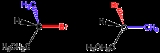
Enantiomer
Overview
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
, an enantiomer (icon ; from the Greek ἐνάντιος, opposite, and μέρος, part or portion) is one of two stereoisomers that are mirror image
Mirror image
A mirror image is a reflected duplication of an object that appears identical but reversed. As an optical effect it results from reflection off of substances such as a mirror or water. It is also a concept in geometry and can be used as a conceptualization process for 3-D structures...
s of each other that are non-superposable (not identical), much as one's left and right hands
Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
are the same except for opposite orientation. It can be clearly understood if you try to place your hands one over the other without touching the back or palm of the left to the same of the right.
Unanswered Questions

