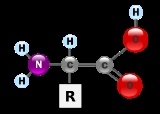
Amino acid
Encyclopedia
Amino acids are molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
s containing an amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
group, a carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
group and a side-chain
Side chain
In organic chemistry and biochemistry, a side chain is a chemical group that is attached to a core part of the molecule called "main chain" or backbone. The placeholder R is often used as a generic placeholder for alkyl group side chains in chemical structure diagrams. To indicate other non-carbon...
that varies between different amino acids. The key elements of an amino acid are carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
, hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
, oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
, and nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
. They are particularly important in biochemistry, where the term usually refers to alpha-amino acids.
An alpha-amino acid has the generic formula
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
H2NCHRCOOH, where R is an organic substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
; the amino group is attached to the carbon atom immediately adjacent to the carboxylate group (the α–carbon
Alpha carbon
The alpha carbon in organic chemistry refers to the first carbon that attaches to a functional group . By extension, the second carbon is the beta carbon, and so on....
). Other types of amino acid exist when the amino group is attached to a different carbon atom; for example, in gamma-amino acids (such as gamma-amino-butyric acid) the carbon atom to which the amino group attaches is separated from the carboxylate group by two other carbon atoms. The various alpha-amino acids differ in which side-chain (R-group) is attached to their alpha carbon, and can vary in size from just one hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
atom in glycine
Glycine
Glycine is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its 'side chain', glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG cf. the genetic code.Glycine is a colourless, sweet-tasting crystalline solid...
to a large heterocyclic group in tryptophan
Tryptophan
Tryptophan is one of the 20 standard amino acids, as well as an essential amino acid in the human diet. It is encoded in the standard genetic code as the codon UGG...
.
Amino acids are critical to life, and have many functions in metabolism
Metabolism
Metabolism is the set of chemical reactions that happen in the cells of living organisms to sustain life. These processes allow organisms to grow and reproduce, maintain their structures, and respond to their environments. Metabolism is usually divided into two categories...
. One particularly important function is to serve as the building blocks of protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s, which are linear chains of amino acids. Amino acids can be linked together in varying sequences to form a vast variety of proteins. Twenty-two amino acids are naturally incorporated into polypeptides and are called proteinogenic or standard amino acids. Of these, 20 are encoded by the universal genetic code
Genetic code
The genetic code is the set of rules by which information encoded in genetic material is translated into proteins by living cells....
. Nine standard amino acids are called "essential" for humans because they cannot be created from other compounds
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
by the human body, and so must be taken in as food.
Due to their central role in biochemistry, amino acids are important in nutrition
Nutrition
Nutrition is the provision, to cells and organisms, of the materials necessary to support life. Many common health problems can be prevented or alleviated with a healthy diet....
and are commonly used in nutrition supplements, fertilizers, food technology
Food technology
Food technology, is a branch of food science which deals with the actual production processes to make foods.-Early history of food technology:...
and industry
Industry
Industry refers to the production of an economic good or service within an economy.-Industrial sectors:There are four key industrial economic sectors: the primary sector, largely raw material extraction industries such as mining and farming; the secondary sector, involving refining, construction,...
. In industry, applications include the production of biodegradable plastic
Biodegradable plastic
Biodegradable plastics are plastics that will decompose in natural aerobic and anaerobic environments. Biodegradation of plastics can be achieved by enabling microorganisms in the environment to metabolize the molecular structure of plastic films to produce an inert humus-like material that is...
s, drug
Drug
A drug, broadly speaking, is any substance that, when absorbed into the body of a living organism, alters normal bodily function. There is no single, precise definition, as there are different meanings in drug control law, government regulations, medicine, and colloquial usage.In pharmacology, a...
s, and chiral catalysts.
History
The first few amino acids were discovered in the early 19th century. In 1806, the French chemists Louis-Nicolas Vauquelin and Pierre Jean RobiquetPierre Jean Robiquet
Pierre Jean Robiquet was a French chemist, who laid founding work in identifying amino acids, the fundamental bricks of proteins, through recognizing the first of them, asparagin, in 1806, in the take up of the industry of industrial dyes, with the identification of alizarin in 1826, and in the...
isolated a compound in asparagus
Asparagus
Asparagus officinalis is a spring vegetable, a flowering perennialplant species in the genus Asparagus. It was once classified in the lily family, like its Allium cousins, onions and garlic, but the Liliaceae have been split and the onion-like plants are now in the family Amaryllidaceae and...
that proved to be asparagine
Asparagine
Asparagine is one of the 20 most common natural amino acids on Earth. It has carboxamide as the side-chain's functional group. It is not an essential amino acid...
, the first amino acid to be discovered. Another amino acid that was discovered in the early 19th century was cystine
Cystine
Cystine is a dimeric amino acid formed by the oxidation of two cysteine residues that covalently link to make a disulfide bond. This organosulfur compound has the formula 2. It is a white solid, and melts at 247-249 °C...
, in 1810, although its monomer, cysteine
Cysteine
Cysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid...
, was discovered much later, in 1884. Glycine
Glycine
Glycine is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its 'side chain', glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG cf. the genetic code.Glycine is a colourless, sweet-tasting crystalline solid...
and leucine
Leucine
Leucine is a branched-chain α-amino acid with the chemical formula HO2CCHCH2CH2. Leucine is classified as a hydrophobic amino acid due to its aliphatic isobutyl side chain. It is encoded by six codons and is a major component of the subunits in ferritin, astacin and other 'buffer' proteins...
were also discovered around this time, in 1820. Usage of the term amino acid in the English language is from 1898.
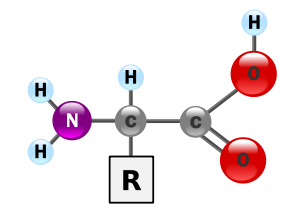
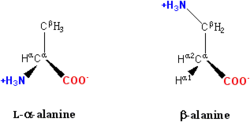
General structure
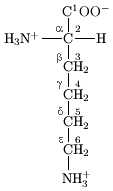
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
atom next to the carboxyl group is called the α–carbon
Alpha carbon
The alpha carbon in organic chemistry refers to the first carbon that attaches to a functional group . By extension, the second carbon is the beta carbon, and so on....
and amino acids with a side-chain bonded to this carbon are referred to as alpha amino acids. These are the most common form found in nature. In the alpha amino acids, the α–carbon is a chiral
Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
carbon atom, with the exception of glycine
Glycine
Glycine is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its 'side chain', glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG cf. the genetic code.Glycine is a colourless, sweet-tasting crystalline solid...
. In amino acids that have a carbon chain attached to the α–carbon (such as lysine
Lysine
Lysine is an α-amino acid with the chemical formula HO2CCH4NH2. It is an essential amino acid, which means that the human body cannot synthesize it. Its codons are AAA and AAG....
, shown to the right) the carbons are labeled in order as α, β, γ, δ, and so on. In some amino acids, the amine group is attached to the β or γ-carbon, and these are therefore referred to as beta or gamma amino acids.
Amino acids are usually classified by the properties
Chemical property
A chemical property is any of a material's properties that becomes evident during a chemical reaction; that is, any quality that can be established only by changing a substance's chemical identity...
of their side-chain into four groups. The side-chain can make an amino acid a weak acid
Weak acid
A weak acid is an acid that dissociates incompletely. It does not release all of its hydrogens in a solution, donating only a partial amount of its protons to the solution...
or a weak
Weak base
In chemistry, a weak base is a chemical base that does not ionize fully in an aqueous solution. As Brønsted–Lowry bases are proton acceptors, a weak base may also be defined as a chemical base in which protonation is incomplete. This results in a relatively low pH compared to strong bases...
base, and a hydrophile
Hydrophile
A hydrophile, from the Greek "water" and φιλια "love," is a molecule or other molecular entity that is attracted to, and tends to be dissolved by water. A hydrophilic molecule or portion of a molecule is one that has a tendency to interact with or be dissolved by, water and other polar substances...
if the side-chain is polar or a hydrophobe
Hydrophobe
In chemistry, hydrophobicity is the physical property of a molecule that is repelled from a mass of water....
if it is nonpolar. The chemical structure
Chemical structure
A chemical structure includes molecular geometry, electronic structure and crystal structure of molecules. Molecular geometry refers to the spatial arrangement of atoms in a molecule and the chemical bonds that hold the atoms together. Molecular geometry can range from the very simple, such as...
s of the 22 standard amino acids, along with their chemical properties, are described more fully in the article on these proteinogenic amino acid
Proteinogenic amino acid
Proteinogenic amino acids are those amino acids that can be found in proteins and require cellular machinery coded for in the genetic code of any organism for their isolated production. There are 22 standard amino acids, but only 21 are found in eukaryotes. Of the 22, 20 are directly encoded by...
s.
The phrase "branched-chain amino acids
Branched-chain amino acids
A branched-chain amino acid is an amino acid having aliphatic side-chains with a branch...
" or BCAA refers to the amino acids having aliphatic side-chains that are non-linear; these are leucine
Leucine
Leucine is a branched-chain α-amino acid with the chemical formula HO2CCHCH2CH2. Leucine is classified as a hydrophobic amino acid due to its aliphatic isobutyl side chain. It is encoded by six codons and is a major component of the subunits in ferritin, astacin and other 'buffer' proteins...
, isoleucine
Isoleucine
Isoleucine is an α-amino acid with the chemical formula HO2CCHCHCH2CH3. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested. Its codons are AUU, AUC and AUA....
, and valine
Valine
Valine is an α-amino acid with the chemical formula HO2CCHCH2. L-Valine is one of 20 proteinogenic amino acids. Its codons are GUU, GUC, GUA, and GUG. This essential amino acid is classified as nonpolar...
. Proline
Proline
Proline is an α-amino acid, one of the twenty DNA-encoded amino acids. Its codons are CCU, CCC, CCA, and CCG. It is not an essential amino acid, which means that the human body can synthesize it. It is unique among the 20 protein-forming amino acids in that the α-amino group is secondary...
is the only proteinogenic amino acid whose side-group links to the α-amino group and, thus, is also the only proteinogenic amino acid containing a secondary amine at this position. In chemical terms, proline is, therefore, an imino acid
Imino acid
In chemistry, an imino acid is any molecule that contains both imino and carboxyl functional groups.Imino acids are related to amino acids, which contain both amino and carboxyl functional groups...
, since it lacks a primary amino group
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
, although it is still classed as an amino acid in the current biochemical nomenclature, and may also be called an "N-alkylated alpha-amino acid".
