chemist. He is known for two major contributions:*The Debye–Hückel theory of electrolytic solutions...
proposes Hückel's rule
Hückel's rule
In organic chemistry, Hückel's rule estimates whether a planar ring molecule will have aromatic properties. The quantum mechanical basis for its formulation was first worked out by physical chemist Erich Hückel in 1931...
, which explains when a planar ring molecule will have aromatic
Aromaticity
In organic chemistry, Aromaticity is a chemical property in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. The earliest use of the term was in an article by August...
properties.
1931:Harold Urey
Harold Urey
Harold Clayton Urey was an American physical chemist whose pioneering work on isotopes earned him the Nobel Prize in Chemistry in 1934...
discovers deuterium
Deuterium
Deuterium, also called heavy hydrogen, is one of two stable isotopes of hydrogen. It has a natural abundance in Earth's oceans of about one atom in of hydrogen . Deuterium accounts for approximately 0.0156% of all naturally occurring hydrogen in Earth's oceans, while the most common isotope ...
by fractionally distilling
Fractional distillation
Fractional distillation is the separation of a mixture into its component parts, or fractions, such as in separating chemical compounds by their boiling point by heating them to a temperature at which several fractions of the compound will evaporate. It is a special type of distillation...
liquid hydrogen.
1932:James Chadwick
James Chadwick
Sir James Chadwick CH FRS was an English Nobel laureate in physics awarded for his discovery of the neutron....
discovers the neutron
Neutron
The neutron is a subatomic hadron particle which has the symbol or , no net electric charge and a mass slightly larger than that of a proton. With the exception of hydrogen, nuclei of atoms consist of protons and neutrons, which are therefore collectively referred to as nucleons. The number of...
.
1932–1934:Linus Pauling and Robert Mulliken quantify electronegativity
Electronegativity
Electronegativity, symbol χ , is a chemical property that describes the tendency of an atom or a functional group to attract electrons towards itself. An atom's electronegativity is affected by both its atomic number and the distance that its valence electrons reside from the charged nucleus...
, devising the scales that now bear their names.
1937:Carlo Perrier
Carlo Perrier
Carlo Perrier was an Italian mineralogist who did extensive research on the element technetium in 1936. He discovered the element along with his colleague, Emilio Segrè , in 1937....
and Emilio Segrè perform the first confirmed synthesis of technetium-97
Technetium
Technetium is the chemical element with atomic number 43 and symbol Tc. It is the lowest atomic number element without any stable isotopes; every form of it is radioactive. Nearly all technetium is produced synthetically and only minute amounts are found in nature...
, the first artificially produced element, filling a gap in the periodic table. Though disputed, the element may have been synthesized as early as 1925 by Walter Noddack
Walter Noddack
Walter Noddack was a German chemist. He, Ida Tacke , and Otto Berg reported the discovery of element 43 and element 75 in 1925.-Rhenium:...
and others.
1937:Eugene Houdry
Eugene Houdry
Eugene Houdry was a French mechanical engineer who invented catalytic cracking of petroleum feed stocks. He originally focused on using lignite as a feedstock, but switched to using heavy liquid tars after moving to the United States in 1930...
develops a method of industrial scale catalytic cracking of petroleum, leading to the development of the first modern oil refinery.
1937:Pyotr Kapitsa
Pyotr Kapitsa
Pyotr Leonidovich Kapitsa was a prominent Soviet/Russian physicist and Nobel laureate.-Biography:Kapitsa was born in the city of Kronstadt and graduated from the Petrograd Polytechnical Institute in 1918. He worked for over ten years with Ernest Rutherford in the Cavendish Laboratory in Cambridge...
, John Allen
John F. Allen (physicist)
John "Jack" Frank Allen was a Canadian-born physicist. Along with Pyotr Leonidovich Kapitsa and Don Misener, Allen discovered the superfluid phase of matter in 1937 using liquid helium in the Royal Society Mond Laboratory in Cambridge, England...
and Don Misener
Don Misener
Don Misener was a physicist. Along with Pyotr Leonidovich Kapitsa and John F. Allen, Misener discovered the superfluid phase of matter in 1937....
produce supercooled helium-4
Helium
Helium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table...
