
Chemical kinetics
Encyclopedia
Reaction rate
The reaction rate or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a reaction takes place...
of chemical processes. Chemical kinetics includes investigations of how different experimental conditions can influence the speed of a chemical reaction and yield information about the reaction's mechanism
Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
and transition state
Transition state
The transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest energy along this reaction coordinate. At this point, assuming a perfectly irreversible reaction, colliding reactant molecules will always...
s, as well as the construction of mathematical models that can describe the characteristics of a chemical reaction. In 1864, Peter Waage
Peter Waage
Peter Waage , the son of a ship's captain, was a significant Norwegian chemist and professor at the Royal Frederick University. Along with his brother-in-law Cato Maximilian Guldberg, he co-discovered and developed the law of mass action between 1864 and 1879.He grew up in Hidra...
and Cato Guldberg pioneered the development of chemical kinetics by formulating the law of mass action, which states that the speed of a chemical reaction is proportional to the quantity of the reacting substances.
Chemical kinetics deals with the experimental determination of reaction rate
Reaction rate
The reaction rate or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a reaction takes place...
s from which rate laws and rate constants are derived. Relatively simple rate laws exist for zero-order reactions (for which reaction rates are independent of concentration), first-order reaction
First-order reaction
First-order reaction may refer to:* Order of reaction, in chemical kinetics, the power to which the concentration term of a certain reactant in the rate equation is raised...
s, and second-order reactions, and can be derived for others. In consecutive reactions, the rate-determining step
Rate-determining step
The rate-determining step is a chemistry term for the slowest step in a chemical reaction. The rate-determining step is often compared to the neck of a funnel; the rate at which water flows through the funnel is determined by the width of the neck, not by the speed at which water is poured in. In...
often determines the kinetics. In consecutive first-order reactions, a steady state
Steady state (chemistry)
In chemistry, a steady state is a situation in which all state variables are constant in spite of ongoing processes that strive to change them. For an entire system to be at steady state, i.e. for all state variables of a system to be constant, there must be a flow through the system...
approximation can simplify the rate law. The activation energy
Activation energy
In chemistry, activation energy is a term introduced in 1889 by the Swedish scientist Svante Arrhenius that is defined as the energy that must be overcome in order for a chemical reaction to occur. Activation energy may also be defined as the minimum energy required to start a chemical reaction...
for a reaction is experimentally determined through the Arrhenius equation
Arrhenius equation
The Arrhenius equation is a simple, but remarkably accurate, formula for the temperature dependence of the reaction rate constant, and therefore, rate of a chemical reaction. The equation was first proposed by the Dutch chemist J. H. van 't Hoff in 1884; five years later in 1889, the Swedish...
and the Eyring equation
Eyring equation
The Eyring equation also known as Eyring–Polanyi equation in chemical kinetics relates the reaction rate to temperature. It was developed almost simultaneously in 1935 by Henry Eyring, M.G. Evans and Michael Polanyi...
. The main factors that influence the reaction rate
Reaction rate
The reaction rate or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a reaction takes place...
include: the physical state of the reactants, the concentrations of the reactants, the temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
at which the reaction occurs, and whether or not any catalysts are present in the reaction.
Nature of the reactants
Depending upon what substances are reacting, the reaction rate varies. Acid/base reactions, the formation of salts, and ion exchangeIon exchange
Ion exchange is an exchange of ions between two electrolytes or between an electrolyte solution and a complex. In most cases the term is used to denote the processes of purification, separation, and decontamination of aqueous and other ion-containing solutions with solid polymeric or mineralic 'ion...
are fast reactions. When covalent bond formation takes place between the molecules and when large molecules are formed, the reactions tend to be very slow.
Nature and strength of bonds in reactant molecules greatly influence the rate of its transformation into products. The reactions that involve lesser bond rearrangement proceed faster than the reactions that involve larger bond rearrangement.
Physical state
The physical state (solidSolid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
, liquid
Liquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
, or gas
Gas
Gas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
) of a reactant is also an important factor of the rate of change. When reactants are in the same phase
Phase (matter)
In the physical sciences, a phase is a region of space , throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, and chemical composition...