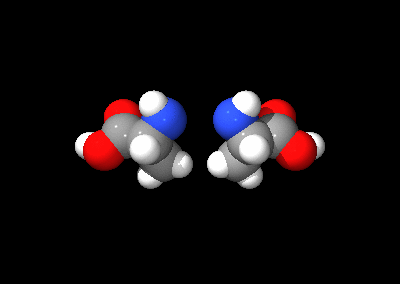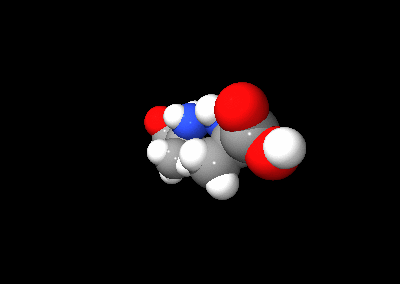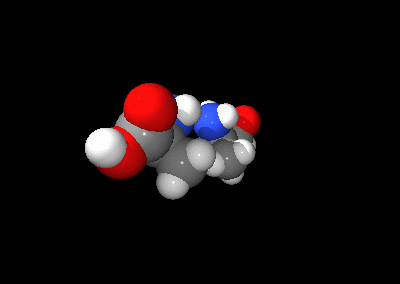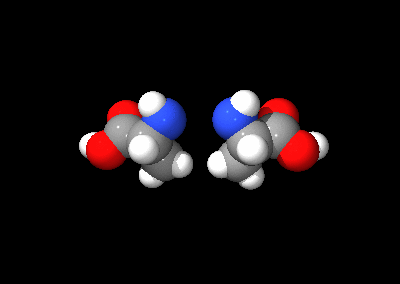
Isomerism
Of the standard α-amino acids, all but glycineGlycine
Glycine is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its 'side chain', glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG cf. the genetic code.Glycine is a colourless, sweet-tasting crystalline solid...
can exist in either of two optical isomers, called L or D amino acids, which are mirror images of each other (see also Chirality
Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
). While L-amino acids represent all of the amino acids found in protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s during translation in the ribosome, D-amino acids are found in some proteins produced by enzyme posttranslational modification
Posttranslational modification
Posttranslational modification is the chemical modification of a protein after its translation. It is one of the later steps in protein biosynthesis, and thus gene expression, for many proteins....
s after translation and translocation to the endoplasmic reticulum, as in exotic sea-dwelling organisms such as cone snail
Cone snail
Conidae is a taxonomic family of minute to quite large sea snails, marine gastropod molluscs in the superfamily Conoidea.The snails within this family are sophisticated predatory animals...
s. They are also abundant components of the peptidoglycan
Peptidoglycan
Peptidoglycan, also known as murein, is a polymer consisting of sugars and amino acids that forms a mesh-like layer outside the plasma membrane of bacteria , forming the cell wall. The sugar component consists of alternating residues of β- linked N-acetylglucosamine and N-acetylmuramic acid...
cell wall
Cell wall
The cell wall is the tough, usually flexible but sometimes fairly rigid layer that surrounds some types of cells. It is located outside the cell membrane and provides these cells with structural support and protection, and also acts as a filtering mechanism. A major function of the cell wall is to...
s of bacteria
Bacteria
Bacteria are a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria have a wide range of shapes, ranging from spheres to rods and spirals...
, and D-serine may act as a neurotransmitter
Neurotransmitter
Neurotransmitters are endogenous chemicals that transmit signals from a neuron to a target cell across a synapse. Neurotransmitters are packaged into synaptic vesicles clustered beneath the membrane on the presynaptic side of a synapse, and are released into the synaptic cleft, where they bind to...
in the brain
Brain
The brain is the center of the nervous system in all vertebrate and most invertebrate animals—only a few primitive invertebrates such as sponges, jellyfish, sea squirts and starfishes do not have one. It is located in the head, usually close to primary sensory apparatus such as vision, hearing,...
. The L and D convention for amino acid configuration refers not to the optical activity of the amino acid itself, but rather to the optical activity of the isomer of glyceraldehyde
Glyceraldehyde
Glyceraldehyde is a triose monosaccharide with chemical formula C3H6O3. It is the simplest of all common aldoses. It is a sweet, colorless, crystalline solid that is an intermediate compound in carbohydrate metabolism...
from which that amino acid can, in theory, be synthesized (D-glyceraldehyde is dextrorotary; L-glyceraldehyde is levorotary).
In alternative fashion, the (S) and (R) designators are used to indicate the absolute stereochemistry
Stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms within molecules. An important branch of stereochemistry is the study of chiral molecules....
. Almost all of the amino acids in proteins
Proteinogenic amino acid
Proteinogenic amino acids are those amino acids that can be found in proteins and require cellular machinery coded for in the genetic code of any organism for their isolated production. There are 22 standard amino acids, but only 21 are found in eukaryotes. Of the 22, 20 are directly encoded by...
are (S) at the α carbon, with cysteine being (R) and glycine non-chiral. Cysteine is unusual since it has a sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
atom at the second position in its side-chain, which has a larger atomic mass
Atomic mass
The atomic mass is the mass of a specific isotope, most often expressed in unified atomic mass units. The atomic mass is the total mass of protons, neutrons and electrons in a single atom....
than the groups attached to the first carbon, which is attached to the α-carbon in the other standard amino acids, thus the (R) instead of (S).
Zwitterions
The amine and carboxylic acid functional groups found in amino acids allow them to have amphiproticAmphoterism
In chemistry, an amphoteric species is a molecule or ion that can react as an acid as well as a base. The word is derived from the Greek word amphoteroi meaning "both"...
properties. Carboxylic acid groups (-CO2H) can be deprotonated to become negative carboxylates (-CO2- ), and α-amino groups (NH2-) can be protonated to become positive α-ammonium groups (+NH3-). At pH values greater than the pKa
PKA
PKA, pKa, or other similar variations may stand for:* pKa, the symbol for the acid dissociation constant at logarithmic scale* Protein kinase A, a class of cAMP-dependent enzymes* Pi Kappa Alpha, the North-American social fraternity...
of the carboxylic acid group (mean for the 20 common amino acids is about 2.2, see the table of amino acid structures above), the negative carboxylate ion predominates. At pH values lower than the pKa of the α-ammonium group (mean for the 20 common α-amino acids is about 9.4), the nitrogen is predominantly protonated as a positively charged α-ammonium group. Thus, at pH between 2.2 and 9.4, the predominant form adopted by α-amino acids contains a negative carboxylate and a positive α-ammonium group, as shown in structure (2) on the right, so has net zero charge. This molecular state is known as a zwitterion
Zwitterion
In chemistry, a zwitterion is a neutral molecule with a positive and a negative electrical charge at different locations within that molecule. Zwitterions are sometimes also called inner salts.-Examples:...
, from the German Zwitter meaning hermaphrodite or hybrid. Below pH 2.2, the predominant form will have a neutral carboxylic acid group and a positive α-ammonium ion (net charge +1), and above pH 9.4, a negative carboxylate and neutral α-amino group (net charge -1). The fully neutral form (structure (1) on the right) is a very minor species in aqueous solution throughout the pH range (less than 1 part in 107). Amino acids also exist as zwitterions in the solid phase, and crystallize with salt-like properties unlike typical organic acids or amines.
Isoelectric point
At pH values between the two pKa values, the zwitterion predominates, but coexists in dynamic equilibriumDynamic equilibrium
A dynamic equilibrium exists once a reversible reaction ceases to change its ratio of reactants/products, but substances move between the chemicals at an equal rate, meaning there is no net change. It is a particular example of a system in a steady state...
with small amounts of net negative and net positive ions. At the exact midpoint between the two pKa values, the trace amount of net negative and trace of net positive ions exactly balance, so that average net charge of all forms present is zero. This pH is known as the isoelectric point
Isoelectric point
The isoelectric point , sometimes abbreviated to IEP, is the pH at which a particular molecule or surface carries no net electrical charge....
pI, so pI = ½(pKa1 + pKa2). The individual amino acids all have slightly different pKa values, so have different isoelectric points. For amino acids with charged side-chains, the pKa of the side-chain is involved. Thus for Asp, Glu with negative side-chains, pI = ½(pKa1 + pKaR), where pKaR is the side-chain pKa. Cysteine also has potentially negative side-chain with pKaR = 8.14, so pI should be calculated as for Asp and Glu, even though the side-chain is not significantly charged at neutral pH. For His, Lys, and Arg with positive side-chains, pI = ½(pKaR + pKa2). Amino acids have zero mobility in electrophoresis at their isoelectric point, although this behaviour is more usually exploited for peptides and proteins than single amino acids. Zwitterions have minimum solubility at their isolectric point and some amino acids (in particular, with non-polar side-chains) can be isolated by precipitation from water by adjusting the pH to the required isoelectric point.
Occurrence and functions in biochemistry
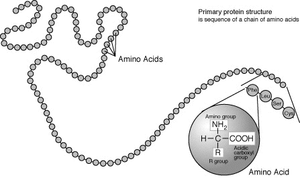
Standard amino acids
Amino acids are the structural units that make up proteins. They join together to form short polymerPolymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
chains called peptide
Peptide
Peptides are short polymers of amino acid monomers linked by peptide bonds. They are distinguished from proteins on the basis of size, typically containing less than 50 monomer units. The shortest peptides are dipeptides, consisting of two amino acids joined by a single peptide bond...
s or longer chains called either polypeptides or protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s. These polymers are linear and unbranched, with each amino acid within the chain attached to two neighboring amino acids. The process of making proteins is called translation and involves the step-by-step addition of amino acids to a growing protein chain by a ribozyme
Ribozyme
A ribozyme is an RNA molecule with a well defined tertiary structure that enables it to catalyze a chemical reaction. Ribozyme means ribonucleic acid enzyme. It may also be called an RNA enzyme or catalytic RNA. Many natural ribozymes catalyze either the hydrolysis of one of their own...
that is called a ribosome
Ribosome
A ribosome is a component of cells that assembles the twenty specific amino acid molecules to form the particular protein molecule determined by the nucleotide sequence of an RNA molecule....
. The order in which the amino acids are added is read through the genetic code
Genetic code
The genetic code is the set of rules by which information encoded in genetic material is translated into proteins by living cells....
from an mRNA
Messenger RNA
Messenger RNA is a molecule of RNA encoding a chemical "blueprint" for a protein product. mRNA is transcribed from a DNA template, and carries coding information to the sites of protein synthesis: the ribosomes. Here, the nucleic acid polymer is translated into a polymer of amino acids: a protein...
template, which is a RNA
RNA
Ribonucleic acid , or RNA, is one of the three major macromolecules that are essential for all known forms of life....
copy of one of the organism's gene
Gene
A gene is a molecular unit of heredity of a living organism. It is a name given to some stretches of DNA and RNA that code for a type of protein or for an RNA chain that has a function in the organism. Living beings depend on genes, as they specify all proteins and functional RNA chains...
s.
Twenty-two amino acids are naturally incorporated into polypeptides and are called proteinogenic or natural amino acids. Of these, 20 are encoded by the universal genetic code
Genetic code
The genetic code is the set of rules by which information encoded in genetic material is translated into proteins by living cells....
. The remaining 2, selenocysteine
Selenocysteine
Selenocysteine is an amino acid that is present in several enzymes .-Nomenclature:...
and pyrrolysine
Pyrrolysine
Pyrrolysine is a naturally occurring, genetically coded amino acid used by some methanogenic archaea and one known bacterium in enzymes that are part of their methane-producing metabolism. It is similar to lysine, but with an added pyrroline ring linked to the end of the lysine side chain...
, are incorporated into proteins by unique synthetic mechanisms. Selenocysteine
Selenocysteine
Selenocysteine is an amino acid that is present in several enzymes .-Nomenclature:...
is incorporated when the mRNA being translated includes a SECIS element
SECIS element
In biology, the SECIS element is an RNA element around 60 nucleotides in length that adopts a stem-loop structure. This structural motif directs the cell to translate UGA codons as selenocysteines...
