Nitric oxide
Encyclopedia
Nitric oxide, also known as nitrogen monoxide, is a diatomic molecule
with chemical formula
N
O
. It is a free radical and is an important intermediate
in the chemical industry
. Nitric oxide is a by-product of combustion of substances in the air, as in automobile
engine
s, fossil fuel power plants, or lightning
.
In mammals including humans, NO is an important cellular signaling molecule
involved in many physiological and pathological processes. Low levels of NO production are important in protecting an organ such as the liver from ischemic damage. Chronic expression of NO is associated with various carcinomas and inflammatory conditions including juvenile diabetes, multiple sclerosis, arthritis and ulcerative colitis.
Nitric oxide should not be confused with nitrous oxide
(N2O), an anaesthetic
and greenhouse gas
, or with nitrogen dioxide
(NO2), a brown toxic gas and a major air pollutant. However, nitric oxide is rapidly oxidised in air to nitrogen dioxide. Humphrey Davy discovered this to his discomfort, when he inhaled the gas early in his career.
Despite being a simple molecule, NO is a fundamental component in the fields of neuroscience
, physiology
, and immunology
, and was proclaimed “Molecule of the Year” in 1992.
at 750 °C to 900 °C (normally at 850 °C) with platinum
as catalyst:
The uncatalyzed endothermic
reaction of O2
and N2
, which is performed at high temperature (>2000 °C) by lightning has not been developed into a practical commercial synthesis (see Birkeland–Eyde process):
In the laboratory, nitric oxide is conveniently generated by reduction of nitric acid
with copper
:
or by the reduction of nitrous acid in the form of sodium nitrite
or potassium nitrite
:
The iron(II) sulfate route is simple and has been used in undergraduate laboratory experiments.
So-called NONOate
compounds are also used for NO generation.
s to give complexes called metal nitrosyl
s. The most common bonding mode of NO is the terminal linear type (M-NO). The angle of the M-N-O group varies from 160° to 180° but is still termed "linear". In this case, the NO group is considered a 3-electron donor under the covalent (neutral) method of electron counting, or a 2-electron donor under the ionic method. In the case of a bent M-N-O conformation, the NO group can be considered a one-electron donor using neutral counting, or a 2-electron donor using ionic counting. One can view such complexes as derived from NO+, which is isoelectronic with CO.
Nitric oxide can serve as a one-electron pseudohalide. In such complexes, the M-N-O group is characterized by an angle between 120° and 140°.
The NO group can also bridge between metal centers through the nitrogen atom in a variety of geometries.
_(white)_in_the_cytoplasm_(green)_of_clusters_of_conifer_cells_one_hour_after_mechanical_agitation.jpg) Nitric oxide concentration can be determined using a simple chemiluminescent reaction involving ozone
Nitric oxide concentration can be determined using a simple chemiluminescent reaction involving ozone
: A sample containing nitric oxide is mixed with a large quantity of ozone. The nitric oxide reacts with the ozone to produce oxygen
and nitrogen dioxide
. This reaction also produces light
(chemiluminescence), which can be measured with a photodetector
. The amount of light produced is proportional to the amount of nitric oxide in the sample.
Other methods of testing include electroanalysis
(amperometric approach), where NO reacts with an electrode to induce a current or voltage change. The detection of NO radicals in biological tissues is particularly difficult due to the short lifetime and concentration of these radicals in tissues. One of the few practical methods is spin trapping
of nitric oxide with iron-dithiocarbamate
complexes and subsequent detection of the mono-nitrosyl-iron complex with electron paramagnetic resonance
(EPR).
A group of fluorescent dye indicators that are also available in acetyl
ated form for intracellular measurements exist. The most common compound is 4,5-diaminofluorescein (DAF-2).
, its synthesis from molecular nitrogen and oxygen requires elevated temperatures above 1000 °C. A major natural source is lightning
. The use of internal combustion engine
s has drastically increased the presence of nitric oxide in the environment. One purpose of catalytic converter
s in cars is to minimize NO emission by catalytic reversion to O2 and N2.
