
Voltaic pile
Encyclopedia
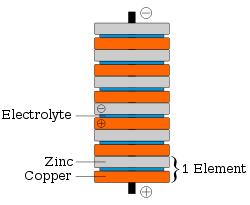

Galvanic cell
A Galvanic cell, or Voltaic cell, named after Luigi Galvani, or Alessandro Volta respectively, is an electrochemical cell that derives electrical energy from spontaneous redox reaction taking place within the cell...
s placed in series. The voltaic pile, invented by Alessandro Volta
Alessandro Volta
Count Alessandro Giuseppe Antonio Anastasio Gerolamo Umberto Volta was a Lombard physicist known especially for the invention of the battery in 1800.-Early life and works:...
in 1800, was the first electric battery
Battery (electricity)
An electrical battery is one or more electrochemical cells that convert stored chemical energy into electrical energy. Since the invention of the first battery in 1800 by Alessandro Volta and especially since the technically improved Daniell cell in 1836, batteries have become a common power...
. Building on Galvani's 1780s discovery of how a circuit of two metals and a frog's leg can cause the frog's leg to respond, Volta demonstrated in 1791 that when two metals and brine-soaked cloth or cardboard are arranged in a circuit they produce an electric current. In 1800, Volta stacked several pairs of alternating copper
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
(or silver
Silver
Silver is a metallic chemical element with the chemical symbol Ag and atomic number 47. A soft, white, lustrous transition metal, it has the highest electrical conductivity of any element and the highest thermal conductivity of any metal...
) and zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
discs (electrode
Electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit...
s) separated by cloth or cardboard soaked in brine
Brine
Brine is water, saturated or nearly saturated with salt .Brine is used to preserve vegetables, fruit, fish, and meat, in a process known as brining . Brine is also commonly used to age Halloumi and Feta cheeses, or for pickling foodstuffs, as a means of preserving them...
(electrolyte
Electrolyte
In chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
) to increase the electrolyte conductivity. When the top and bottom contacts were connected by a wire, an electric current flowed through the voltaic pile and the connecting wire.
Electromotive force
The strength of the pile is expressed in terms of its electromotive forceElectromotive force
In physics, electromotive force, emf , or electromotance refers to voltage generated by a battery or by the magnetic force according to Faraday's Law, which states that a time varying magnetic field will induce an electric current.It is important to note that the electromotive "force" is not a...
, or emf, given in volts. Volta characterized the emf of a pair of metals in terms of the difference in their voltages, which he could measure. His theory of contact tension considered that the emf, which drives the electric current through a circuit containing a voltaic cell, occurs at the contact between the two metals.
Sir Humphry Davy
Humphry Davy
Sir Humphry Davy, 1st Baronet FRS MRIA was a British chemist and inventor. He is probably best remembered today for his discoveries of several alkali and alkaline earth metals, as well as contributions to the discoveries of the elemental nature of chlorine and iodine...
and Andrew Crosse
Andrew Crosse
Andrew Crosse was a British amateur scientist who was born and died at Fyne Court, Broomfield, Somerset. Crosse was an early pioneer and experimenter in the use of electricity and one of the last of the 'gentlemen scientists'...
were among the first to develop large voltaic piles.
Applications
On March 20, 1800, Volta wrote to the LondonLondon
London is the capital city of :England and the :United Kingdom, the largest metropolitan area in the United Kingdom, and the largest urban zone in the European Union by most measures. Located on the River Thames, London has been a major settlement for two millennia, its history going back to its...
Royal Society
Royal Society
The Royal Society of London for Improving Natural Knowledge, known simply as the Royal Society, is a learned society for science, and is possibly the oldest such society in existence. Founded in November 1660, it was granted a Royal Charter by King Charles II as the "Royal Society of London"...
to describe the technique for producing electric current using his pile. On learning of the voltaic pile, William Nicholson
William Nicholson (chemist)
William Nicholson was a renowned English chemist and writer on "natural philosophy" and chemistry, as well as a translator, journalist, publisher, scientist, and inventor.-Early life:...
and Anthony Carlisle
Anthony Carlisle
Sir Anthony Carlisle FRCS, FRS was an English surgeon.He was born in Stillington, County Durham, the third son of Thomas Carlisle and his first wife, and the half-brother of Nicholas Carlisle, FRS. He was apprenticed to medical practitioners in York and Durham, including his uncle Anthony Hubback...
used it to discover the electrolysis
Electrolysis
In chemistry and manufacturing, electrolysis is a method of using a direct electric current to drive an otherwise non-spontaneous chemical reaction...
of water. Humphry Davy
Humphry Davy
Sir Humphry Davy, 1st Baronet FRS MRIA was a British chemist and inventor. He is probably best remembered today for his discoveries of several alkali and alkaline earth metals, as well as contributions to the discoveries of the elemental nature of chlorine and iodine...
showed that the electromotive force
Electromotive force
In physics, electromotive force, emf , or electromotance refers to voltage generated by a battery or by the magnetic force according to Faraday's Law, which states that a time varying magnetic field will induce an electric current.It is important to note that the electromotive "force" is not a...