, which causes the UGA codon to encode selenocysteine instead of a stop codon
Stop codon
In the genetic code, a stop codon is a nucleotide triplet within messenger RNA that signals a termination of translation. Proteins are based on polypeptides, which are unique sequences of amino acids. Most codons in messenger RNA correspond to the addition of an amino acid to a growing polypeptide...
. Pyrrolysine
Pyrrolysine
Pyrrolysine is a naturally occurring, genetically coded amino acid used by some methanogenic archaea and one known bacterium in enzymes that are part of their methane-producing metabolism. It is similar to lysine, but with an added pyrroline ring linked to the end of the lysine side chain...
is used by some methanogen
Methanogen
Methanogens are microorganisms that produce methane as a metabolic byproduct in anoxic conditions. They are classified as archaea, a group quite distinct from bacteria...
ic archaea
Archaea
The Archaea are a group of single-celled microorganisms. A single individual or species from this domain is called an archaeon...
in enzymes that they use to produce methane
Methane
Methane is a chemical compound with the chemical formula . It is the simplest alkane, the principal component of natural gas, and probably the most abundant organic compound on earth. The relative abundance of methane makes it an attractive fuel...
. It is coded for with the codon UAG, which is normally a stop codon in other organisms. This UAG codon is followed by a PYLIS downstream sequence
PYLIS downstream sequence
In biology, the PYLIS downstream sequence is a stem-loop structure which appears on some mRNA sequences. This structural motif causes the UAG stop codon to be translated to the amino acid pyrrolysine instead of ending the protein translation...
.
Non-standard amino acids
Aside from the 22 standard amino acids, there are many other amino acids that are called non-proteinogenic or non-standard. Those either are not found in proteins (for example carnitineCarnitine
Carnitine is a quaternary ammonium compound biosynthesized from the amino acids lysine and methionine. In living cells, it is required for the transport of fatty acids from the cytosol into the mitochondria during the breakdown of lipids for the generation of metabolic energy. It is widely...
, GABA
Gamma-aminobutyric acid
γ-Aminobutyric acid is the chief inhibitory neurotransmitter in the mammalian central nervous system. It plays a role in regulating neuronal excitability throughout the nervous system...
), or are not produced directly and in isolation by standard cellular machinery (for example, hydroxyproline
Hydroxyproline
-4-Hydroxyproline, or L-hydroxyproline , is a common non-proteinogenic amino acid, abbreviated as HYP, e.g., in Protein Data Bank.-Structure and discovery:...
and selenomethionine
Selenomethionine
Selenomethionine is an amino acid containing selenium. The L-enantiomer of selenomethionine, known as Se-met and Sem, is a common natural food source of selenium. In vivo, selenomethionine is randomly incorporated instead of methionine and is readily oxidized. Its antioxidant activity arises from...
).
Non-standard amino acids that are found in proteins are formed by post-translational modification, which is modification after translation during protein synthesis. These modifications are often essential for the function or regulation of a protein; for example, the carboxylation
Carboxylation
Carboxylation in chemistry is a chemical reaction in which a carboxylic acid group is introduced in a substrate. The opposite reaction is decarboxylation.-Carboxylation in organic chemistry:In organic chemistry many different protocols exist for carboxylation...
of glutamate allows for better binding of calcium cations
Calcium in biology
Calcium plays a pivotal role in the physiology and biochemistry of organisms and the cell. It plays an important role in signal transduction pathways, where it acts as a second messenger, in neurotransmitter release from neurons, contraction of all muscle cell types, and fertilization...
, and the hydroxylation
Hydroxylation
Hydroxylation is a chemical process that introduces a hydroxyl group into an organic compound. In biochemistry, hydroxylation reactions are often facilitated by enzymes called hydroxylases. Hydroxylation is the first step in the oxidative degradation of organic compounds in air...
of proline
Proline
Proline is an α-amino acid, one of the twenty DNA-encoded amino acids. Its codons are CCU, CCC, CCA, and CCG. It is not an essential amino acid, which means that the human body can synthesize it. It is unique among the 20 protein-forming amino acids in that the α-amino group is secondary...
is critical for maintaining connective tissues
Collagen
Collagen is a group of naturally occurring proteins found in animals, especially in the flesh and connective tissues of mammals. It is the main component of connective tissue, and is the most abundant protein in mammals, making up about 25% to 35% of the whole-body protein content...
. Another example is the formation of hypusine
Hypusine
Hypusine is an unusual amino acid found in all eukaryotes and in some archaea, but not in bacteria. The only known protein containing hypusine is eukaryotic translation initiation factor 5A and a similar protein found in archaebacteria. In humans, two isoforms of eIF-5A have been described:...
in the translation initiation factor
Eukaryotic initiation factor
Eukaryotic initiation factors are proteins involved in the initiation phase of eukaryotic translation. They function in forming a complex with the 40S ribosomal subunit and Met-tRNAi called the 43S preinitation complex , recognizing the 5' cap structure of mRNA and recruiting the 43S PIC to mRNA,...
EIF5A
EIF5A
Eukaryotic translation initiation factor 5A-1 is a protein that in humans is encoded by the EIF5A gene.It is the only known protein to contain the unusual amino acid hypusine [N - -lysine], which is formed from the polyamine spermidine by two catalytic steps.-Further reading:...
, through modification of a lysine residue. Such modifications can also determine the localization of the protein, e.g., the addition of long hydrophobic groups can cause a protein to bind to a phospholipid
Phospholipid
Phospholipids are a class of lipids that are a major component of all cell membranes as they can form lipid bilayers. Most phospholipids contain a diglyceride, a phosphate group, and a simple organic molecule such as choline; one exception to this rule is sphingomyelin, which is derived from...
membrane.
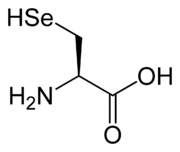
Lanthionine
Lanthionine is a nonproteinogenic amino acid with the chemical formula . As the monosulfide analog of cystine, lanthionine is composed of two alanine residues that are crosslinked on their β-carbon atoms by a thioether linkage...
, 2-aminoisobutyric acid
2-Aminoisobutyric acid
2-Aminoisobutyric acid, or α-aminoisobutyric acid or α-methylalanine or 2-methylalanine, is an amino acid with the structural formula is H2N-C2-COOH. It is contained in some antibiotics of fungal origin, e.g. alamethicin and some lantibiotics. It is not one of the proteinogenic amino acids and...
, dehydroalanine
Dehydroalanine
Dehydroalanine is an uncommon amino acid found in peptides of microbial origin ....
, and the neurotransmitter gamma-aminobutyric acid
Gamma-aminobutyric acid
γ-Aminobutyric acid is the chief inhibitory neurotransmitter in the mammalian central nervous system. It plays a role in regulating neuronal excitability throughout the nervous system...
. Nonstandard amino acids often occur as intermediates in the metabolic pathway
Metabolic pathway
In biochemistry, metabolic pathways are series of chemical reactions occurring within a cell. In each pathway, a principal chemical is modified by a series of chemical reactions. Enzymes catalyze these reactions, and often require dietary minerals, vitamins, and other cofactors in order to function...
s for standard amino acids — for example, ornithine
Ornithine
Ornithine is an amino acid that plays a role in the urea cycle.-Role in urea cycle:L-Ornithine is one of the products of the action of the enzyme arginase on L-arginine, creating urea. Therefore, ornithine is a central part of the urea cycle, which allows for the disposal of excess nitrogen....
and citrulline
Citrulline
The organic compound citrulline is an α-amino acid. Its name is derived from citrullus, the Latin word for watermelon, from which it was first isolated in 1930.It has the idealized formula H2NCNH3CHCO2H...
occur in the urea cycle
Urea cycle
The urea cycle is a cycle of biochemical reactions occurring in many animals that produces urea from ammonia . This cycle was the first metabolic cycle discovered , five years before the discovery of the TCA cycle...
, part of amino acid catabolism
Catabolism
Catabolism is the set of metabolic pathways that break down molecules into smaller units and release energy. In catabolism, large molecules such as polysaccharides, lipids, nucleic acids and proteins are broken down into smaller units such as monosaccharides, fatty acids, nucleotides, and amino...
(see below). A rare exception to the dominance of α-amino acids in biology is the β-amino acid beta alanine (3-aminopropanoic acid), which is used in plants and microorganisms in the synthesis of pantothenic acid
Pantothenic acid
Pantothenic acid, also called pantothenate or vitamin B5 , is a water-soluble vitamin. For many animals, pantothenic acid is an essential nutrient. Animals require pantothenic acid to synthesize coenzyme-A , as well as to synthesize and metabolize proteins, carbohydrates, and fats.Pantothenic acid...
(vitamin B5), a component of coenzyme A
Coenzyme A
Coenzyme A is a coenzyme, notable for its role in the synthesis and oxidation of fatty acids, and the oxidation of pyruvate in the citric acid cycle. All sequenced genomes encode enzymes that use coenzyme A as a substrate, and around 4% of cellular enzymes use it as a substrate...
.
In human nutrition
When taken up into the human body from the diet, the 22 standard amino acids either are used to synthesize proteins and other biomolecules or are oxidized to ureaUrea
Urea or carbamide is an organic compound with the chemical formula CO2. The molecule has two —NH2 groups joined by a carbonyl functional group....
and carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
as a source of energy. The oxidation pathway starts with the removal of the amino group by a transaminase
Transaminase
In biochemistry, a transaminase or an aminotransferase is an enzyme that catalyzes a type of reaction between an amino acid and an α-keto acid. To be specific, this reaction involves removing the amino group from the amino acid, leaving behind an α-keto acid, and transferring it to the...
, the amino group is then fed into the urea cycle
Urea cycle
The urea cycle is a cycle of biochemical reactions occurring in many animals that produces urea from ammonia . This cycle was the first metabolic cycle discovered , five years before the discovery of the TCA cycle...
. The other product of transamidation is a keto acid
Keto acid
Keto acids are organic compounds that contain a carboxylic acid group and a ketone group. The alpha-keto acids are especially important in biology as they are involved in the Krebs citric acid cycle and in glycolysis...
that enters the citric acid cycle
Citric acid cycle
The citric acid cycle — also known as the tricarboxylic acid cycle , the Krebs cycle, or the Szent-Györgyi-Krebs cycle — is a series of chemical reactions which is used by all aerobic living organisms to generate energy through the oxidization of acetate derived from carbohydrates, fats and...
. Glucogenic amino acid
Glucogenic amino acid
A glucogenic amino acid is an amino acid that can be converted into glucose through gluconeogenesis. This is in contrast to the ketogenic amino acids, which are converted into ketone bodies....
s can also be converted into glucose, through gluconeogenesis
Gluconeogenesis
Gluconeogenesis is a metabolic pathway that results in the generation of glucose from non-carbohydrate carbon substrates such as lactate, glycerol, and glucogenic amino acids....
.
