Carboxybenzyl
Encyclopedia
Carboxybenzyl or Cbz or Z is an amine
protecting group
in organic synthesis
. It is commonly used in peptide synthesis
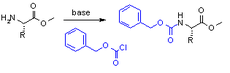
and is formed by reacting an amine with benzyl chloroformate
and a weak base
:
It is used to protect amines from electrophile
s. The protected amine can be deprotected by catalytic hydrogenation
or treatment with HBr
, yielding a terminal carbamic acid
that then readily decarboxylates to yield the free amine.
The method was first used by Max Bergmann
in 1932 for the synthesis of peptides.
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
protecting group
Protecting group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group in order to obtain chemoselectivity in a subsequent chemical reaction...
in organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
. It is commonly used in peptide synthesis
Peptide synthesis
In organic chemistry, peptide synthesis is the production of peptides, which are organic compounds in which multiple amino acids are linked via amide bonds which are also known as peptide bonds...
and is formed by reacting an amine with benzyl chloroformate
Benzyl chloroformate
Benzyl chloroformate is the benzyl ester of chloroformic acid. It is also known as benzyl chlorocarbonate is an oily liquid whose color is anywhere from yellow to colorless. It is also known for its pungent odor...
and a weak base
Weak base
In chemistry, a weak base is a chemical base that does not ionize fully in an aqueous solution. As Brønsted–Lowry bases are proton acceptors, a weak base may also be defined as a chemical base in which protonation is incomplete. This results in a relatively low pH compared to strong bases...
:
It is used to protect amines from electrophile
Electrophile
In general electrophiles are positively charged species that are attracted to an electron rich centre. In chemistry, an electrophile is a reagent attracted to electrons that participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile...
s. The protected amine can be deprotected by catalytic hydrogenation
Hydrogenation
Hydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
or treatment with HBr
Hydrogen bromide
Hydrogen bromide is the diatomic molecule HBr. HBr is a gas at standard conditions. Hydrobromic acid forms upon dissolving HBr in water. Conversely, HBr can be liberated from hydrobromic acid solutions with the addition of a dehydration agent, but not by distillation. Hydrogen bromide and...
, yielding a terminal carbamic acid
Carbamic acid
Carbamic acid is a compound that is unstable under normal circumstances. It is technically the simplest amino acid, though its instability allows glycine to assume this title. Its importance is due more to its relevance in identifying the names of larger compounds...
that then readily decarboxylates to yield the free amine.
The method was first used by Max Bergmann
Max Bergmann
Max Bergmann was a Jewish-German biochemist. He was the first to use the Carboxybenzyl protecting group for the synthesis of oligopeptides.-Life and work:Bergmann was born in Fürth, Bavaria, Germany on February 12, 1886....
in 1932 for the synthesis of peptides.