, which has been implicated in acid rain
. Furthermore, both NO and NO2 participate in ozone layer depletion. Nitric oxide is a small highly diffusible gas and a ubiquitous bioactive molecule.
for the synthesis of nitric acid
from ammonia
. In 2005, the US alone produced 6 million metric tons of nitric acid. It finds use in the semiconductor
industry for various processes. In one of its applications it is used along with nitrous oxide
to form oxynitride
gates in CMOS
devices.
with nitric oxide results in incorporation of nitrogen, which can be quantified by means of X-ray photoelectron spectroscopy
.
biological messenger
, playing a role in a variety of biological processes. Nitric oxide, known as the 'endothelium-derived relaxing factor
', or 'EDRF', is biosynthesized endogenously from L-arginine, oxygen
and NADPH by various nitric oxide synthase
(NOS) enzyme
s. Reduction of inorganic nitrate may also serve to make nitric oxide. The endothelium
(inner lining) of blood vessel
s uses nitric oxide to signal the surrounding smooth muscle
to relax, thus resulting in vasodilation
and increasing blood flow. Nitric oxide is highly reactive (having a lifetime of a few seconds), yet diffuses freely across membranes. These attributes make nitric oxide ideal for a transient paracrine (between adjacent cells) and autocrine (within a single cell) signaling molecule.
The production of nitric oxide is elevated in populations living at high altitudes, which helps these people avoid hypoxia
by aiding in pulmonary vasculature vasodilation
. Effects include vasodilatation, neurotransmission
(see gasotransmitters
), modulation of the hair cycle
, production of reactive nitrogen intermediates and penile erections
(through its ability to vasodilate
). Nitroglycerin and amyl nitrite
serve as vasodilators because they are converted to nitric oxide in the body. The vasodialating antihypertensive drug minoxidil
contains an NO moity and may act as an NO agonist. Similarly, Sildenafil citrate
, popularly known by the trade name Viagra, stimulates erections primarily by enhancing signaling through the nitric oxide pathway in the penis.
Nitric oxide (NO) contributes to vessel homeostasis by inhibiting vascular smooth muscle contraction and growth, platelet aggregation, and leukocyte adhesion to
the endothelium. Humans with atherosclerosis
, diabetes, or hypertension
often show impaired NO pathways. A high salt intake was demonstrated to attenuate NO production, although bioavailability remains unregulated.
Nitric oxide is also generated by phagocytes (monocyte
s, macrophage
s, and neutrophils) as part of the human immune response. Phagocytes are armed with inducible nitric oxide synthase (iNOS), which is activated by interferon-gamma
(IFN-γ) as a single signal or by tumor necrosis factor
(TNF) along with a second signal. On the other hand, transforming growth factor-beta (TGF-β) provides a strong inhibitory signal to iNOS, whereas interleukin
-4 (IL-4) and IL-10 provide weak inhibitory signals. In this way the immune system may regulate the armamentarium of phagocytes that play a role in inflammation and immune responses. Nitric oxide secreted as an immune response is as free radicals and is toxic to bacteria; the mechanism for this includes DNA damage and degradation of iron sulfur centers into iron ions and iron-nitrosyl
compounds. In response, however, many bacterial pathogens have evolved mechanisms for nitric oxide resistance. Because nitric oxide might serve as an inflammometer in conditions like asthma
, there has been increasing interest in the use of exhaled nitric oxide
as a breath test
in diseases with airway
inflammation. Reduced levels of exhaled NO have been associated with exposure to air pollution.
Nitric oxide can contribute to reperfusion injury
when an excessive amount produced during reperfusion (following a period of ischemia
) reacts with superoxide
to produce the damaging oxidant peroxynitrite
. In contrast, inhaled nitric oxide has been shown to help survival and recovery from paraquat
poisoning, which produces lung tissue–damaging superoxide and hinders NOS metabolism.
In plants, nitric oxide can be produced by any of four routes: (i) L-arginine-dependent nitric oxide synthase, (although the existence of animal NOS homologs in plants is debated), (ii) by plasma membrane-bound nitrate reductase
, (iii) by mitochondrial electron transport chain, or (iv) by non-enzymatic reactions. It is a signaling molecule, acts mainly against oxidative stress and also plays a role in plant pathogen interactions. Treating cut flowers and other plants with nitric oxide has been shown to lengthen the time before wilting.