Pyrrolysine trait is restricted to several microbes, and only one organism has both Pyl and Sec. Of the 22 standard amino acids, 9 are called essential amino acid
Essential amino acid
An essential amino acid or indispensable amino acid is an amino acid that cannot be synthesized de novo by the organism , and therefore must be supplied in the diet.-Essentiality vs. conditional essentiality in humans:...
s because the human body
Human body
The human body is the entire structure of a human organism, and consists of a head, neck, torso, two arms and two legs.By the time the human reaches adulthood, the body consists of close to 100 trillion cells, the basic unit of life...
cannot synthesize
Biosynthesis
Biosynthesis is an enzyme-catalyzed process in cells of living organisms by which substrates are converted to more complex products. The biosynthesis process often consists of several enzymatic steps in which the product of one step is used as substrate in the following step...
them from other compounds
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
at the level needed for normal growth, so they must be obtained from food. In addition, cysteine
Cysteine
Cysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid...
, taurine, tyrosine
Tyrosine
Tyrosine or 4-hydroxyphenylalanine, is one of the 22 amino acids that are used by cells to synthesize proteins. Its codons are UAC and UAU. It is a non-essential amino acid with a polar side group...
, histidine
Histidine
Histidine Histidine, an essential amino acid, has a positively charged imidazole functional group. It is one of the 22 proteinogenic amino acids. Its codons are CAU and CAC. Histidine was first isolated by German physician Albrecht Kossel in 1896. Histidine is an essential amino acid in humans...
, and arginine
Arginine
Arginine is an α-amino acid. The L-form is one of the 20 most common natural amino acids. At the level of molecular genetics, in the structure of the messenger ribonucleic acid mRNA, CGU, CGC, CGA, CGG, AGA, and AGG, are the triplets of nucleotide bases or codons that codify for arginine during...
are semiessential amino-acids in children, because the metabolic pathways that synthesize these amino acids are not fully developed. The amounts required also depend on the age and health of the individual, so it is hard to make general statements about the dietary requirement for some amino acids.
| Essential | Nonessential |
|---|---|
| Histidine Histidine Histidine Histidine, an essential amino acid, has a positively charged imidazole functional group. It is one of the 22 proteinogenic amino acids. Its codons are CAU and CAC. Histidine was first isolated by German physician Albrecht Kossel in 1896. Histidine is an essential amino acid in humans... |
Alanine Alanine Alanine is an α-amino acid with the chemical formula CH3CHCOOH. The L-isomer is one of the 20 amino acids encoded by the genetic code. Its codons are GCU, GCC, GCA, and GCG. It is classified as a nonpolar amino acid... |
| Isoleucine Isoleucine Isoleucine is an α-amino acid with the chemical formula HO2CCHCHCH2CH3. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested. Its codons are AUU, AUC and AUA.... |
Arginine Arginine Arginine is an α-amino acid. The L-form is one of the 20 most common natural amino acids. At the level of molecular genetics, in the structure of the messenger ribonucleic acid mRNA, CGU, CGC, CGA, CGG, AGA, and AGG, are the triplets of nucleotide bases or codons that codify for arginine during... * |
| Leucine Leucine Leucine is a branched-chain α-amino acid with the chemical formula HO2CCHCH2CH2. Leucine is classified as a hydrophobic amino acid due to its aliphatic isobutyl side chain. It is encoded by six codons and is a major component of the subunits in ferritin, astacin and other 'buffer' proteins... |
Asparagine Asparagine Asparagine is one of the 20 most common natural amino acids on Earth. It has carboxamide as the side-chain's functional group. It is not an essential amino acid... |
| Lysine Lysine Lysine is an α-amino acid with the chemical formula HO2CCH4NH2. It is an essential amino acid, which means that the human body cannot synthesize it. Its codons are AAA and AAG.... |
Aspartic acid Aspartic acid Aspartic acid is an α-amino acid with the chemical formula HOOCCHCH2COOH. The carboxylate anion, salt, or ester of aspartic acid is known as aspartate. The L-isomer of aspartate is one of the 20 proteinogenic amino acids, i.e., the building blocks of proteins... |
| Methionine Methionine Methionine is an α-amino acid with the chemical formula HO2CCHCH2CH2SCH3. This essential amino acid is classified as nonpolar. This amino-acid is coded by the codon AUG, also known as the initiation codon, since it indicates mRNA's coding region where translation into protein... |
Cysteine Cysteine Cysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid... * |
| Phenylalanine Phenylalanine Phenylalanine is an α-amino acid with the formula C6H5CH2CHCOOH. This essential amino acid is classified as nonpolar because of the hydrophobic nature of the benzyl side chain. L-Phenylalanine is an electrically neutral amino acid, one of the twenty common amino acids used to biochemically form... |
Glutamic acid Glutamic acid Glutamic acid is one of the 20 proteinogenic amino acids, and its codons are GAA and GAG. It is a non-essential amino acid. The carboxylate anions and salts of glutamic acid are known as glutamates... |
| Threonine Threonine Threonine is an α-amino acid with the chemical formula HO2CCHCHCH3. Its codons are ACU, ACA, ACC, and ACG. This essential amino acid is classified as polar... |
Glutamine Glutamine Glutamine is one of the 20 amino acids encoded by the standard genetic code. It is not recognized as an essential amino acid but may become conditionally essential in certain situations, including intensive athletic training or certain gastrointestinal disorders... * |
| Tryptophan Tryptophan Tryptophan is one of the 20 standard amino acids, as well as an essential amino acid in the human diet. It is encoded in the standard genetic code as the codon UGG... |
Glycine Glycine Glycine is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its 'side chain', glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG cf. the genetic code.Glycine is a colourless, sweet-tasting crystalline solid... |
| Valine Valine Valine is an α-amino acid with the chemical formula HO2CCHCH2. L-Valine is one of 20 proteinogenic amino acids. Its codons are GUU, GUC, GUA, and GUG. This essential amino acid is classified as nonpolar... |
Ornithine Ornithine Ornithine is an amino acid that plays a role in the urea cycle.-Role in urea cycle:L-Ornithine is one of the products of the action of the enzyme arginase on L-arginine, creating urea. Therefore, ornithine is a central part of the urea cycle, which allows for the disposal of excess nitrogen.... * |
| Proline Proline Proline is an α-amino acid, one of the twenty DNA-encoded amino acids. Its codons are CCU, CCC, CCA, and CCG. It is not an essential amino acid, which means that the human body can synthesize it. It is unique among the 20 protein-forming amino acids in that the α-amino group is secondary... * |
|
| Selenocysteine Selenocysteine Selenocysteine is an amino acid that is present in several enzymes .-Nomenclature:... * |
|
| Serine Serine Serine is an amino acid with the formula HO2CCHCH2OH. It is one of the proteinogenic amino acids. By virtue of the hydroxyl group, serine is classified as a polar amino acid.-Occurrence and biosynthesis:... * |
|
| Taurine* | |
| Tyrosine Tyrosine Tyrosine or 4-hydroxyphenylalanine, is one of the 22 amino acids that are used by cells to synthesize proteins. Its codons are UAC and UAU. It is a non-essential amino acid with a polar side group... * |
(*) Essential only in certain cases.
Non-protein functions
In humans, non-protein amino acids also have important roles as metabolic intermediateMetabolic intermediate
Metabolic intermediates refers to molecules which are the precursors or metabolites of biologically significant molecules.Although these intermediates are of relatively minor direct importance to cellular function, they can play important roles in the allosteric regulation of enzymes.-Clinical...
s, such as in the biosynthesis of the neurotransmitter
Neurotransmitter
Neurotransmitters are endogenous chemicals that transmit signals from a neuron to a target cell across a synapse. Neurotransmitters are packaged into synaptic vesicles clustered beneath the membrane on the presynaptic side of a synapse, and are released into the synaptic cleft, where they bind to...
gamma-aminobutyric acid
Gamma-aminobutyric acid
γ-Aminobutyric acid is the chief inhibitory neurotransmitter in the mammalian central nervous system. It plays a role in regulating neuronal excitability throughout the nervous system...
. Many amino acids are used to synthesize other molecules, for example:
- TryptophanTryptophanTryptophan is one of the 20 standard amino acids, as well as an essential amino acid in the human diet. It is encoded in the standard genetic code as the codon UGG...
is a precursor of the neurotransmitter serotoninSerotoninSerotonin or 5-hydroxytryptamine is a monoamine neurotransmitter. Biochemically derived from tryptophan, serotonin is primarily found in the gastrointestinal tract, platelets, and in the central nervous system of animals including humans...
. - TyrosineTyrosineTyrosine or 4-hydroxyphenylalanine, is one of the 22 amino acids that are used by cells to synthesize proteins. Its codons are UAC and UAU. It is a non-essential amino acid with a polar side group...
is a precursor of the neurotransmitterNeurotransmitterNeurotransmitters are endogenous chemicals that transmit signals from a neuron to a target cell across a synapse. Neurotransmitters are packaged into synaptic vesicles clustered beneath the membrane on the presynaptic side of a synapse, and are released into the synaptic cleft, where they bind to...
dopamineDopamineDopamine is a catecholamine neurotransmitter present in a wide variety of animals, including both vertebrates and invertebrates. In the brain, this substituted phenethylamine functions as a neurotransmitter, activating the five known types of dopamine receptors—D1, D2, D3, D4, and D5—and their...
. - GlycineGlycineGlycine is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its 'side chain', glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG cf. the genetic code.Glycine is a colourless, sweet-tasting crystalline solid...
is a precursor of porphyrins such as hemeHemeA heme or haem is a prosthetic group that consists of an iron atom contained in the center of a large heterocyclic organic ring called a porphyrin. Not all porphyrins contain iron, but a substantial fraction of porphyrin-containing metalloproteins have heme as their prosthetic group; these are...
. - ArginineArginineArginine is an α-amino acid. The L-form is one of the 20 most common natural amino acids. At the level of molecular genetics, in the structure of the messenger ribonucleic acid mRNA, CGU, CGC, CGA, CGG, AGA, and AGG, are the triplets of nucleotide bases or codons that codify for arginine during...
is a precursor of nitric oxideNitric oxideNitric oxide, also known as nitrogen monoxide, is a diatomic molecule with chemical formula NO. It is a free radical and is an important intermediate in the chemical industry...
. - OrnithineOrnithineOrnithine is an amino acid that plays a role in the urea cycle.-Role in urea cycle:L-Ornithine is one of the products of the action of the enzyme arginase on L-arginine, creating urea. Therefore, ornithine is a central part of the urea cycle, which allows for the disposal of excess nitrogen....
and S-adenosylmethionineS-Adenosyl methionineS-Adenosyl methionine is a common cosubstrate involved in methyl group transfers. SAM was first discovered in Italy by G. L. Cantoni in 1952. It is made from adenosine triphosphate and methionine by methionine adenosyltransferase . Transmethylation, transsulfuration, and aminopropylation are the...
are precursors of polyaminePolyamineA polyamine is an organic compound having two or more primary amino groups .This class of compounds includes several synthetic substances that are important feedstocks for the chemical industry, such as ethylene diamine , 1,3-diaminopropane , and hexamethylenediamine...
s. - Aspartate, glycineGlycineGlycine is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its 'side chain', glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG cf. the genetic code.Glycine is a colourless, sweet-tasting crystalline solid...