, as in aqueous solution
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
, thermal motion brings them into contact. However, when they are in different phases, the reaction is limited to the interface between the reactants. Reaction can occur only at their area of contact; in the case of a liquid and a gas, at the surface of the liquid. Vigorous shaking and stirring may be needed to bring the reaction to completion. This means that the more finely divided a solid or liquid reactant the greater its surface area
Surface area
Surface area is the measure of how much exposed area a solid object has, expressed in square units. Mathematical description of the surface area is considerably more involved than the definition of arc length of a curve. For polyhedra the surface area is the sum of the areas of its faces...
per unit volume
Volume
Volume is the quantity of three-dimensional space enclosed by some closed boundary, for example, the space that a substance or shape occupies or contains....
and the more contact it makes with the other reactant, thus the faster the reaction. To make an analogy, for example, when one starts a fire, one uses wood chips and small branches — one does not start with large logs right away. In organic chemistry, on water reaction
On water reaction
On water reactions are a group of organic reactions that take place as an emulsion in water and that exhibit an unusual reaction rate acceleration compared to the same reaction in an organic solvent or compared to the corresponding dry media reaction. This effect has been known for many years but...
s are the exception to the rule that homogeneous reactions take place faster than heterogeneous reactions.
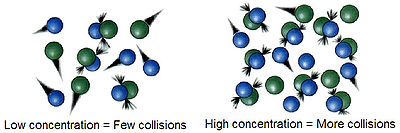
Concentration
ConcentrationConcentration
In chemistry, concentration is defined as the abundance of a constituent divided by the total volume of a mixture. Four types can be distinguished: mass concentration, molar concentration, number concentration, and volume concentration...
plays a very important role in reactions, because, according to the collision theory
Collision theory
Collision theory is a theory proposed by Max Trautz and William Lewis in 1916 and 1918, that qualitatively explains how chemical reactions occur and why reaction rates differ for different reactions. For a reaction to occur the reactant particles must collide. Only a certain fraction of the total...
of chemical reactions, molecules must collide in order to react together. As the concentration of the reactants increases, the frequency
Frequency
Frequency is the number of occurrences of a repeating event per unit time. It is also referred to as temporal frequency.The period is the duration of one cycle in a repeating event, so the period is the reciprocal of the frequency...
of the molecules colliding increases, striking each other more frequently by being in closer contact at any given point in time. One may think of two reactants being in a closed container: All the molecules contained within are colliding constantly. By increasing the amount of one or more of the reactants, these collisions happen more often, increasing the reaction rate.
Temperature
TemperatureTemperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
usually has a major effect on the rate of a chemical reaction. Molecules at a higher temperature have more thermal energy
Thermal energy
Thermal energy is the part of the total internal energy of a thermodynamic system or sample of matter that results in the system's temperature....
. Although collision frequency is greater at higher temperatures, this alone contributes only a very small proportion to the increase in rate of reaction. Much more important is the fact that the proportion of reactant molecules with sufficient energy to react (energy greater than activation energy
Activation energy
In chemistry, activation energy is a term introduced in 1889 by the Swedish scientist Svante Arrhenius that is defined as the energy that must be overcome in order for a chemical reaction to occur. Activation energy may also be defined as the minimum energy required to start a chemical reaction...
: E > Ea) is significantly higher and is explained in detail by the Maxwell–Boltzmann distribution of molecular energies.
The 'rule of thumb' that the rate of chemical reactions doubles for every 10 °C temperature rise is a common misconception. This may have been generalized from the special case of biological systems, where the α (temperature coefficient)
Q10 (temperature coefficient)
The Q10 temperature coefficient is a measure of the rate of change of a biological or chemical system as a consequence of increasing the temperature by 10 °C. There are many examples where the Q10 is used, one being the calculation of the nerve conduction velocity and another being calculating the...
is often between 1.5 and 2.5.
A reaction's kinetics can also be studied with a temperature jump
Temperature Jump
Temperature jump is a technique used in the study of chemical kinetics. It usually involves the discharging of a capacitor through a small volume Temperature jump is a technique used in the study of chemical kinetics. It usually involves the discharging of a capacitor (in the kV range) through a...
approach. This involves using a sharp rise in temperature and observing the relaxation time
Relaxation time
In the physical sciences, relaxation usually means the return of a perturbed system into equilibrium.Each relaxation process can be characterized by a relaxation time τ...
of the return to equilibrium.
Catalysts
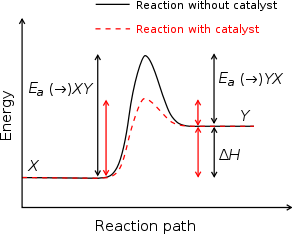
Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
to occur with a lower activation energy
Activation energy
In chemistry, activation energy is a term introduced in 1889 by the Swedish scientist Svante Arrhenius that is defined as the energy that must be overcome in order for a chemical reaction to occur. Activation energy may also be defined as the minimum energy required to start a chemical reaction...
