Uranium
Overview
Uranium is a silvery-white metal
lic chemical element
in the actinide
series of the periodic table
, with atomic number
92. It is assigned the chemical symbol
U. A uranium atom has 92 proton
s and 92 electron
s, of which 6 are valence electron
s. The uranium nucleus binds between 141 and 146 neutron
s, establishing six isotopes (U-233 through U-238), the most common of which are uranium-238
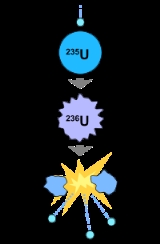
(146 neutrons) and uranium-235
(143 neutrons). All isotopes are unstable and uranium is weakly radioactive
.
Metal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
lic chemical element
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
in the actinide
Actinide
The actinide or actinoid series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium.The actinide series derives its name from the group 3 element actinium...
series of the periodic table
Periodic table
The periodic table of the chemical elements is a tabular display of the 118 known chemical elements organized by selected properties of their atomic structures. Elements are presented by increasing atomic number, the number of protons in an atom's atomic nucleus...
, with atomic number
Atomic number
In chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element...
92. It is assigned the chemical symbol
Chemical symbol
A chemical symbol is a 1- or 2-letter internationally agreed code for a chemical element, usually derived from the name of the element, often in Latin. Only the first letter is capitalised...
U. A uranium atom has 92 proton
Proton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
s and 92 electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s, of which 6 are valence electron
Valence electron
In chemistry, valence electrons are the electrons of an atom that can participate in the formation of chemical bonds with other atoms. Valence electrons are the "own" electrons, present in the free neutral atom, that combine with valence electrons of other atoms to form chemical bonds. In a single...
s. The uranium nucleus binds between 141 and 146 neutron
Neutron
The neutron is a subatomic hadron particle which has the symbol or , no net electric charge and a mass slightly larger than that of a proton. With the exception of hydrogen, nuclei of atoms consist of protons and neutrons, which are therefore collectively referred to as nucleons. The number of...
s, establishing six isotopes (U-233 through U-238), the most common of which are uranium-238
Uranium-238
Uranium-238 is the most common isotope of uranium found in nature. It is not fissile, but is a fertile material: it can capture a slow neutron and after two beta decays become fissile plutonium-239...
(146 neutrons) and uranium-235
Uranium-235
- References :* .* DOE Fundamentals handbook: Nuclear Physics and Reactor theory , .* A piece of U-235 the size of a grain of rice can produce energy equal to that contained in three tons of coal or fourteen barrels of oil. -External links:* * * one of the earliest articles on U-235 for the...
(143 neutrons). All isotopes are unstable and uranium is weakly radioactive
Radioactive decay
Radioactive decay is the process by which an atomic nucleus of an unstable atom loses energy by emitting ionizing particles . The emission is spontaneous, in that the atom decays without any physical interaction with another particle from outside the atom...
.

