
Quantum chemistry
Encyclopedia
Quantum chemistry is a branch of chemistry
whose primary focus is the application of quantum mechanics
in physical models and experiments of chemical systems. It involves heavy interplay of experimental and theoretical methods:
In these ways, quantum chemists investigate chemical phenomena.
, the 1859 statement of the black body radiation problem by Gustav Kirchhoff
, the 1877 suggestion by Ludwig Boltzmann
that the energy states of a physical system could be discrete, and the 1900 quantum hypothesis by Max Planck
that any energy radiating atomic system can theoretically be divided into a number of discrete energy elements ε such that each of these energy elements is proportional to the frequency
ν with which they each individually radiate energy
, as defined by the following formula:
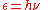
where h is a numerical value called Planck’s Constant. Then, in 1905, to explain the photoelectric effect
(1839), i.e., that shining light on certain materials can function to eject electrons from the material, Albert Einstein
postulated, based on Planck’s quantum hypothesis, that light
itself consists of individual quantum particles, which later came to be called photons (1926). In the years to follow, this theoretical basis slowly began to be applied to chemical structure, reactivity, and bonding.
(or Dirac equation
in relativistic quantum chemistry
) with the electronic molecular Hamiltonian. This is called determining the electronic structure of the molecule. It can be said that the electronic structure of a molecule or crystal implies essentially its chemical properties. An exact solution for the Schrödinger equation can only be obtained for the hydrogen atom. Since all other atomic, or molecular systems, involve the motions of three or more "particles", their Schrödinger equations cannot be solved exactly and so approximate solutions must be sought.
surrounded by electrons. Unlike the earlier Bohr model
of the atom, however, the wave model describes electrons as "clouds" moving in orbitals, and their positions are represented by probability distributions
rather than discrete points. The strength of this model lies in its predictive power
. Specifically, it predicts the pattern of chemically similar elements found in the periodic table. The wave model is so named because electrons exhibit properties (such as interference) traditionally associated with waves. See wave-particle duality.
Although the mathematical basis of quantum chemistry had been laid by Schrödinger
in 1926, it is generally accepted that the first true calculation in quantum chemistry was that of the German physicists Walter Heitler
and Fritz London
on the hydrogen (H2) molecule in 1927. Heitler and London's method was extended by the American theoretical physicist John C. Slater
and the American theoretical chemist Linus Pauling
to become the Valence-Bond (VB) [or Heitler-London-Slater-Pauling (HLSP)] method. In this method, attention is primarily devoted to the pairwise interactions between atoms, and this method therefore correlates closely with classical chemists' drawings of bonds
.
An alternative approach was developed in 1929 by Friedrich Hund
and Robert S. Mulliken
, in which electron
s are described by mathematical functions delocalized over an entire molecule
. The Hund-Mulliken approach or molecular orbital (MO) method is less intuitive to chemists, but has turned out capable of predicting spectroscopic properties
better than the VB method. This approach is the conceptional basis of the Hartree-Fock
method and further post Hartree-Fock methods.
The Thomas-Fermi model
was developed independently by Thomas and Fermi
in 1927. This was the first attempt to describe many-electron systems on the basis of electronic density
instead of wave functions, although it was not very successful in the treatment of entire molecules. The method did provide the basis for what is now known as density functional theory. Though this method is less developed than post Hartree-Fock methods, its significantly lower computational requirements (scaling typically no worse than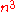 with respect to
with respect to  basis functions) allow it to tackle larger polyatomic molecules and even macromolecule
basis functions) allow it to tackle larger polyatomic molecules and even macromolecule
s. This computational affordability and often comparable accuracy to MP2 and CCSD (post-Hartree–Fock methods) has made it one of the most popular methods in computational chemistry
at present.
with the total molecular Hamiltonian
in order to study the motion of molecules. Direct solution of the Schrödinger equation is called quantum molecular dynamics, within the semiclassical approximation semiclassical molecular dynamics, and within the classical mechanics
framework molecular dynamics
(MD). Statistical approaches, using for example Monte Carlo method
s, are also possible.
potential
s called potential energy surface
s. This is the Born-Oppenheimer approximation
introduced by Born
and Oppenheimer
in 1927. Pioneering applications of this in chemistry were performed by Rice and Ramsperger in 1927 and Kassel in 1928, and generalized into the RRKM theory in 1952 by Marcus
who took the transition state
theory developed by Eyring
in 1935 into account. These methods enable simple estimates of unimolecular reaction rates from a few characteristics of the potential surface.