, and glutamineGlutamineGlutamine is one of the 20 amino acids encoded by the standard genetic code. It is not recognized as an essential amino acid but may become conditionally essential in certain situations, including intensive athletic training or certain gastrointestinal disorders...
are precursors of nucleotideNucleotideNucleotides are molecules that, when joined together, make up the structural units of RNA and DNA. In addition, nucleotides participate in cellular signaling , and are incorporated into important cofactors of enzymatic reactions...
s. - PhenylalaninePhenylalaninePhenylalanine is an α-amino acid with the formula C6H5CH2CHCOOH. This essential amino acid is classified as nonpolar because of the hydrophobic nature of the benzyl side chain. L-Phenylalanine is an electrically neutral amino acid, one of the twenty common amino acids used to biochemically form...
is a precursor of various phenylpropanoidPhenylpropanoidThe phenylpropanoids are a diverse family of organic compounds that are synthesized by plants from the amino acid phenylalanine. Their name is derived from the six-carbon, aromatic phenyl group and the three-carbon propene tail of cinnamic acid, which is synthesized from phenylalanine in the first...
s, which are important in plant metabolism.
However, not all of the functions of other abundant non-standard amino acids are known. For example, taurine is a major amino acid in muscle and brain tissues, but, although many functions have been proposed, its precise role in the body has not been determined.
Some non-standard amino acids are used as defenses against herbivores
Plant defense against herbivory
Plant defense against herbivory or host-plant resistance describes a range of adaptations evolved by plants which improve their survival and reproduction by reducing the impact of herbivores. Plants use several strategies to defend against damage caused by herbivores...
in plants. For example canavanine
Canavanine
L---Canavanine is a non-proteinogenic α-amino acid found in certain leguminous plants. It is structurally related to the proteinogenic α-amino acid L-arginine, the sole difference being the replacement of a methylene group in arginine with an oxa group in canavanine...
is an analogue of arginine
Arginine
Arginine is an α-amino acid. The L-form is one of the 20 most common natural amino acids. At the level of molecular genetics, in the structure of the messenger ribonucleic acid mRNA, CGU, CGC, CGA, CGG, AGA, and AGG, are the triplets of nucleotide bases or codons that codify for arginine during...
that is found in many legumes, and in particularly large amounts in Canavalia gladiata
Canavalia gladiata
Canavalia gladiata, usually called Sword Bean, is a domesticated plant species in the legume family. The legume is a used as a vegetable in interiors of central and south central India, though not commercially farmed. In telugu it is called 'chamma kaya'The fruits are eaten as a vegetable in...
(sword bean). This amino acid protects the plants from predators such as insect
Insect
Insects are a class of living creatures within the arthropods that have a chitinous exoskeleton, a three-part body , three pairs of jointed legs, compound eyes, and two antennae...
s and can cause illness in people if some types of legumes are eaten without processing. The non-protein amino acid mimosine
Mimosine
Mimosine or leucenol is an alkaloid, β-3-hydroxy-4 pyridone amino acid. It is a toxic non-protein free amino acid otherwise chemically similar to tyrosine, and was first isolated from Mimosa pudica. It occurs in a few other Mimosa spp...
is found in other species of legume, particularly Leucaena leucocephala. This compound is an analogue of tyrosine
Tyrosine
Tyrosine or 4-hydroxyphenylalanine, is one of the 22 amino acids that are used by cells to synthesize proteins. Its codons are UAC and UAU. It is a non-essential amino acid with a polar side group...
and can poison animals that graze on these plants.
Uses in technology
Amino acids are used for a variety of applications in industry, but their main use is as additives to animal feedCompound feed
Compound feeds are feedstuffs that are blended from various raw materials and additives. These blends are formulated according to the specific requirements of the target animal...
. This is necessary, since many of the bulk components of these feeds, such as soybean
Soybean
The soybean or soya bean is a species of legume native to East Asia, widely grown for its edible bean which has numerous uses...
s, either have low levels or lack some of the essential amino acid
Essential amino acid
An essential amino acid or indispensable amino acid is an amino acid that cannot be synthesized de novo by the organism , and therefore must be supplied in the diet.-Essentiality vs. conditional essentiality in humans:...
s: Lysine, methionine, threonine, and tryptophan are most important in the production of these feeds. In this industry, amino acids are also used to chelate metal cations in order to improve the absorption of minerals from supplements, which may be required to improve the health or production of these animals.
The food industry
Food industry
The food production is a complex, global collective of diverse businesses that together supply much of the food energy consumed by the world population...
is also a major consumer of amino acids, in particular, glutamic acid
Glutamic acid
Glutamic acid is one of the 20 proteinogenic amino acids, and its codons are GAA and GAG. It is a non-essential amino acid. The carboxylate anions and salts of glutamic acid are known as glutamates...
, which is used as a flavor enhancer, and Aspartame
Aspartame
Aspartame is an artificial, non-saccharide sweetener used as a sugar substitute in some foods and beverages. In the European Union, it is codified as E951. Aspartame is a methyl ester of the aspartic acid/phenylalanine dipeptide. It was first sold under the brand name NutraSweet; since 2009 it...
(aspartyl-phenylalanine-1-methyl ester) as a low-calorie artificial sweetener. Similar technology to that used for animal nutrition is employed in the human nutrition industry to alleviate symptoms of mineral deficiencies, such as anemia, by improving mineral absorption and reducing negative side effects from inorganic mineral supplementation.
The chelating ability of amino acids has been used in fertilizers for agriculture to facilitate the delivery of minerals to plants in order to correct mineral deficiencies, such as iron chlorosis. These fertilizers are also used to prevent deficiencies from occurring and improving the overall health of the plants. The remaining production of amino acids is used in the synthesis of drug
Drug
A drug, broadly speaking, is any substance that, when absorbed into the body of a living organism, alters normal bodily function. There is no single, precise definition, as there are different meanings in drug control law, government regulations, medicine, and colloquial usage.In pharmacology, a...
s and cosmetics
Cosmetics
Cosmetics are substances used to enhance the appearance or odor of the human body. Cosmetics include skin-care creams, lotions, powders, perfumes, lipsticks, fingernail and toe nail polish, eye and facial makeup, towelettes, permanent waves, colored contact lenses, hair colors, hair sprays and...
.
| Amino acid derivative | Pharmaceutical application |
|---|---|
| 5-HTP (5-hydroxytryptophan) | Experimental treatment for depression. |
| L-DOPA (L-dihydroxyphenylalanine) | Treatment for Parkinsonism Parkinsonism Parkinsonism is a neurological syndrome characterized by tremor, hypokinesia, rigidity, and postural instability. The underlying causes of parkinsonism are numerous, and diagnosis can be complex... . |
| Eflornithine Eflornithine Eflornithine is a drug found to be effective in the treatment of facial hirsutism as well as in African trypanosomiasis... |
Drug Drug A drug, broadly speaking, is any substance that, when absorbed into the body of a living organism, alters normal bodily function. There is no single, precise definition, as there are different meanings in drug control law, government regulations, medicine, and colloquial usage.In pharmacology, a... that inhibits ornithine decarboxylase Ornithine decarboxylase The enzyme ornithine decarboxylase catalyzes the decarboxylation of ornithine to form putrescine. This reaction is the committed step in polyamine synthesis. In humans, this protein has 461 amino acids and forms a homodimer.... and is used in the treatment of sleeping sickness. |
Expanded genetic code
Since 2001, 40 non-natural amino acids have been added into protein by creating a unique codon (recoding) and a corresponding transfer-RNA:aminoacyl – tRNA-synthetase pair to encode it with diverse physicochemical and biological properties in order to be used as a tool to exploring protein structureProtein structure
Proteins are an important class of biological macromolecules present in all organisms. Proteins are polymers of amino acids. Classified by their physical size, proteins are nanoparticles . Each protein polymer – also known as a polypeptide – consists of a sequence formed from 20 possible L-α-amino...
and function or to create novel or enhanced proteins.
Chemical building blocks
Amino acids are important as low-cost feedstocks. These compounds are used in chiral pool synthesisChiral pool synthesis
Chiral pool synthesis is a strategy that aims to improve the efficiency of chiral synthesis. It starts the organic synthesis of a complex enantiopure chemical compound from a stock of readily available enantiopure substances. Common chiral starting materials include monosaccharides and amino acids...
as enantiomerically-pure
Enantiomer
In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable , much as one's left and right hands are the same except for opposite orientation. It can be clearly understood if you try to place your hands one over the other without...
building blocks.
Amino acids have been investigated as precursors chiral catalysts, e.g., for asymmetric hydrogenation
Hydrogenation
Hydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
reactions, although no commercial applications exist.
Biodegradable plastics
Amino acids are under development as components of a range of biodegradable polymers. These materials have applications as environmentally friendly packaging and in medicine in drug deliveryDrug delivery
Drug delivery is the method or process of administering a pharmaceutical compound to achieve a therapeutic effect in humans or animals. Drug delivery technologies modify drug release profile, absorption, distribution and elimination for the benefit of improving product efficacy and safety, as well...
and the construction of prosthetic implants
Prosthesis
In medicine, a prosthesis, prosthetic, or prosthetic limb is an artificial device extension that replaces a missing body part. It is part of the field of biomechatronics, the science of using mechanical devices with human muscle, skeleton, and nervous systems to assist or enhance motor control...
. These polymers include polypeptides, polyamides, polyesters, polysulfides, and polyurethanes with amino acids either forming part of their main chains or bonded as side-chains. These modifications alter the physical properties and reactivities of the polymers. An interesting example of such materials is polyaspartate
Sodium poly(aspartate)
Sodium poly is a condensation polymer based on the amino acid aspartic acid.-Polymerization:The polymerization reaction is an example of a step-growth polymerization to a polyamide and in one practical procedure aspartic acid is simply heated to 180 °C resulting in water release and the formation...
, a water-soluble biodegradable polymer that may have applications in disposable diaper
Diaper
A nappy or a diaper is a kind of pant that allows one to defecate or urinate on oneself discreetly. When diapers become soiled, they require changing; this process is often performed by a second person such as a parent or caregiver...
s and agriculture. Due to its solubility and ability to chelate
Chelation
Chelation is the formation or presence of two or more separate coordinate bonds between apolydentate ligand and a single central atom....
metal ions, polyaspartate is also being used as a biodegradeable anti-scaling
Fouling
Fouling refers to the accumulation of unwanted material on solid surfaces, most often in an aquatic environment. The fouling material can consist of either living organisms or a non-living substance...
agent and a corrosion inhibitor
Corrosion inhibitor
A corrosion inhibitor is a chemical compound that, when added to a liquid or gas, decreases the corrosion rate of a material, typically a metal or an alloy. The effectiveness of a corrosion inhibitor depends on fluid composition, quantity of water, and flow regime...