, the first zero-viscosity superfluid
Superfluid
Superfluidity is a state of matter in which the matter behaves like a fluid without viscosity and with extremely high thermal conductivity. The substance, which appears to be a normal liquid, will flow without friction past any surface, which allows it to continue to circulate over obstructions and...
, a substance that displays quantum mechanical properties on a macroscopic scale.
1938:Otto Hahn
Otto Hahn
Otto Hahn FRS was a German chemist and Nobel laureate, a pioneer in the fields of radioactivity and radiochemistry. He is regarded as "the father of nuclear chemistry". Hahn was a courageous opposer of Jewish persecution by the Nazis and after World War II he became a passionate campaigner...
discovers the process of nuclear fission
Nuclear fission
In nuclear physics and nuclear chemistry, nuclear fission is a nuclear reaction in which the nucleus of an atom splits into smaller parts , often producing free neutrons and photons , and releasing a tremendous amount of energy...
in uranium
Uranium
Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons...
and thorium
Thorium
Thorium is a natural radioactive chemical element with the symbol Th and atomic number 90. It was discovered in 1828 and named after Thor, the Norse god of thunder....
.
1939:Linus Pauling publishes The Nature of the Chemical Bond, a compilation of a decades worth of work on chemical bonding. It is one of the most important modern chemical texts. It explains hybridization theory, covalent bonding and ionic bonding as explained through electronegativity, and resonance
Resonance (chemistry)
In chemistry, resonance or mesomerism is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by one single Lewis formula...
as a means to explain, among other things, the structure of benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
.
1940:Edwin McMillan
Edwin McMillan
Edwin Mattison McMillan was an American physicist and Nobel laureate credited with being the first ever to produce a transuranium element. He shared the Nobel Prize in Chemistry with Glenn Seaborg in 1951....
and Philip H. Abelson identify neptunium
Neptunium
Neptunium is a chemical element with the symbol Np and atomic number 93. A radioactive metal, neptunium is the first transuranic element and belongs to the actinide series. Its most stable isotope, 237Np, is a by-product of nuclear reactors and plutonium production and it can be used as a...
, the lightest and first synthesized transuranium element
Transuranium element
In chemistry, transuranium elements are the chemical elements with atomic numbers greater than 92...
, found in the products of uranium fission
Nuclear fission
In nuclear physics and nuclear chemistry, nuclear fission is a nuclear reaction in which the nucleus of an atom splits into smaller parts , often producing free neutrons and photons , and releasing a tremendous amount of energy...
. McMillan would found a lab at Berkley that would be involved in the discovery of many new elements and isotopes.
1941:Glenn T. Seaborg
Glenn T. Seaborg
Glenn Theodore Seaborg was an American scientist who won the 1951 Nobel Prize in Chemistry for "discoveries in the chemistry of the transuranium elements", contributed to the discovery and isolation of ten elements, and developed the actinide concept, which led to the current arrangement of the...
takes over McMillan's work creating new atomic nuclei. Pioneers method of neutron capture
Neutron capture
Neutron capture is a kind of nuclear reaction in which an atomic nucleus collides with one or more neutrons and they merge to form a heavier nucleus. Since neutrons have no electric charge they can enter a nucleus more easily than positively charged protons, which are repelled...
and later through other nuclear reactions. Would become the principal or co-discoverer of nine new chemical elements, and dozens of new isotopes of existing elements.
1945:Jacob A. Marinsky
Jacob A. Marinsky
Jacob Akiba Marinsky was a chemist who was the co-discoverer of the element promethium.Marinsky was born in Buffalo, New York, and attended the University at Buffalo, entering at age 16 and receiving a bachelor's degree in chemistry in 1939.During World War II he was employed as a chemist for the...