Non-adiabatic dynamics consists of taking the interaction between several coupled potential energy surface (corresponding to different electronic quantum states of the molecule). The coupling terms are called vibronic couplings. The pioneering work in this field was done by Stueckelberg
, Landau, and Zener
in the 1930s, in their work on what is now known as the Landau-Zener transition. Their formula allows the transition probability between two diabatic
potential curves in the neighborhood of an avoided crossing
to be calculated.
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
whose primary focus is the application of quantum mechanics
Quantum mechanics
Quantum mechanics, also known as quantum physics or quantum theory, is a branch of physics providing a mathematical description of much of the dual particle-like and wave-like behavior and interactions of energy and matter. It departs from classical mechanics primarily at the atomic and subatomic...
in physical models and experiments of chemical systems. It involves heavy interplay of experimental and theoretical methods:
- Experimental quantum chemists rely heavily on spectroscopySpectroscopySpectroscopy is the study of the interaction between matter and radiated energy. Historically, spectroscopy originated through the study of visible light dispersed according to its wavelength, e.g., by a prism. Later the concept was expanded greatly to comprise any interaction with radiative...
, through which information regarding the quantizationQuantizationQuantization is the procedure of constraining something from a relatively large or continuous set of values to a relatively small discrete set...
of energy on a molecular scale can be obtained. Common methods are infra-red (IR) spectroscopy and nuclear magnetic resonance (NMR) spectroscopy.
- Theoretical quantum chemistry, the workings of which also tend to fall under the category of computational chemistryComputational chemistryComputational chemistry is a branch of chemistry that uses principles of computer science to assist in solving chemical problems. It uses the results of theoretical chemistry, incorporated into efficient computer programs, to calculate the structures and properties of molecules and solids...
, seeks to calculate the predictions of quantum theory; as this task, when applied to polyatomic species, invokes the many-body problemMany-body problemThe many-body problem is a general name for a vast category of physical problems pertaining to the properties of microscopic systems made of a large number of interacting particles. Microscopic here implies that quantum mechanics has to be used to provide an accurate description of the system...
, these calculations are performed using computers rather than by back-of-the-envelope calculations.
In these ways, quantum chemists investigate chemical phenomena.
- In reactions, quantum chemistry studies the ground state of individual atoms and molecules, the excited states, and the transition states that occur during chemical reactions.
- On the calculations: quantum chemical studies use also semi-empirical and other methods based on quantum mechanical principles, and deal with time dependent problems. Many quantum chemical studies assume the nuclei are at rest (Born-Oppenheimer approximation). Many calculations involve iterative methods that include self-consistent field methods. Major goals of quantum chemistry include increasing the accuracy of the results for small molecular systems, and increasing the size of large molecules that can be processed, which is limited by scaling considerations—the computation time increases as a power of the number of atoms.
History
The history of quantum chemistry essentially began with the 1838 discovery of cathode rays by Michael FaradayMichael Faraday
Michael Faraday, FRS was an English chemist and physicist who contributed to the fields of electromagnetism and electrochemistry....
, the 1859 statement of the black body radiation problem by Gustav Kirchhoff
Gustav Kirchhoff
Gustav Robert Kirchhoff was a German physicist who contributed to the fundamental understanding of electrical circuits, spectroscopy, and the emission of black-body radiation by heated objects...
, the 1877 suggestion by Ludwig Boltzmann
Ludwig Boltzmann
Ludwig Eduard Boltzmann was an Austrian physicist famous for his founding contributions in the fields of statistical mechanics and statistical thermodynamics...
that the energy states of a physical system could be discrete, and the 1900 quantum hypothesis by Max Planck
Max Planck
Max Karl Ernst Ludwig Planck, ForMemRS, was a German physicist who actualized the quantum physics, initiating a revolution in natural science and philosophy. He is regarded as the founder of the quantum theory, for which he received the Nobel Prize in Physics in 1918.-Life and career:Planck came...
that any energy radiating atomic system can theoretically be divided into a number of discrete energy elements ε such that each of these energy elements is proportional to the frequency
Frequency
Frequency is the number of occurrences of a repeating event per unit time. It is also referred to as temporal frequency.The period is the duration of one cycle in a repeating event, so the period is the reciprocal of the frequency...