. In addition, the aromatic amino acid tyrosine
Tyrosine
Tyrosine or 4-hydroxyphenylalanine, is one of the 22 amino acids that are used by cells to synthesize proteins. Its codons are UAC and UAU. It is a non-essential amino acid with a polar side group...
is being developed as a possible replacement for toxic phenol
Phenol
Phenol, also known as carbolic acid, phenic acid, is an organic compound with the chemical formula C6H5OH. It is a white crystalline solid. The molecule consists of a phenyl , bonded to a hydroxyl group. It is produced on a large scale as a precursor to many materials and useful compounds...
s such as bisphenol A
Bisphenol A
Bisphenol A is an organic compound with two phenol functional groups. It is used to make polycarbonate plastic and epoxy resins, along with other applications....
in the manufacture of polycarbonate
Polycarbonate
PolycarbonatePhysical PropertiesDensity 1.20–1.22 g/cm3Abbe number 34.0Refractive index 1.584–1.586FlammabilityV0-V2Limiting oxygen index25–27%Water absorption – Equilibrium0.16–0.35%Water absorption – over 24 hours0.1%...
s.
Reactions
As amino acids have both a primary amineAmine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
group and a primary carboxyl group, these chemicals can undergo most of the reactions associated with these functional groups. These include nucleophilic addition
Nucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where in a chemical compound a π bond is removed by the creation of two new covalent bonds by the addition of a nucleophile....
, amide bond
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
formation and imine formation
Alkylimino-de-oxo-bisubstitution
Alkylimino-de-oxo-bisubstitution in organic chemistry is the organic reaction of carbonyl compounds with amines to imines . The reaction name is based on the IUPAC Nomenclature for Transformations...
for the amine group and esterification, amide bond
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
formation and decarboxylation
Decarboxylation
Decarboxylation is a chemical reaction that releases carbon dioxide . Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is the first chemical step in photosynthesis, is called carbonation, the addition of CO2 to...
for the carboxylic acid group. The combination of these functional groups allow amino acids to be effective polydentate ligands for metal-amino acid chelates.
The multiple side-chains of amino acids can also undergo chemical reactions. The types of these reactions are determined by the groups on these side-chains and are, therefore, different between the various types of amino acid.
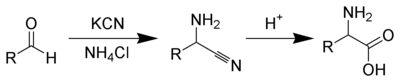
Chemical synthesis
Several methods exist to synthesize amino acids. One of the oldest methods begins with the brominationHell-Volhard-Zelinsky halogenation
The Hell-Volhard-Zelinsky halogenation reaction halogenates carboxylic acids at the α carbon. The reaction is named after three chemists, the German chemists Carl Magnus von Hell and Jacob Volhard and the Russian chemist Nikolay Zelinsky .- Scheme :Unlike other halogenation reactions, this...
at the α-carbon of a carboxylic acid. Nucleophilic substitution with ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
then converts the alkyl bromide to the amino acid. In alternative fashion, the Strecker amino acid synthesis
Strecker amino acid synthesis
The Strecker amino acid synthesis, devised by Adolph Strecker, is a series of chemical reactions that synthesize an amino acid from an aldehyde . The aldehyde is condensed with ammonium chloride in the presence of potassium cyanide to form an α-aminonitrile, which is subsequently hydrolyzed to give...
involves the treatment of an aldehyde with potassium cyanide
Potassium cyanide
Potassium cyanide is an inorganic compound with the formula KCN. This colorless crystalline compound, similar in appearance to sugar, is highly soluble in water. Most KCN is used in gold mining, organic synthesis, and electroplating. Smaller applications include jewelry for chemical gilding and...
and ammonia, this produces an α-amino nitrile as an intermediate. Hydrolysis of the nitrile in acid then yields a α-amino acid. Using ammonia or ammonium salts in this reaction gives unsubstituted amino acids, while substituting primary and secondary amines will yield substituted amino acids. Likewise, using ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s, instead of aldehydes, gives α,α-disubstituted amino acids. The classical synthesis gives racemic mixtures of α-amino acids as products, but several alternative procedures using asymmetric auxiliaries or asymmetric catalysts have been developed.
At the current time, the most-adopted method is an automated synthesis on a solid support (e.g., polystyrene
Polystyrene
Polystyrene ) also known as Thermocole, abbreviated following ISO Standard PS, is an aromatic polymer made from the monomer styrene, a liquid hydrocarbon that is manufactured from petroleum by the chemical industry...
beads), using protecting group
Protecting group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group in order to obtain chemoselectivity in a subsequent chemical reaction...
s (e.g., Fmoc and t-Boc
Di-tert-butyl dicarbonate
Di-tert-butyl dicarbonate is a reagent widely used in organic synthesis. This carbonate ester reacts with amines to give N-tert-butoxycarbonyl or so-called t-BOC derivatives. These derivatives do not behave as amines, which allows certain subsequent transformations to occur that would have...
) and activating groups (e.g., DCC
Dicyclohexylcarbodiimide
N,N-Dicyclohexylcarbodiimide is an organic compound with chemical formula C13H22N2 whose primary use is to couple amino acids during artificial peptide synthesis. Under standard conditions, it exists in the form of white crystals with a heavy, sweet odor. The low melting point of this material...
and DIC).
Peptide bond formation
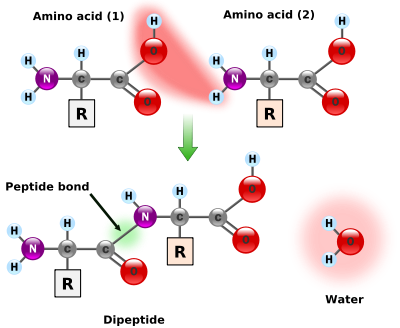
Polymerization
In polymer chemistry, polymerization is a process of reacting monomer molecules together in a chemical reaction to form three-dimensional networks or polymer chains...
of amino acids is what creates proteins. This condensation reaction
Condensation reaction
A condensation reaction is a chemical reaction in which two molecules or moieties combine to form one single molecule, together with the loss of a small molecule. When this small molecule is water, it is known as a dehydration reaction; other possible small molecules lost are hydrogen chloride,...
yields the newly formed peptide bond
Peptide bond
This article is about the peptide link found within biological molecules, such as proteins. A similar article for synthetic molecules is being created...
and a molecule of water. In cells, this reaction does not occur directly; instead the amino acid is first activated by attachment to a transfer RNA
Transfer RNA
Transfer RNA is an adaptor molecule composed of RNA, typically 73 to 93 nucleotides in length, that is used in biology to bridge the three-letter genetic code in messenger RNA with the twenty-letter code of amino acids in proteins. The role of tRNA as an adaptor is best understood by...
molecule through an ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
bond. This aminoacyl-tRNA is produced in an ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
-dependent reaction carried out by an aminoacyl tRNA synthetase
Aminoacyl tRNA synthetase
An aminoacyl tRNA synthetase is an enzyme that catalyzes the esterification of a specific amino acid or its precursor to one of all its compatible cognate tRNAs to form an aminoacyl-tRNA. This is sometimes called "charging" the tRNA with the amino acid...
. This aminoacyl-tRNA is then a substrate for the ribosome
Ribosome
A ribosome is a component of cells that assembles the twenty specific amino acid molecules to form the particular protein molecule determined by the nucleotide sequence of an RNA molecule....
, which catalyzes the attack of the amino group of the elongating protein chain on the ester bond. As a result of this mechanism, all proteins made by ribosomes are synthesized starting at their N-terminus and moving towards their C-terminus.
However, not all peptide bonds are formed in this way. In a few cases, peptides are synthesized by specific enzymes. For example, the tripeptide glutathione
Glutathione
Glutathione is a tripeptide that contains an unusual peptide linkage between the amine group of cysteine and the carboxyl group of the glutamate side-chain...
is an essential part of the defenses of cells against oxidative stress. This peptide is synthesized in two steps from free amino acids. In the first step gamma-glutamylcysteine synthetase
Gamma-glutamylcysteine synthetase
Gamma-glutamylcysteine synthetase is the first enzyme in the glutathione biosynthesis pathway.-Function:...
condenses cysteine
Cysteine
Cysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid...
and glutamic acid
Glutamic acid
Glutamic acid is one of the 20 proteinogenic amino acids, and its codons are GAA and GAG. It is a non-essential amino acid. The carboxylate anions and salts of glutamic acid are known as glutamates...
through a peptide bond formed between the side-chain carboxyl of the glutamate (the gamma carbon of this side-chain) and the amino group of the cysteine. This dipeptide is then condensed with glycine
Glycine
Glycine is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its 'side chain', glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG cf. the genetic code.Glycine is a colourless, sweet-tasting crystalline solid...
by glutathione synthetase
Glutathione synthetase
Glutathione synthetase is the second enzyme in the glutathione biosynthesis pathway. It catalyses the condensation of gamma-glutamylcysteine and glycine, to form glutathione.In eukaryotes, this is a homodimeric enzyme...
to form glutathione.
In chemistry, peptides are synthesized by a variety of reactions. One of the most-used in solid-phase peptide synthesis
Peptide synthesis
In organic chemistry, peptide synthesis is the production of peptides, which are organic compounds in which multiple amino acids are linked via amide bonds which are also known as peptide bonds...
uses the aromatic oxime derivatives of amino acids as activated units. These are added in sequence onto the growing peptide chain, which is attached to a solid resin support. The ability to easily synthesize vast numbers of different peptides by varying the types and order of amino acids (using combinatorial chemistry
Combinatorial chemistry
Combinatorial chemistry involves the rapid synthesis or the computer simulation of a large number of different but structurally related molecules or materials...
) has made peptide synthesis particularly important in creating libraries of peptides for use in drug discovery through high-throughput screening
High-throughput screening
High-throughput screening is a method for scientific experimentation especially used in drug discovery and relevant to the fields of biology and chemistry. Using robotics, data processing and control software, liquid handling devices, and sensitive detectors, High-Throughput Screening allows a...
.
Biosynthesis
In plants, nitrogen is first assimilated into organic compounds in the form of glutamate, formed from alpha-ketoglutarate and ammonia in the mitochondrion. In order to form other amino acids, the plant uses transaminaseTransaminase
In biochemistry, a transaminase or an aminotransferase is an enzyme that catalyzes a type of reaction between an amino acid and an α-keto acid. To be specific, this reaction involves removing the amino group from the amino acid, leaving behind an α-keto acid, and transferring it to the...
s to move the amino group to another alpha-keto carboxylic acid. For example, aspartate aminotransferase converts glutamate and oxaloacetate to alpha-ketoglutarate and aspartate. Other organisms use transaminases for amino acid synthesis, too.
Nonstandard amino acids are usually formed through modifications to standard amino acids. For example, homocysteine
Homocysteine
Homocysteine is a non-protein amino acid with the formula HSCH2CH2CHCO2H. It is a homologue of the amino acid cysteine, differing by an additional methylene group. It is biosynthesized from methionine by the removal of its terminal Cε methyl group...
is formed through the transsulfuration pathway
Transsulfuration pathway
The transsulfuration pathway is a metabolic pathway involving the interconversion of cysteine and homocysteine, through the intermediate cystathionine. In eukarotes, such as humans, the transsulfuration pathway is critical for creating cysteine from the essential amino acid methionine...
or by the demethylation of methionine via the intermediate metabolite S-adenosyl methionine
S-Adenosyl methionine
S-Adenosyl methionine is a common cosubstrate involved in methyl group transfers. SAM was first discovered in Italy by G. L. Cantoni in 1952. It is made from adenosine triphosphate and methionine by methionine adenosyltransferase . Transmethylation, transsulfuration, and aminopropylation are the...
