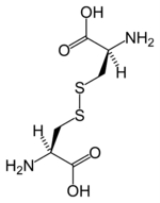
Disulfide bond
Overview
In chemistry
, a disulfide bond (Br.E. disulphide bond) is a covalent bond
, usually derived by the coupling of two thiol
group
s. The linkage is also called an SS-bond or disulfide bridge. The overall connectivity is therefore R-S-S-R. The terminology is widely used in biochemistry. In formal terms, the connection is a persulfide, in analogy to its congener
, peroxide
(R-O-O-R), but this terminology is obscure and is no longer used (except in reference to R-S-S-H or H-S-S-H compounds).
The disulfide bond is strong, with a typical bond dissociation energy
of 60 kcal/mole.
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
, a disulfide bond (Br.E. disulphide bond) is a covalent bond
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
, usually derived by the coupling of two thiol
Thiol
In organic chemistry, a thiol is an organosulfur compound that contains a carbon-bonded sulfhydryl group...
group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
s. The linkage is also called an SS-bond or disulfide bridge. The overall connectivity is therefore R-S-S-R. The terminology is widely used in biochemistry. In formal terms, the connection is a persulfide, in analogy to its congener
Congener
Congener has several different meanings depending on the field in which it is used. Colloquially, it is used to mean a person or thing like another, in character or action.-Biology:In biology, congeners are organisms within the same genus...
, peroxide
Organic peroxide
Organic peroxides are organic compounds containing the peroxide functional group . If the R' is hydrogen, the compound is called an organic hydroperoxide. Peresters have general structure RCOOR. The O-O bond easily breaks and forms free radicals of the form RO·...
(R-O-O-R), but this terminology is obscure and is no longer used (except in reference to R-S-S-H or H-S-S-H compounds).
The disulfide bond is strong, with a typical bond dissociation energy
Bond dissociation energy
In chemistry, bond-dissociation energy or D0, is one measure of the bond strength in a chemical bond. It is defined as the standard enthalpy change when a bond is cleaved by homolysis, with reactants and products of the homolysis reaction at 0 K...
of 60 kcal/mole.