ν with which they each individually radiate energy
Energy
In physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
, as defined by the following formula:
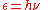
where h is a numerical value called Planck’s Constant. Then, in 1905, to explain the photoelectric effect
Photoelectric effect
In the photoelectric effect, electrons are emitted from matter as a consequence of their absorption of energy from electromagnetic radiation of very short wavelength, such as visible or ultraviolet light. Electrons emitted in this manner may be referred to as photoelectrons...
(1839), i.e., that shining light on certain materials can function to eject electrons from the material, Albert Einstein
Albert Einstein
Albert Einstein was a German-born theoretical physicist who developed the theory of general relativity, effecting a revolution in physics. For this achievement, Einstein is often regarded as the father of modern physics and one of the most prolific intellects in human history...
postulated, based on Planck’s quantum hypothesis, that light
Light
Light or visible light is electromagnetic radiation that is visible to the human eye, and is responsible for the sense of sight. Visible light has wavelength in a range from about 380 nanometres to about 740 nm, with a frequency range of about 405 THz to 790 THz...
itself consists of individual quantum particles, which later came to be called photons (1926). In the years to follow, this theoretical basis slowly began to be applied to chemical structure, reactivity, and bonding.
Electronic structure
The first step in solving a quantum chemical problem is usually solving the Schrödinger equationSchrödinger equation
The Schrödinger equation was formulated in 1926 by Austrian physicist Erwin Schrödinger. Used in physics , it is an equation that describes how the quantum state of a physical system changes in time....
(or Dirac equation
Dirac equation
The Dirac equation is a relativistic quantum mechanical wave equation formulated by British physicist Paul Dirac in 1928. It provided a description of elementary spin-½ particles, such as electrons, consistent with both the principles of quantum mechanics and the theory of special relativity, and...
in relativistic quantum chemistry
Relativistic quantum chemistry
Relativistic quantum chemistry invokes quantum chemical and relativistic mechanical arguments to explain elemental properties and structure, especially for heavy elements of the periodic table....
) with the electronic molecular Hamiltonian. This is called determining the electronic structure of the molecule. It can be said that the electronic structure of a molecule or crystal implies essentially its chemical properties. An exact solution for the Schrödinger equation can only be obtained for the hydrogen atom. Since all other atomic, or molecular systems, involve the motions of three or more "particles", their Schrödinger equations cannot be solved exactly and so approximate solutions must be sought.
Wave model
The foundation of quantum mechanics and quantum chemistry is the wave model, in which the atom is a small, dense, positively charged nucleusAtomic nucleus
The nucleus is the very dense region consisting of protons and neutrons at the center of an atom. It was discovered in 1911, as a result of Ernest Rutherford's interpretation of the famous 1909 Rutherford experiment performed by Hans Geiger and Ernest Marsden, under the direction of Rutherford. The...
surrounded by electrons. Unlike the earlier Bohr model
Bohr model
In atomic physics, the Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with electrostatic forces providing attraction,...
of the atom, however, the wave model describes electrons as "clouds" moving in orbitals, and their positions are represented by probability distributions
Probability amplitude
In quantum mechanics, a probability amplitude is a complex number whose modulus squared represents a probability or probability density.For example, if the probability amplitude of a quantum state is \alpha, the probability of measuring that state is |\alpha|^2...
rather than discrete points. The strength of this model lies in its predictive power
Predictive power
The predictive power of a scientific theory refers to its ability to generate testable predictions. Theories with strong predictive power are highly valued, because the predictions can often encourage the falsification of the theory...
. Specifically, it predicts the pattern of chemically similar elements found in the periodic table. The wave model is so named because electrons exhibit properties (such as interference) traditionally associated with waves. See wave-particle duality.