, while hydroxyproline
Hydroxyproline
-4-Hydroxyproline, or L-hydroxyproline , is a common non-proteinogenic amino acid, abbreviated as HYP, e.g., in Protein Data Bank.-Structure and discovery:...
is made by a posttranslational modification
Posttranslational modification
Posttranslational modification is the chemical modification of a protein after its translation. It is one of the later steps in protein biosynthesis, and thus gene expression, for many proteins....
of proline
Proline
Proline is an α-amino acid, one of the twenty DNA-encoded amino acids. Its codons are CCU, CCC, CCA, and CCG. It is not an essential amino acid, which means that the human body can synthesize it. It is unique among the 20 protein-forming amino acids in that the α-amino group is secondary...
.
Microorganism
Microorganism
A microorganism or microbe is a microscopic organism that comprises either a single cell , cell clusters, or no cell at all...
s and plants can synthesize many uncommon amino acids. For example, some microbes make 2-aminoisobutyric acid
2-Aminoisobutyric acid
2-Aminoisobutyric acid, or α-aminoisobutyric acid or α-methylalanine or 2-methylalanine, is an amino acid with the structural formula is H2N-C2-COOH. It is contained in some antibiotics of fungal origin, e.g. alamethicin and some lantibiotics. It is not one of the proteinogenic amino acids and...
and lanthionine
Lanthionine
Lanthionine is a nonproteinogenic amino acid with the chemical formula . As the monosulfide analog of cystine, lanthionine is composed of two alanine residues that are crosslinked on their β-carbon atoms by a thioether linkage...
, which is a sulfide-bridged derivative of alanine. Both of these amino acids are found in peptidic lantibiotics
Lantibiotics
Lantibiotics are a class of peptide antibiotics that contain the characteristic polycyclic thioether amino acids lanthionine or methyllanthionine, as well as the unsaturated amino acids dehydroalanine and 2-aminoisobutyric acid....
such as alamethicin
Alamethicin
Alamethicin is a peptide antibiotic, produced by the fungus Trichoderma viride. It contains the non-proteinogenic amino acid 2-aminoisobutyric acid , which strongly induces helical peptide structures...
. While in plants, 1-aminocyclopropane-1-carboxylic acid
1-Aminocyclopropane-1-carboxylic acid
1-Aminocyclopropane-1-carboxylic acid is a disubstituted cyclic alpha-amino acid in which a three-membered cyclopropane ring is fuzed to the C-atom of the amino acid....
is a small disubstituted cyclic amino acid that is a key intermediate in the production of the plant hormone ethylene.
Catabolism
Degradation of an amino acid often involves deaminationDeamination
Deamination is the removal of an amine group from a molecule. Enzymes which catalyse this reaction are called deaminases.In the human body, deamination takes place primarily in the liver, however glutamate is also deaminated in the kidneys. Deamination is the process by which amino acids are...
by moving its amino group to alpha-ketoglutarate, forming glutamate. This process involves transaminases, often the same as those used in amination during synthesis. In many vertebrates, the amino group is then removed through the urea cycle
Urea cycle
The urea cycle is a cycle of biochemical reactions occurring in many animals that produces urea from ammonia . This cycle was the first metabolic cycle discovered , five years before the discovery of the TCA cycle...
and is excreted in the form of urea
Urea
Urea or carbamide is an organic compound with the chemical formula CO2. The molecule has two —NH2 groups joined by a carbonyl functional group....
. However, amino acid degradation can produce uric acid
Uric acid
Uric acid is a heterocyclic compound of carbon, nitrogen, oxygen, and hydrogen with the formula C5H4N4O3. It forms ions and salts known as urates and acid urates such as ammonium acid urate. Uric acid is created when the body breaks down purine nucleotides. High blood concentrations of uric acid...
or ammonia instead. For example, serine dehydratase
Serine dehydratase
Serine Dehydratase or L-Serine Ammonia Lyase is in the ß-family of Pyridoxal Phosphate-dependent enzymes. SDH is found widely in nature, but its structural and chemical properties vary greatly among species. SDH is found in yeast, bacteria, and the cytoplasm of mammalian hepatocytes...
converts serine to pyruvate and ammonia.
Physicochemical properties of amino acids
The 20 amino acids encoded directly by the genetic code can be divided into several groups based on their properties. Important factors are charge, hydrophilicityHydrophile
A hydrophile, from the Greek "water" and φιλια "love," is a molecule or other molecular entity that is attracted to, and tends to be dissolved by water. A hydrophilic molecule or portion of a molecule is one that has a tendency to interact with or be dissolved by, water and other polar substances...
or hydrophobicity
Hydrophobe
In chemistry, hydrophobicity is the physical property of a molecule that is repelled from a mass of water....
, size, and functional groups. These properties are important for protein structure
Protein structure
Proteins are an important class of biological macromolecules present in all organisms. Proteins are polymers of amino acids. Classified by their physical size, proteins are nanoparticles . Each protein polymer – also known as a polypeptide – consists of a sequence formed from 20 possible L-α-amino...
and protein–protein interactions. The water-soluble proteins tend to have their hydrophobic residues (Leu, Ile, Val, Phe, and Trp) buried in the middle of the protein, whereas hydrophilic side-chains are exposed to the aqueous solvent. The integral membrane protein
Integral membrane protein
An integral membrane protein is a protein molecule that is permanently attached to the biological membrane. Proteins that cross the membrane are surrounded by "annular" lipids, which are defined as lipids that are in direct contact with a membrane protein...
s tend to have outer rings of exposed hydrophobic amino acids that anchor them into the lipid bilayer
Lipid bilayer
The lipid bilayer is a thin membrane made of two layers of lipid molecules. These membranes are flat sheets that form a continuous barrier around cells. The cell membrane of almost all living organisms and many viruses are made of a lipid bilayer, as are the membranes surrounding the cell nucleus...
. In the case part-way between these two extremes, some peripheral membrane protein
Peripheral membrane protein
Peripheral membrane proteins are proteins that adhere only temporarily to the biological membrane with which they are associated. These molecules attach to integral membrane proteins, or penetrate the peripheral regions of the lipid bilayer. The regulatory protein subunits of many ion channels and...
s have a patch of hydrophobic amino acids on their surface that locks onto the membrane. In similar fashion, proteins that have to bind to positively-charged molecules have surfaces rich with negatively charged amino acids like glutamate and aspartate, while proteins binding to negatively-charged molecules have surfaces rich with positively charged chains like lysine
Lysine
Lysine is an α-amino acid with the chemical formula HO2CCH4NH2. It is an essential amino acid, which means that the human body cannot synthesize it. Its codons are AAA and AAG....
and arginine
Arginine
Arginine is an α-amino acid. The L-form is one of the 20 most common natural amino acids. At the level of molecular genetics, in the structure of the messenger ribonucleic acid mRNA, CGU, CGC, CGA, CGG, AGA, and AGG, are the triplets of nucleotide bases or codons that codify for arginine during...
. There are different hydrophobicity scales of amino acid residues.
Some amino acids have special properties such as cysteine
Cysteine
Cysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid...
, that can form covalent disulfide bond
Disulfide bond
In chemistry, a disulfide bond is a covalent bond, usually derived by the coupling of two thiol groups. The linkage is also called an SS-bond or disulfide bridge. The overall connectivity is therefore R-S-S-R. The terminology is widely used in biochemistry...
s to other cysteine residues, proline
Proline
Proline is an α-amino acid, one of the twenty DNA-encoded amino acids. Its codons are CCU, CCC, CCA, and CCG. It is not an essential amino acid, which means that the human body can synthesize it. It is unique among the 20 protein-forming amino acids in that the α-amino group is secondary...
that forms a cycle
Cyclic compound
In chemistry, a cyclic compound is a compound in which a series of atoms is connected to form a loop or ring.While the vast majority of cyclic compounds are organic, a few inorganic substances form cyclic compounds as well, including sulfur, silanes, phosphanes, phosphoric acid, and triboric acid. ...
to the polypeptide backbone, and glycine
Glycine
Glycine is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its 'side chain', glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG cf. the genetic code.Glycine is a colourless, sweet-tasting crystalline solid...
that is more flexible than other amino acids.
Many proteins undergo a range of posttranslational modification
Posttranslational modification
Posttranslational modification is the chemical modification of a protein after its translation. It is one of the later steps in protein biosynthesis, and thus gene expression, for many proteins....
s, when additional chemical groups are attached to the amino acids in proteins. Some modifications can produce hydrophobic lipoprotein
Lipoprotein
A lipoprotein is a biochemical assembly that contains both proteins and lipids water-bound to the proteins. Many enzymes, transporters, structural proteins, antigens, adhesins, and toxins are lipoproteins...
s, or hydrophilic glycoprotein
Glycoprotein
Glycoproteins are proteins that contain oligosaccharide chains covalently attached to polypeptide side-chains. The carbohydrate is attached to the protein in a cotranslational or posttranslational modification. This process is known as glycosylation. In proteins that have segments extending...
s. These type of modification allow the reversible targeting of a protein to a membrane. For example, the addition and removal of the fatty acid palmitic acid
Palmitic acid
Palmitic acid, or hexadecanoic acid in IUPAC nomenclature, is one of the most common saturated fatty acids found in animals and plants. Its molecular formula is CH314CO2H. As its name indicates, it is a major component of the oil from palm trees . Palmitate is a term for the salts and esters of...
to cysteine residues in some signaling proteins causes the proteins to attach and then detach from cell membranes.