. In autocatalysis
Autocatalysis
A single chemical reaction is said to have undergone autocatalysis, or be autocatalytic, if the reaction product itself is the catalyst for that reaction....
a reaction product is itself a catalyst for that reaction leading to positive feedback
Positive feedback
Positive feedback is a process in which the effects of a small disturbance on a system include an increase in the magnitude of the perturbation. That is, A produces more of B which in turn produces more of A. In contrast, a system that responds to a perturbation in a way that reduces its effect is...
. Proteins that act as catalysts in biochemical reactions are called enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
s. Michaelis-Menten kinetics
Michaelis-Menten kinetics
In biochemistry, Michaelis–Menten kinetics is one of the simplest and best-known models of enzyme kinetics. It is named after German biochemist Leonor Michaelis and Canadian physician Maud Menten. The model takes the form of an equation describing the rate of enzymatic reactions, by relating...
describe the rate of enzyme mediated reactions
Enzyme kinetics
Enzyme kinetics is the study of the chemical reactions that are catalysed by enzymes. In enzyme kinetics, the reaction rate is measured and the effects of varying the conditions of the reaction investigated...
. A catalyst does not affect the position of the equilibria, as the catalyst speeds up the backward and forward reactions equally.
In certain organic molecules, specific substituents can have an influence on reaction rate in neighbouring group participation
Neighbouring group participation
Neighbouring group participation or NGP in organic chemistry has been defined by IUPAC as the interaction of a reaction centre with a lone pair of electrons in an atom or the electrons present in a sigma bond or pi bond . When NGP is in operation it is normal for the reaction rate to be increased...
.
Agitating or mixing a solution will also accelerate the rate of a chemical reaction, as this gives the particles greater kinetic energy, increasing the number of collisions between reactants and, therefore, the possibility of successful collisions.
Pressure
Increasing the pressure in a gaseous reaction will increase the number of collisions between reactants, increasing the rate of reaction. This is because the activityActivity (chemistry)
In chemical thermodynamics, activity is a measure of the “effective concentration” of a species in a mixture, meaning that the species' chemical potential depends on the activity of a real solution in the same way that it would depend on concentration for an ideal solution.By convention, activity...
of a gas is directly proportional to the partial pressure of the gas. This is similar to the effect of increasing the concentration of a solution.
A reaction's kinetics can also be studied with a pressure jump
Pressure jump
Pressure jump is a technique used in the study of chemical kinetics. It involves making rapid changes to the pressure of an experimental system and observing the return to equilibrium or steady state...
approach. This involves making fast changes in pressure and observing the relaxation time
Relaxation time
In the physical sciences, relaxation usually means the return of a perturbed system into equilibrium.Each relaxation process can be characterized by a relaxation time τ...
of the return to equilibrium.
Equilibrium
While chemical kinetics is concerned with the rate of a chemical reaction, thermodynamicsThermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
determines the extent to which reactions occur. In a reversible reaction
Reversible reaction
A reversible reaction is a chemical reaction that results in an equilibrium mixture of reactants and products. For a reaction involving two reactants and two products this can be expressed symbolically as...
, chemical equilibrium is reached when the rates of the forward and reverse reactions are equal and the concentrations of the reactants and products
Product (chemistry)
Product are formed during chemical reactions as reagents are consumed. Products have lower energy than the reagents and are produced during the reaction according to the second law of thermodynamics. The released energy comes from changes in chemical bonds between atoms in reagent molecules and...
no longer change. This is demonstrated by, for example, the Haber–Bosch process for combining nitrogen and hydrogen to produce ammonia. Chemical clock
Chemical clock
A chemical clock is a complex mixture of reacting chemical compounds in which the concentration of one or more components exhibits periodic changes....
reactions such as the Belousov–Zhabotinsky reaction demonstrate that component concentrations can oscillate for a long time before finally attaining the equilibrium.
Free energy
In general terms, the free energy change (ΔG)Thermodynamic free energy
The thermodynamic free energy is the amount of work that a thermodynamic system can perform. The concept is useful in the thermodynamics of chemical or thermal processes in engineering and science. The free energy is the internal energy of a system less the amount of energy that cannot be used to...
of a reaction determines whether a chemical change will take place, but kinetics describes how fast the reaction is. A reaction can be very exothermic
Exothermic
In thermodynamics, the term exothermic describes a process or reaction that releases energy from the system, usually in the form of heat, but also in the form of light , electricity , or sound...
and have a very positive entropy
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
change but will not happen in practice if the reaction is too slow. If a reactant can produce two different products, the thermodynamically most stable one will in general form, except in special circumstances when the reaction is said to be under kinetic reaction control. The Curtin–Hammett principle applies when determining the product ratio for two reactants interconverting rapidly, each going to a different product. It is possible to make predictions about reaction rate constants for a reaction from free-energy relationship
Free-energy relationship
In physical organic chemistry, a free-energy relationship or linear Gibbs energy relation relates the logarithm of a reaction rate constant or equilibrium constant for one series of reactions with the logarithm of the rate or equilibrium constant for a related series of reactions...
s.