Valence bond
Although the mathematical basis of quantum chemistry had been laid by Schrödinger
Erwin Schrödinger
Erwin Rudolf Josef Alexander Schrödinger was an Austrian physicist and theoretical biologist who was one of the fathers of quantum mechanics, and is famed for a number of important contributions to physics, especially the Schrödinger equation, for which he received the Nobel Prize in Physics in 1933...
in 1926, it is generally accepted that the first true calculation in quantum chemistry was that of the German physicists Walter Heitler
Walter Heitler
Walter Heinrich Heitler was a German physicist who made contributions to quantum electrodynamics and quantum field theory...
and Fritz London
Fritz London
Fritz Wolfgang London was a German theoretical physicist. His fundamental contributions to the theories of chemical bonding and of intermolecular forces are today considered classic and are discussed in standard textbooks of physical chemistry.With his brother Heinz, he made a significant...
on the hydrogen (H2) molecule in 1927. Heitler and London's method was extended by the American theoretical physicist John C. Slater
John C. Slater
John Clarke Slater was a noted American physicist who made major contributions to the theory of the electronic structure of atoms, molecules and solids. This work is of ongoing importance in chemistry, as well as in many areas of physics. He also made major contributions to microwave electronics....
and the American theoretical chemist Linus Pauling
Linus Pauling
Linus Carl Pauling was an American chemist, biochemist, peace activist, author, and educator. He was one of the most influential chemists in history and ranks among the most important scientists of the 20th century...
to become the Valence-Bond (VB) [or Heitler-London-Slater-Pauling (HLSP)] method. In this method, attention is primarily devoted to the pairwise interactions between atoms, and this method therefore correlates closely with classical chemists' drawings of bonds
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
.
Molecular orbital
An alternative approach was developed in 1929 by Friedrich Hund
Friedrich Hund
Friedrich Hermann Hund was a German physicist from Karlsruhe known for his work on atoms and molecules.Hund worked at the Universities of Rostock, Leipzig, Jena, Frankfurt am Main, and Göttingen....
and Robert S. Mulliken
Robert S. Mulliken
Robert Sanderson Mulliken was an American physicist and chemist, primarily responsible for the early development of molecular orbital theory, i.e. the elaboration of the molecular orbital method of computing the structure of molecules. Dr. Mulliken received the Nobel Prize for chemistry in 1966...
, in which electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s are described by mathematical functions delocalized over an entire molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
. The Hund-Mulliken approach or molecular orbital (MO) method is less intuitive to chemists, but has turned out capable of predicting spectroscopic properties
Spectroscopy
Spectroscopy is the study of the interaction between matter and radiated energy. Historically, spectroscopy originated through the study of visible light dispersed according to its wavelength, e.g., by a prism. Later the concept was expanded greatly to comprise any interaction with radiative...
better than the VB method. This approach is the conceptional basis of the Hartree-Fock
Hartree-Fock
In computational physics and chemistry, the Hartree–Fock method is an approximate method for the determination of the ground-state wave function and ground-state energy of a quantum many-body system....
method and further post Hartree-Fock methods.
Density functional theory
The Thomas-Fermi model
Gas in a box
In quantum mechanics, the results of the quantum particle in a box can be used to look at the equilibrium situation for a quantum ideal gas in a box which is a box containing a large number of molecules which do not interact with each other except for instantaneous thermalizing collisions...
was developed independently by Thomas and Fermi
Enrico Fermi
Enrico Fermi was an Italian-born, naturalized American physicist particularly known for his work on the development of the first nuclear reactor, Chicago Pile-1, and for his contributions to the development of quantum theory, nuclear and particle physics, and statistical mechanics...
in 1927. This was the first attempt to describe many-electron systems on the basis of electronic density
Electronic density
In quantum mechanics, and in particular quantum chemistry, the electronic density is a measure of the probability of an electron occupying an infinitesimal element of space surrounding any given point. It is a scalar quantity depending upon three spatial variables and is typically denoted as either...
instead of wave functions, although it was not very successful in the treatment of entire molecules. The method did provide the basis for what is now known as density functional theory. Though this method is less developed than post Hartree-Fock methods, its significantly lower computational requirements (scaling typically no worse than
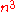 with respect to
with respect to  basis functions) allow it to tackle larger polyatomic molecules and even macromolecule
basis functions) allow it to tackle larger polyatomic molecules and even macromoleculeMacromolecule
A macromolecule is a very large molecule commonly created by some form of polymerization. In biochemistry, the term is applied to the four conventional biopolymers , as well as non-polymeric molecules with large molecular mass such as macrocycles...
s. This computational affordability and often comparable accuracy to MP2 and CCSD (post-Hartree–Fock methods) has made it one of the most popular methods in computational chemistry
Computational chemistry
Computational chemistry is a branch of chemistry that uses principles of computer science to assist in solving chemical problems. It uses the results of theoretical chemistry, incorporated into efficient computer programs, to calculate the structures and properties of molecules and solids...
at present.