, Lawrence E. Glendenin
Lawrence E. Glendenin
Lawrence Elgin Glendenin was an American chemist who co-discovered the element promethium.- Biography :Glendenin was born in Bay City, Michigan on November 8, 1918...
, and Charles D. Coryell
Charles D. Coryell
Charles DuBois Coryell was an American chemist who was one of the discoverers of the element promethium....
perform the first confirmed synthesis of Promethium
Promethium
Promethium is a chemical element with the symbol Pm and atomic number 61. It is notable for being the only exclusively radioactive element besides technetium that is followed by chemical elements with stable isotopes.- Prediction :...
, filling in the last "gap" in the periodic table.
1945–1946: Felix Bloch
Felix Bloch
Felix Bloch was a Swiss physicist, working mainly in the U.S.-Life and work:Bloch was born in Zürich, Switzerland to Jewish parents Gustav and Agnes Bloch. He was educated there and at the Eidgenössische Technische Hochschule, also in Zürich. Initially studying engineering he soon changed to physics...
and Edward Mills Purcell
Edward Mills Purcell
Edward Mills Purcell was an American physicist who shared the 1952 Nobel Prize for Physics for his independent discovery of nuclear magnetic resonance in liquids and in solids. Nuclear magnetic resonance has become widely used to study the molecular structure of pure materials and the...
develop the process of Nuclear Magnetic Resonance
Nuclear magnetic resonance
Nuclear magnetic resonance is a physical phenomenon in which magnetic nuclei in a magnetic field absorb and re-emit electromagnetic radiation...
, an analytical
Analytical chemistry
Analytical chemistry is the study of the separation, identification, and quantification of the chemical components of natural and artificial materials. Qualitative analysis gives an indication of the identity of the chemical species in the sample and quantitative analysis determines the amount of...
technique important in elucidating structures of molecules, especially in organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
.
1951:Linus Pauling uses X-ray crystallography to deduce the secondary structure
Secondary structure
In biochemistry and structural biology, secondary structure is the general three-dimensional form of local segments of biopolymers such as proteins and nucleic acids...
of proteins.
1952:Alan Walsh
Alan Walsh (scientist)
Alan Walsh FRS was a British and Australian physicist who won the Royal Medal in 1976. He was most notable for his work on Atomic absorption spectroscopy. In 1977, he was created a Knight Bachelor for 'his distinguished service to science'...
pioneers the field of atomic absorption spectroscopy
Atomic absorption spectroscopy
Atomic absorption spectroscopy is a spectroanalytical procedure for the qualitative and quantitative determination of chemical elements employing the absorption of optical radiation by free atoms in the gaseous state. In analytical chemistry the technique is used for determining the concentration...
, an important quantitative
Quantitative property
A quantitative property is one that exists in a range of magnitudes, and can therefore be measured with a number. Measurements of any particular quantitative property are expressed as a specific quantity, referred to as a unit, multiplied by a number. Examples of physical quantities are distance,...
spectroscopy method that allows one to measure specific concentrations of a material in a mixture.
1952:Robert Burns Woodward
Robert Burns Woodward
Robert Burns Woodward was an American organic chemist, considered by many to be the preeminent organic chemist of the twentieth century...
, Geoffrey Wilkinson
Geoffrey Wilkinson
Sir Geoffrey Wilkinson FRS was a Nobel laureate English chemist who pioneered inorganic chemistry and homogeneous transition metal catalysis.-Biography:...
, and Ernst Otto Fischer
Ernst Otto Fischer
Ernst Otto Fischer was a German chemist who won the Nobel Prize for pioneering work in the area of organometallic chemistry.-Early life:...
discover the structure of ferrocene
Ferrocene
Ferrocene is an organometallic compound with the formula Fe2. It is the prototypical metallocene, a type of organometallic chemical compound consisting of two cyclopentadienyl rings bound on opposite sides of a central metal atom. Such organometallic compounds are also known as sandwich compounds...