, which drives the electric current through a circuit containing a single voltaic cell, was caused by a chemical reaction, not by the voltage difference between the two metals. He also used the voltaic pile to decompose chemicals and to produce new chemicals. William Hyde Wollaston
William Hyde Wollaston
William Hyde Wollaston FRS was an English chemist and physicist who is famous for discovering two chemical elements and for developing a way to process platinum ore.-Biography:...
showed that electricity from voltaic piles had identical effects to those of electricity produced by friction
Friction
Friction is the force resisting the relative motion of solid surfaces, fluid layers, and/or material elements sliding against each other. There are several types of friction:...
. In 1802 Vasily Petrov
Vasily Vladimirovich Petrov
Vasily Vladimirovich Petrov was a Russian experimental physicist, self-taught electrical technician, academician of Russian Academy of Sciences ....
used voltaic piles in the discovery and research of electric arc
Electric arc
An electric arc is an electrical breakdown of a gas which produces an ongoing plasma discharge, resulting from a current flowing through normally nonconductive media such as air. A synonym is arc discharge. An arc discharge is characterized by a lower voltage than a glow discharge, and relies on...
effects.
Electrochemistry
Because Volta believed that the emfElectromotive force
In physics, electromotive force, emf , or electromotance refers to voltage generated by a battery or by the magnetic force according to Faraday's Law, which states that a time varying magnetic field will induce an electric current.It is important to note that the electromotive "force" is not a...
occurred at the contact between the two metals, Volta's piles had a different design than the modern design illustrated on this page. His piles had one extra disc of copper at the top, in contact with the zinc, and one extra disc of zinc at the bottom, in contact with the copper. Expanding on the work of his mentor Davy, in the early 1830s, Faraday studied voltaic cells in detail. This led to his founding of the area of electrochemistry
Electrochemistry
Electrochemistry is a branch of chemistry that studies chemical reactions which take place in a solution at the interface of an electron conductor and an ionic conductor , and which involve electron transfer between the electrode and the electrolyte or species in solution.If a chemical reaction is...
. The words "electrode" and "electrolyte", used above to describe Volta's work, are due to Faraday.
Dry pile
A number of high-voltage dry piles were invented between the early 19th century and the 1830s in an attempt to determine the source of electricityElectricity
Electricity is a general term encompassing a variety of phenomena resulting from the presence and flow of electric charge. These include many easily recognizable phenomena, such as lightning, static electricity, and the flow of electrical current in an electrical wire...
of the wet voltaic pile, and specifically to support Volta’s hypothesis of contact tension. Indeed, Volta himself experimented with a pile whose cardboard discs had dried out, probably accidentally.
The first to publish was Johann Wilhelm Ritter
Johann Wilhelm Ritter
Johann Wilhelm Ritter was a German chemist, physicist and philosopher. He was born in Samitz near Haynau in Silesia , and died in Munich.-Life and work:...
in 1802, albeit in an obscure journal, but over the next decade, it was announced repeatedly as a new discovery. One form of dry pile is the Zamboni pile
Zamboni pile
The Zamboni pile is an early electric battery, invented by Giuseppe Zamboni in 1812.A Zamboni pile is an "electrostatic battery" and is constructed from discs of silver foil, zinc foil, and paper...
. The dry pile was the ancestor of the modern dry cell
Dry Cell
-Dry Cell's formation:Part of the band formed in 1998 when guitarist Danny Hartwell and drummer Brandon Brown met at the Ratt Show on the Sunset Strip. They later met up with then-vocalist Judd Gruenbaum. The original name of the band was "Beyond Control"....
.
External links
- Voltaic Pile - Interactive Java Tutorial National High Magnetic Field Laboratory
- "The invention of the Battery". Association for Overseas Technical Scholarship.
- "The Voltaic Pile". Electricity. Kenyon.edu.
- "A Voltaic Pile (c. 1800)". Professor Taylor's Ohm Page Featuring Grade Sheets & Less Important Matters.
- An Encyclopedia Article
- Lewis, Nancy D., "Alesandro Volta The Voltaic Pile".
- Lewis, Nancy D., "Humphry Davy Electrochemistry".
- "Battery Chemistry: Voltaic Pile. How Batteries Work. HowStuffWorks, Inc. 2004.