Chemical dynamics
A further step can consist of solving the Schrödinger equationSchrödinger equation
The Schrödinger equation was formulated in 1926 by Austrian physicist Erwin Schrödinger. Used in physics , it is an equation that describes how the quantum state of a physical system changes in time....
with the total molecular Hamiltonian
Molecular Hamiltonian
In atomic, molecular, and optical physics as well as in quantum chemistry, molecular Hamiltonian is the name given to the Hamiltonian representing the energy of the electrons and nuclei in a molecule...
in order to study the motion of molecules. Direct solution of the Schrödinger equation is called quantum molecular dynamics, within the semiclassical approximation semiclassical molecular dynamics, and within the classical mechanics
Classical mechanics
In physics, classical mechanics is one of the two major sub-fields of mechanics, which is concerned with the set of physical laws describing the motion of bodies under the action of a system of forces...
framework molecular dynamics
Molecular dynamics
Molecular dynamics is a computer simulation of physical movements of atoms and molecules. The atoms and molecules are allowed to interact for a period of time, giving a view of the motion of the atoms...
(MD). Statistical approaches, using for example Monte Carlo method
Monte Carlo method
Monte Carlo methods are a class of computational algorithms that rely on repeated random sampling to compute their results. Monte Carlo methods are often used in computer simulations of physical and mathematical systems...
s, are also possible.
Adiabatic chemical dynamics
In adiabatic dynamics, interatomic interactions are represented by single scalarScalar (physics)
In physics, a scalar is a simple physical quantity that is not changed by coordinate system rotations or translations , or by Lorentz transformations or space-time translations . This is in contrast to a vector...
potential
Potential
*In linguistics, the potential mood*The mathematical study of potentials is known as potential theory; it is the study of harmonic functions on manifolds...
s called potential energy surface
Potential energy surface
A potential energy surface is generally used within the adiabatic or Born–Oppenheimer approximation in quantum mechanics and statistical mechanics to model chemical reactions and interactions in simple chemical and physical systems...
s. This is the Born-Oppenheimer approximation
Born-Oppenheimer approximation
In quantum chemistry, the computation of the energy and wavefunction of an average-size molecule is a formidable task that is alleviated by the Born–Oppenheimer approximation, named after Max Born and J. Robert Oppenheimer. For instance the benzene molecule consists of 12 nuclei and 42...
introduced by Born
Max Born
Max Born was a German-born physicist and mathematician who was instrumental in the development of quantum mechanics. He also made contributions to solid-state physics and optics and supervised the work of a number of notable physicists in the 1920s and 30s...
and Oppenheimer
Robert Oppenheimer
Julius Robert Oppenheimer was an American theoretical physicist and professor of physics at the University of California, Berkeley. Along with Enrico Fermi, he is often called the "father of the atomic bomb" for his role in the Manhattan Project, the World War II project that developed the first...
in 1927. Pioneering applications of this in chemistry were performed by Rice and Ramsperger in 1927 and Kassel in 1928, and generalized into the RRKM theory in 1952 by Marcus
Rudolph A. Marcus
Rudolph "Rudy" Arthur Marcus is a Canadian-born chemist who received the 1992 Nobel Prize in Chemistry for his theory of electron transfer. Marcus theory, named after him, provides a thermodynamic and kinetic framework for describing one electron outer-sphere electron transfer.He was born in...
who took the transition state
Transition state
The transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest energy along this reaction coordinate. At this point, assuming a perfectly irreversible reaction, colliding reactant molecules will always...
theory developed by Eyring
Henry Eyring
Henry Eyring was a Mexican-born American theoretical chemist whose primary contribution was in the study of chemical reaction rates and intermediates....
in 1935 into account. These methods enable simple estimates of unimolecular reaction rates from a few characteristics of the potential surface.
Non-adiabatic chemical dynamics
Non-adiabatic dynamics consists of taking the interaction between several coupled potential energy surface (corresponding to different electronic quantum states of the molecule). The coupling terms are called vibronic couplings. The pioneering work in this field was done by Stueckelberg
Ernst Stueckelberg
Ernst Carl Gerlach Stueckelberg was a Swiss mathematician and physicist.- Career :In 1927 Stueckelberg got his Ph. D. at the University of Basel under August Hagenbach...