The kinetic isotope effect
Kinetic isotope effect
The kinetic isotope effect is the ratio of reaction rates of two different isotopically labeled molecules in a chemical reaction. It is also called "isotope fractionation," although this term is somewhat broader in meaning...
is the difference in the rate of a chemical reaction when an atom in one of the reactants is replaced by one of its isotope
Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
s.
Chemical kinetics provides information on residence time
Residence time
Residence time is the average amount of time that a particle spends in a particular system. This measurement varies directly with the amount of substance that is present in the system....
and heat transfer
Heat transfer
Heat transfer is a discipline of thermal engineering that concerns the exchange of thermal energy from one physical system to another. Heat transfer is classified into various mechanisms, such as heat conduction, convection, thermal radiation, and phase-change transfer...
in a chemical reactor
Chemical reactor
In chemical engineering, chemical reactors are vessels designed to contain chemical reactions. The design of a chemical reactor deals with multiple aspects of chemical engineering. Chemical engineers design reactors to maximize net present value for the given reaction...
in chemical engineering
Chemical engineering
Chemical engineering is the branch of engineering that deals with physical science , and life sciences with mathematics and economics, to the process of converting raw materials or chemicals into more useful or valuable forms...
and the molar mass distribution
Molar mass distribution
In linear polymers the individual polymer chains rarely have exactly the same degree of polymerization and molar mass, and there is always a distribution around an average value. The molar mass distribution in a polymer describes the relationship between the number of moles of each polymer species...
in polymer chemistry
Polymer chemistry
Polymer chemistry or macromolecular chemistry is a multidisciplinary science that deals with the chemical synthesis and chemical properties of polymers or macromolecules. According to IUPAC recommendations, macromolecules refer to the individual molecular chains and are the domain of chemistry...
.
Applications
The mathematical models that describe chemical reaction kinetics provide chemists and chemical engineers with tools to better understand and describe chemical processes such as food decomposition, microorganism growth, stratospheric ozone decomposition, and the complex chemistry of biological systems. These models can also be used in the design or modification of chemical reactors to optimize product yield, more efficiently separate products, and eliminate environmentally harmful by-products. When performing catalytic cracking of heavy hydrocarbons into gasoline and light gas, for example, kinetic models can be used to find the temperature and pressure at which the highest yield of heavy hydrocarbons into gasoline will occur. Kinetics is also a basic aspect of chemistry.See also
- Collision theoryCollision theoryCollision theory is a theory proposed by Max Trautz and William Lewis in 1916 and 1918, that qualitatively explains how chemical reactions occur and why reaction rates differ for different reactions. For a reaction to occur the reactant particles must collide. Only a certain fraction of the total...
- Arrhenius equationArrhenius equationThe Arrhenius equation is a simple, but remarkably accurate, formula for the temperature dependence of the reaction rate constant, and therefore, rate of a chemical reaction. The equation was first proposed by the Dutch chemist J. H. van 't Hoff in 1884; five years later in 1889, the Swedish...
- Autocatalytic reactions and order creation
- Flame speedFlame speedThe flame speed is the measured rate of expansion of the flame front in a combustion reaction. Whereas flame speed is generally used for a fuel, a related term is explosive velocity, which is the same relationship measured for an explosive. Combustion engineers differentiate between the laminar...
- DetonationDetonationDetonation involves a supersonic exothermic front accelerating through a medium that eventually drives a shock front propagating directly in front of it. Detonations are observed in both conventional solid and liquid explosives, as well as in reactive gases...
- Two-dimensional gasTwo-dimensional gasA two-dimensional gas is a collection of N objects which are constrained to move in a planar or other two-dimensional space in a gaseous state. The objects can be: ideal gas elements such as rigid disks undergoing elastic collisions; elementary particles, or any object in physics which obeys laws...
- EnthalpyEnthalpyEnthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
External links
- Chemical Kinetics
- Chemistry applets
- University of Waterloo
- Washington state university
- Chemical Kinetics of Gas Phase Reactions
- Chemical Kinetics Summary
- Kinpy: Python code generator for solving kinetic equations
- PottersWheelPottersWheelPottersWheel is a MATLAB toolbox for mathematical modeling of time-dependent dynamical systems that can be expressed as chemical reaction networks or ordinary differential equations . It allows the automatic calibration of model parameters by fitting the model to experimental measurements...
Matlab toolbox to fit chemical rate constants to experimental data