, one of the founding discoveries of the field of organometallic chemistry
Organometallic chemistry
Organometallic chemistry is the study of chemical compounds containing bonds between carbon and a metal. Since many compounds without such bonds are chemically similar, an alternative may be compounds containing metal-element bonds of a largely covalent character...
.
1953:James D. Watson
James D. Watson
James Dewey Watson is an American molecular biologist, geneticist, and zoologist, best known as one of the co-discoverers of the structure of DNA in 1953 with Francis Crick...
and Francis Crick
Francis Crick
Francis Harry Compton Crick OM FRS was an English molecular biologist, biophysicist, and neuroscientist, and most noted for being one of two co-discoverers of the structure of the DNA molecule in 1953, together with James D. Watson...
propose the structure of DNA
DNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
, opening the door to the field of molecular biology
Molecular biology
Molecular biology is the branch of biology that deals with the molecular basis of biological activity. This field overlaps with other areas of biology and chemistry, particularly genetics and biochemistry...
.
1957: Jens Skou discovers Na⁺/K⁺-ATPase, the first ion-transporting enzyme.
1958:Max Perutz
Max Perutz
Max Ferdinand Perutz, OM, CH, CBE, FRS was an Austrian-born British molecular biologist, who shared the 1962 Nobel Prize for Chemistry with John Kendrew, for their studies of the structures of hemoglobin and globular proteins...
and John Kendrew
John Kendrew
Sir John Cowdery Kendrew, CBE, FRS was an English biochemist and crystallographer who shared the 1962 Nobel Prize in Chemistry with Max Perutz; their group in the Cavendish Laboratory investigated the structure of heme-containing proteins.-Biography:He was born in Oxford, son of Wilford George...
use X-ray crystallography to elucidate a protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
structure, specifically Sperm Whale
Sperm Whale
The sperm whale, Physeter macrocephalus, is a marine mammal species, order Cetacea, a toothed whale having the largest brain of any animal. The name comes from the milky-white waxy substance, spermaceti, found in the animal's head. The sperm whale is the only living member of genus Physeter...
myoglobin
Myoglobin
Myoglobin is an iron- and oxygen-binding protein found in the muscle tissue of vertebrates in general and in almost all mammals. It is related to hemoglobin, which is the iron- and oxygen-binding protein in blood, specifically in the red blood cells. The only time myoglobin is found in the...
.
1962:Neil Bartlett synthesizes xenon hexafluoroplatinate
Xenon hexafluoroplatinate
Xenon hexafluoroplatinate is the name of the product of the reaction of platinum hexafluoride and xenon, in an experiment that proved the chemical reactivity of the noble gases...
, showing for the first time that the noble gases can form chemical compounds.
1962:George Olah observes carbocation
Carbocation
A carbocation is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability . Therefore carbocations are often reactive,...
s via superacid
Superacid
According to the classical definition superacid is an acid with an acidity greater than that of 100% pure sulfuric acid, which has a Hammett acidity function of −12. According to the modern definition, superacid is a medium, in which the chemical potential of the proton is higher than in pure...
reactions.
1964:Richard R. Ernst
Richard R. Ernst
Richard Robert Ernst is a Swiss physical chemist and Nobel Laureate.Born in Winterthur, Switzerland, Ernst was awarded the Nobel Prize in Chemistry in 1991 for his contributions towards the development of Fourier Transform nuclear magnetic resonance spectroscopy while at Varian Associates, Palo...
performs experiments that will lead to the development of the technique of Fourier Transform
Fourier transform
In mathematics, Fourier analysis is a subject area which grew from the study of Fourier series. The subject began with the study of the way general functions may be represented by sums of simpler trigonometric functions...
NMR. This would greatly increase the sensitivity of the technique, and open the door for magnetic resonance imaging
Magnetic resonance imaging
Magnetic resonance imaging , nuclear magnetic resonance imaging , or magnetic resonance tomography is a medical imaging technique used in radiology to visualize detailed internal structures...
or MRI.