Table of standard amino acid abbreviations and properties
| Amino Acid | 3-Letter | 1-Letter | Side-chain polarity | Side-chain charge (pH 7.4) | Hydropathy index | Absorbance λmax(nm) | ε at λmax (x10−3 M−1 cm−1) |
|---|---|---|---|---|---|---|---|
| Alanine Alanine Alanine is an α-amino acid with the chemical formula CH3CHCOOH. The L-isomer is one of the 20 amino acids encoded by the genetic code. Its codons are GCU, GCC, GCA, and GCG. It is classified as a nonpolar amino acid... |
Ala | A | nonpolar | neutral | 1.8 | ||
| Arginine Arginine Arginine is an α-amino acid. The L-form is one of the 20 most common natural amino acids. At the level of molecular genetics, in the structure of the messenger ribonucleic acid mRNA, CGU, CGC, CGA, CGG, AGA, and AGG, are the triplets of nucleotide bases or codons that codify for arginine during... |
Arg | R | polar | positive | −4.5 | ||
| Asparagine Asparagine Asparagine is one of the 20 most common natural amino acids on Earth. It has carboxamide as the side-chain's functional group. It is not an essential amino acid... |
Asn | N | polar | neutral | −3.5 | ||
| Aspartic acid Aspartic acid Aspartic acid is an α-amino acid with the chemical formula HOOCCHCH2COOH. The carboxylate anion, salt, or ester of aspartic acid is known as aspartate. The L-isomer of aspartate is one of the 20 proteinogenic amino acids, i.e., the building blocks of proteins... |
Asp | D | polar | negative | −3.5 | ||
| Cysteine Cysteine Cysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid... |
Cys | C | polar | neutral | 2.5 | 250 | 0.3 |
| Glutamic acid Glutamic acid Glutamic acid is one of the 20 proteinogenic amino acids, and its codons are GAA and GAG. It is a non-essential amino acid. The carboxylate anions and salts of glutamic acid are known as glutamates... |
Glu | E | polar | negative | −3.5 | ||
| Glutamine Glutamine Glutamine is one of the 20 amino acids encoded by the standard genetic code. It is not recognized as an essential amino acid but may become conditionally essential in certain situations, including intensive athletic training or certain gastrointestinal disorders... |
Gln | Q | polar | neutral | −3.5 | ||
| Glycine Glycine Glycine is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its 'side chain', glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG cf. the genetic code.Glycine is a colourless, sweet-tasting crystalline solid... |
Gly | G | nonpolar | neutral | −0.4 | ||
| Histidine Histidine Histidine Histidine, an essential amino acid, has a positively charged imidazole functional group. It is one of the 22 proteinogenic amino acids. Its codons are CAU and CAC. Histidine was first isolated by German physician Albrecht Kossel in 1896. Histidine is an essential amino acid in humans... |
His | H | polar | positive(10%) neutral(90%) |
−3.2 | 211 | 5.9 |
| Isoleucine Isoleucine Isoleucine is an α-amino acid with the chemical formula HO2CCHCHCH2CH3. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested. Its codons are AUU, AUC and AUA.... |
Ile | I | nonpolar | neutral | 4.5 | ||
| Leucine Leucine Leucine is a branched-chain α-amino acid with the chemical formula HO2CCHCH2CH2. Leucine is classified as a hydrophobic amino acid due to its aliphatic isobutyl side chain. It is encoded by six codons and is a major component of the subunits in ferritin, astacin and other 'buffer' proteins... |
Leu | L | nonpolar | neutral | 3.8 | ||
| Lysine Lysine Lysine is an α-amino acid with the chemical formula HO2CCH4NH2. It is an essential amino acid, which means that the human body cannot synthesize it. Its codons are AAA and AAG.... |
Lys | K | polar | positive | −3.9 | ||
| Methionine Methionine Methionine is an α-amino acid with the chemical formula HO2CCHCH2CH2SCH3. This essential amino acid is classified as nonpolar. This amino-acid is coded by the codon AUG, also known as the initiation codon, since it indicates mRNA's coding region where translation into protein... |
Met | M | nonpolar | neutral | 1.9 | ||
| Phenylalanine Phenylalanine Phenylalanine is an α-amino acid with the formula C6H5CH2CHCOOH. This essential amino acid is classified as nonpolar because of the hydrophobic nature of the benzyl side chain. L-Phenylalanine is an electrically neutral amino acid, one of the twenty common amino acids used to biochemically form... |
Phe | F | nonpolar | neutral | 2.8 | 257, 206, 188 | 0.2, 9.3, 60.0 |
| Proline Proline Proline is an α-amino acid, one of the twenty DNA-encoded amino acids. Its codons are CCU, CCC, CCA, and CCG. It is not an essential amino acid, which means that the human body can synthesize it. It is unique among the 20 protein-forming amino acids in that the α-amino group is secondary... |
Pro | P | nonpolar | neutral | −1.6 | ||
| Serine Serine Serine is an amino acid with the formula HO2CCHCH2OH. It is one of the proteinogenic amino acids. By virtue of the hydroxyl group, serine is classified as a polar amino acid.-Occurrence and biosynthesis:... |
Ser | S | polar | neutral | −0.8 | ||
| Threonine Threonine Threonine is an α-amino acid with the chemical formula HO2CCHCHCH3. Its codons are ACU, ACA, ACC, and ACG. This essential amino acid is classified as polar... |
Thr | T | polar | neutral | −0.7 | ||
| Tryptophan Tryptophan Tryptophan is one of the 20 standard amino acids, as well as an essential amino acid in the human diet. It is encoded in the standard genetic code as the codon UGG... |
Trp | W | nonpolar | neutral | −0.9 | 280, 219 | 5.6, 47.0 |
| Tyrosine Tyrosine Tyrosine or 4-hydroxyphenylalanine, is one of the 22 amino acids that are used by cells to synthesize proteins. Its codons are UAC and UAU. It is a non-essential amino acid with a polar side group... |
Tyr | Y | polar | neutral | −1.3 | 274, 222, 193 | 1.4, 8.0, 48.0 |
| Valine Valine Valine is an α-amino acid with the chemical formula HO2CCHCH2. L-Valine is one of 20 proteinogenic amino acids. Its codons are GUU, GUC, GUA, and GUG. This essential amino acid is classified as nonpolar... |
Val | V | nonpolar | neutral | 4.2 |
In addition, there are two additional amino acids that are incorporated by overriding stop codons:
| 21st and 22nd amino acids | 3-Letter | 1-Letter |
|---|---|---|
| Selenocysteine Selenocysteine Selenocysteine is an amino acid that is present in several enzymes .-Nomenclature:... |
Sec | U |
| Pyrrolysine Pyrrolysine Pyrrolysine is a naturally occurring, genetically coded amino acid used by some methanogenic archaea and one known bacterium in enzymes that are part of their methane-producing metabolism. It is similar to lysine, but with an added pyrroline ring linked to the end of the lysine side chain... |
Pyl | O |
In addition to the specific amino acid codes, placeholders are used in cases where chemical
Protein sequencing
Protein sequencing is a technique to determine the amino acid sequence of a protein, as well as which conformation the protein adopts and the extent to which it is complexed with any non-peptide molecules...
or crystallographic
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
analysis of a peptide or protein cannot conclusively determine the identity of a residue.
| Ambiguous Amino Acids | 3-Letter | 1-Letter |
|---|---|---|
| Asparagine or aspartic acid | Asx | B |
| Glutamine or glutamic acid | Glx | Z |
| Leucine or Isoleucine | Xle | J |
| Unspecified or unknown amino acid | Xaa | X |
Unk is sometimes used instead of Xaa, but is less standard.
In addition, many non-standard amino acids have a specific code. For example, several peptide drugs, such as Bortezomib
Bortezomib
Bortezomib is the first therapeutic proteasome inhibitor to be tested in humans. It is approved in the U.S. for treating relapsed multiple myeloma and mantle cell lymphoma...
and MG132
MG132
MG132 is a specific, potent, reversible, and cell-permeable proteasome inhibitor . Reduces the degradation of ubiquitin-conjugated proteins in mammalian cells and permeable strains of yeast by the 26S complex without affecting its ATPase or isopeptidase activities. MG132 activates c-Jun N-terminal...
, are artificially synthesized
Peptide synthesis
In organic chemistry, peptide synthesis is the production of peptides, which are organic compounds in which multiple amino acids are linked via amide bonds which are also known as peptide bonds...
and retain their protecting group
Protecting group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group in order to obtain chemoselectivity in a subsequent chemical reaction...
s, which have specific codes. Bortezomib is Pyz
Pyrazinoic acid
Pyrazinoic acid is a pyrazinamide metabolite....
-Phe-boroLeu, and MG132 is Z
Carboxybenzyl
Carboxybenzyl or Cbz or Z is an amine protecting group in organic synthesis. It is commonly used in peptide synthesis and is formed by reacting an amine with benzyl chloroformate and a weak base:It is used to protect amines from electrophiles...
-Leu-Leu-Leu-al. To aid in the analysis of protein structure, photocrosslinking amino acid analogues
Photo-reactive amino acid analog
Photo-reactive amino acid analogs for in-vivo crosslinking of protein complexes were introduced in 2005 by researchers from the Max Planck Institute. In this method, cells are grown with photoreactive diazirine analogs to leucine and methionine, which are incorporated into proteins...
are available. These include photoleucine (pLeu) and photomethionine (pMet).
See also
- Amino acid datingAmino acid datingAmino acid dating is a dating technique used to estimate the age of a specimen in paleobiology, archaeology, forensic science, taphonomy, sedimentary geology and other fields. This technique relates changes in amino acid molecules to the time elapsed since they were formed.-Principle:All...
- Beta amino acid
- DegronDegronA degron is a specific sequence of amino acids in a protein that directs the starting place of degradation. A degron sequence can occur at either the N or C-terminal region, these are called N-Degrons or C-degrons respectively....
- Glucogenic amino acidGlucogenic amino acidA glucogenic amino acid is an amino acid that can be converted into glucose through gluconeogenesis. This is in contrast to the ketogenic amino acids, which are converted into ketone bodies....
- HomochiralityHomochiralityHomochirality is a term used to refer to a group of molecules that possess the same sense of chirality. Molecules involved are not necessarily the same compound, but similar groups are arranged in the same way around a central atom. In biology homochirality is found in the chemical building blocks...
- LeucinesLeucinesThe leucines are primarily the four isomeric amino acids: leucine, isoleucine, tert-leucine and norleucine. Being compared with the four butanols, they could be classified as butyl-substituted glycines; they represent all four possible variations....
- Miller–Urey experiment
- Proteinogenic amino acidProteinogenic amino acidProteinogenic amino acids are those amino acids that can be found in proteins and require cellular machinery coded for in the genetic code of any organism for their isolated production. There are 22 standard amino acids, but only 21 are found in eukaryotes. Of the 22, 20 are directly encoded by...
(including chemical structures) - Table of codons, 3-nucleotide sequences that encode each amino acid
- PolyamidePolyamideA polyamide is a polymer containing monomers of amides joined by peptide bonds. They can occur both naturally and artificially, examples being proteins, such as wool and silk, and can be made artificially through step-growth polymerization or solid-phase synthesis, examples being nylons, aramids,...
Further reading
- Doolittle, R.F. (1989) Redundancies in protein sequences. In Predictions of Protein Structure and the Principles of Protein Conformation (Fasman, G.D. ed) Plenum Press, New York, pp. 599–623
- David L. Nelson and Michael M. Cox, Lehninger Principles of Biochemistry, 3rd edition, 2000, Worth Publishers, ISBN 1-57259-153-6
- Meierhenrich, U.J.: Amino acids and the asymmetry of life, Springer-Verlag, Berlin, New York, 2008. ISBN 978-3-540-76885-2
- Morelli, Robert J. "Studies of amino acid absorption from the small intestine." San Francisco: Morelli, 1952.
External links
- Amino acids overview physical-chemistry properties, 3D structures, etc.
- List of Standard Amino Acids The Detailed PDF List of Standard Amino Acids (including 3D depictions)
- Nomenclature and Symbolism for Amino Acids and Peptides IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN)
- Molecular Expressions: The Amino Acid Collection – Has detailed information and microscopy photographs of each amino acid.
- Amino acid properties – Properties of the amino acids (a tool aimed mostly at molecular geneticists trying to understand the meaning of mutations)
- Synthesis of Amino Acids and Derivatives
- Learn the 20 proteinogenic amino acids online
- The origin of the single-letter code for the amino acids
- Amino acid solution’s pH, titration and isoelectric point calculation free spreadsheet
- Interactive Amino Map Web Application at DNA.UTAH.EDU