, Landau, and Zener
Clarence Zener
Clarence Melvin Zener was the American physicist who first described the property concerning the breakdown of electrical insulators. These findings were later exploited by Bell Labs in the development of the Zener diode, which was duly named after him...
in the 1930s, in their work on what is now known as the Landau-Zener transition. Their formula allows the transition probability between two diabatic
Diabatic
A diabatic process is one in which heat transfer takes place, which is the opposite of an adiabatic process. In quantum chemistry, the potential energy surfaces are obtained within the adiabatic or Born-Oppenheimer approximation...
potential curves in the neighborhood of an avoided crossing
Avoided crossing
[Image:Avoided_crossing.png|thumb|right|300px|An avoided energy level crossing in a two level system subjected to an external magnetic field. Note the energies of the diabatic states, \scriptstyle...
to be calculated.
See also
- Atomic physicsAtomic physicsAtomic physics is the field of physics that studies atoms as an isolated system of electrons and an atomic nucleus. It is primarily concerned with the arrangement of electrons around the nucleus and...
- Computational chemistryComputational chemistryComputational chemistry is a branch of chemistry that uses principles of computer science to assist in solving chemical problems. It uses the results of theoretical chemistry, incorporated into efficient computer programs, to calculate the structures and properties of molecules and solids...
- Condensed matter physicsCondensed matter physicsCondensed matter physics deals with the physical properties of condensed phases of matter. These properties appear when a number of atoms at the supramolecular and macromolecular scale interact strongly and adhere to each other or are otherwise highly concentrated in a system. The most familiar...
- International Academy of Quantum Molecular ScienceInternational Academy of Quantum Molecular ScienceThe International Academy of Quantum Molecular Science is an international scientific learned society covering all applications of quantum theory to chemistry and chemical physics. It was created in Menton in 1967. The founding members were Raymond Daudel, Per-Olov Löwdin, Robert G. Parr, John...
- Molecular modellingMolecular modellingMolecular modelling encompasses all theoretical methods and computational techniques used to model or mimic the behaviour of molecules. The techniques are used in the fields of computational chemistry, computational biology and materials science for studying molecular systems ranging from small...
- Physical chemistryPhysical chemistryPhysical chemistry is the study of macroscopic, atomic, subatomic, and particulate phenomena in chemical systems in terms of physical laws and concepts...
- Quantum chemistry computer programsQuantum chemistry computer programsQuantum chemistry computer programs are used in computational chemistry to implement the methods of quantum chemistry. Most include the Hartree–Fock and some post-Hartree–Fock methods. They may also include density functional theory , molecular mechanics or semi-empirical quantum...
- Quantum electrochemistryQuantum electrochemistryThe scientific school of Quantum electrochemistry began to form in the 1960s under Revaz Dogonadze. Generally speaking, the field comprises the notions arising in electrodynamics, quantum mechanics, and electrochemistry; and so is studied by a very large array of different professional researchers...
- QMC@HomeQMC@HomeQMC@Home is a distributed computing project for the BOINC client aimed at further developing and testing Quantum Monte Carlo for use in quantum chemistry. It is hosted by the University of Münster with participation by the Cavendish Laboratory...
- Theoretical physicsTheoretical physicsTheoretical physics is a branch of physics which employs mathematical models and abstractions of physics to rationalize, explain and predict natural phenomena...
- Electron localization functionElectron localization functionIn quantum chemistry, the electron localization function is a measure of the likelihood of finding an electron in the neighborhood space of a reference electron located at a given point and with the same spin...
Further reading
Considers the extent to which chemistry and especially the periodic system has been reduced to quantum mechanics.- Karplus M., Porter R.N. (1971). Atoms and Molecules. An introduction for students of physical chemistry , Benjamin-Cummings Publishing Company, ISBN 978-0-8053-5218-4
- Attila Szabo, Neil S. Ostlund. (1996). Modern Quantum Chemistry: Introduction to Advanced Electronic Structure Theory , Dover, ISBN 0-486-69-186-1
External links
- The Sherrill Group - Notes
- ChemViz Curriculum Support Resources
- Early ideas in the history of quantum chemistry
- The Particle Adventure