1965:Robert Burns Woodward and Roald Hoffmann
Roald Hoffmann
Roald Hoffmann is an American theoretical chemist who won the 1981 Nobel Prize in Chemistry. He currently teaches at Cornell University in Ithaca, New York.-Escape from the Holocaust:...
propose the Woodward-Hoffmann rules
Woodward-Hoffmann rules
The Woodward–Hoffmann rules devised by Robert Burns Woodward and Roald Hoffmann are a set of rules in organic chemistry predicting the stereochemistry of pericyclic reactions based on orbital symmetry. These include electrocyclic reactions, cycloadditions , sigmatropic reactions, and group transfer...
, which use the symmetry of molecular orbital
Molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term "orbital" was first...
s to explain the stereochemistry of chemical reactions.
1966:Hotosi Nozaki and Ryōji Noyori
Ryoji Noyori
is a Japanese chemist. He won the Nobel Prize in Chemistry in 2001. Noyori shared half of the prize with William S. Knowles for the study of chirally catalyzed hydrogenations; the second half of the Prize went to K. Barry Sharpless for his study in chirally catalyzed oxidation reactions...
discovered the first example of asymmetric catalysis (hydrogenation
Hydrogenation
Hydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
) using a structurally well-defined chiral
Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
transition metal
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
complex.
1970: John Pople
John Pople
Sir John Anthony Pople, KBE, FRS, was a Nobel-Prize winning theoretical chemist. Born in Burnham-on-Sea, Somerset, England, he attended Bristol Grammar School. He won a scholarship to Trinity College, Cambridge in 1943. He received his B. A. in 1946. Between 1945 and 1947 he worked at the Bristol...
develops the GAUSSIAN
GAUSSIAN
Gaussian is a computational chemistry software program initially released in 1970 by John Pople and his research group at Carnegie-Mellon University as Gaussian 70. It has been continuously updated since then...
program greatly easing computational chemistry
Computational chemistry
Computational chemistry is a branch of chemistry that uses principles of computer science to assist in solving chemical problems. It uses the results of theoretical chemistry, incorporated into efficient computer programs, to calculate the structures and properties of molecules and solids...
calculations.
1971:Yves Chauvin
Yves Chauvin
Yves Chauvin is a French chemist and Nobel Prize laureate. He is honorary research director at the Institut français du pétrole and a member of the French Academy of Science. Chauvin received his degree from the Lyon School of Chemistry, Physics and Electronics in 1954.He was awarded the 2005...
offered an explanation of the reaction mechanism of olefin metathesis
Olefin metathesis
Olefin metathesis or transalkylidenation is an organic reaction that entails redistribution of alkylene fragments by the scission of carbon - carbon double bonds in olefins . Its advantages include the creation of fewer sideproducts and hazardous wastes. Yves Chauvin, Robert H. Grubbs, and Richard R...
reactions.
1975:Karl Barry Sharpless and group discover a stereoselective oxidation reactions including Sharpless epoxidation
Sharpless epoxidation
The Sharpless Epoxidation reaction is an enantioselective chemical reaction to prepare 2,3-epoxyalcohols from primary and secondary allylic alcohols....
, Sharpless asymmetric dihydroxylation
Sharpless asymmetric dihydroxylation
Sharpless asymmetric dihydroxylation is the chemical reaction of an alkene with osmium tetroxide in the presence of a chiral quinine ligand to form a vicinal diol....
, and Sharpless oxyamination
Sharpless oxyamination
The Sharpless oxyamination is the chemical reaction of alkenes with alkyl imido osmium compounds to form vicinal amino-alcohols. A comprehensive review of this reaction was authored by McLeod et al. in 2002.Vicinal amino-alcohols are important products in organic synthesis and recurring...
.

Harold Kroto
Sir Harold Walter Kroto, FRS , born Harold Walter Krotoschiner, is a British chemist and one of the three recipients to share the 1996 Nobel Prize in Chemistry with Robert Curl and Richard Smalley....
, Robert Curl
Robert Curl
Robert Floyd Curl, Jr. the son of a Methodist Minister is a graduate of Thomas Jefferson High School in San Antonio, Texas and is an emeritus professor of chemistry at Rice University....
and Richard Smalley
Richard Smalley
Richard Errett Smalley was the Gene and Norman Hackerman Professor of Chemistry and a Professor of Physics and Astronomy at Rice University, in Houston, Texas...
discover fullerenes, a class of large carbon molecules superficially resembling the geodesic dome
Geodesic dome
A geodesic dome is a spherical or partial-spherical shell structure or lattice shell based on a network of great circles on the surface of a sphere. The geodesics intersect to form triangular elements that have local triangular rigidity and also distribute the stress across the structure. When...
designed by architect R. Buckminster Fuller.
1991:Sumio Iijima
Sumio Iijima
Sumio Iijima is a Japanese physicist, often cited as the discoverer of carbon nanotubes. Although carbon nanotubes had been observed prior to his "discovery", Iijima's 1991 paper generated unprecedented interest in the carbon nanostructures and has since fueled intense research in the area of...
uses electron microscopy to discover a type of cylindrical fullerene known as a carbon nanotube
Carbon nanotube
Carbon nanotubes are allotropes of carbon with a cylindrical nanostructure. Nanotubes have been constructed with length-to-diameter ratio of up to 132,000,000:1, significantly larger than for any other material...
, though earlier work had been done in the field as early as 1951. This material is an important component in the field of nanotechnology
Nanotechnology
Nanotechnology is the study of manipulating matter on an atomic and molecular scale. Generally, nanotechnology deals with developing materials, devices, or other structures possessing at least one dimension sized from 1 to 100 nanometres...
.
1994:First total synthesis of Taxol
Holton Taxol total synthesis
The Holton Taxol total synthesis, published by Robert A. Holton and his group at Florida State University in 1994 was the first total synthesis of Taxol ....
by Robert A. Holton
Robert A. Holton
Robert A. Holton is an American academic chemist who is known for his work regarding the chemical synthesis for Taxol , a widely-utilized and highly-effective anti-cancer drug. He is a Professor of Chemistry at Florida State University. Dr. Holton’s research group has accomplished the total...
and his group.
1995:Eric Cornell and Carl Wieman
Carl Wieman
Carl Edwin Wieman is an American physicist at the University of British Columbia and recipient of the Nobel Prize in Physics for the production, in 1995 with Eric Allin Cornell, of the first true Bose–Einstein condensate.-Biography:...
produce the first Bose–Einstein condensate
Bose–Einstein condensate
A Bose–Einstein condensate is a state of matter of a dilute gas of weakly interacting bosons confined in an external potential and cooled to temperatures very near absolute zero . Under such conditions, a large fraction of the bosons occupy the lowest quantum state of the external potential, at...
, a substance that displays quantum mechanical properties on the macroscopic scale.
See also
- History of chemistryHistory of chemistryBy 1000 BC, ancient civilizations used technologies that would eventually form the basis of the various branches of chemistry. Examples include extracting metals from ores, making pottery and glazes, fermenting beer and wine, making pigments for cosmetics and painting, extracting chemicals from...
- Discoveries of the chemical elementsDiscoveries of the chemical elementsThe discovery of the elements known to exist today is presented here in chronological order. The elements are listed generally in the order in which each was first defined as the pure element, as the exact date of discovery of most elements cannot be accurately defined.Given is each element's name,...
- Nobel Prize in chemistryNobel Prize in ChemistryThe Nobel Prize in Chemistry is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for outstanding contributions in chemistry, physics, literature,...
Further reading
- Servos, John W.John ServosJohn William Servos is an American professor and historian of science. His research centers on the historical development of science as a discourse and in the form of institutions and on how science has situated itself historically in the culture at large.Servos is the Anson D...
, Physical chemistry from Ostwald to Pauling : the making of a science in America, Princeton, N.J. : Princeton University Press, 1990. ISBN 0691085668