An important biological reaction of nitric oxide is S-nitrosylation
, the conversion of thiol
groups, including cysteine
residues in proteins, to form S-nitrosothiols (RSNOs). S-Nitrosylation
is a mechanism for dynamic, post-translational regulation of most or all major classes of protein.
and aconitase
, activation of the soluble guanylate cyclase
, ADP ribosylation of proteins, protein sulfhydryl group nitrosylation
, and iron regulatory factor activation. NO has been demonstrated to activate NF-κB in peripheral blood mononuclear cells, an important transcription factor in iNOS gene expression in response to inflammation. It was found that NO acts through the stimulation of the soluble guanylate cyclase, which is a heterodimeric enzyme with subsequent formation of cyclic GMP. Cyclic GMP activates protein kinase G, which causes phosphorylation of myosin light chain phosphatase, and therefore inactivation of myosin light-chain kinase
, and leads ultimately to the dephosphorylation of the myosin light chain, causing smooth muscle relaxation.
in neonatal patients post-meconium aspiration and related to birth defects. These are often a last-resort gas mixture before the use of extracorporeal membrane oxygenation
(ECMO). Nitric oxide therapy has the potential to significantly increase the quality of life and, in some cases, save the lives of infants at risk for pulmonary vascular disease.
in patients with acute right ventricular failure secondary to pulmonary embolism
.
anginal drug: it causes vasodilation
, which can help with ischemic pain, known as angina, by decreasing the cardiac workload. By dilating (expanding) the veins, nitric oxide drugs lower arterial pressure and left ventricular filling pressure. This vasodilation does not decrease the volume of blood the heart pumps, but rather it decreases the force the heart muscle must exert to pump the same volume of blood. Nitroglycerin pills, taken sublingually (under the tongue), are used to prevent or treat acute chest pain. The nitroglycerin reacts with a sulfhydryl
group (–SH) to produce nitric oxide, which eases the pain by causing vasodilation. Recent evidence suggests that nitrates may be beneficial for treatment of angina due to reduced myocardial oxygen consumption both by decreasing preload and afterload and by some direct vasodilation of coronary vessels.
Mainly for its utility as a vasodilator, NO has entered the fitness industry as an array of supplements geared towards accelerated muscle growth and prolonged stamina during endurance activities.
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
with chemical formula
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
N
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
O
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
. It is a free radical and is an important intermediate
Reaction intermediate
A reaction intermediate or an intermediate is a molecular entity that is formed from the reactants and reacts further to give the directly observed products of a chemical reaction. Most chemical reactions are stepwise, that is they take more than one elementary step to complete...
in the chemical industry
Chemical industry
The chemical industry comprises the companies that produce industrial chemicals. Central to the modern world economy, it converts raw materials into more than 70,000 different products.-Products:...
. Nitric oxide is a by-product of combustion of substances in the air, as in automobile
Automobile
An automobile, autocar, motor car or car is a wheeled motor vehicle used for transporting passengers, which also carries its own engine or motor...
engine
Engine
An engine or motor is a machine designed to convert energy into useful mechanical motion. Heat engines, including internal combustion engines and external combustion engines burn a fuel to create heat which is then used to create motion...
s, fossil fuel power plants, or lightning
Lightning
Lightning is an atmospheric electrostatic discharge accompanied by thunder, which typically occurs during thunderstorms, and sometimes during volcanic eruptions or dust storms...
.
In mammals including humans, NO is an important cellular signaling molecule
Signaling molecule
A signaling molecule is a chemical involved in transmitting information between cells. Such molecules are released from the cell sending the signal, cross over the gap between cells by diffusion, and interact with specific receptors in another cell, triggering a response in that cell by activating...
involved in many physiological and pathological processes. Low levels of NO production are important in protecting an organ such as the liver from ischemic damage. Chronic expression of NO is associated with various carcinomas and inflammatory conditions including juvenile diabetes, multiple sclerosis, arthritis and ulcerative colitis.
Nitric oxide should not be confused with nitrous oxide
Nitrous oxide
Nitrous oxide, commonly known as laughing gas or sweet air, is a chemical compound with the formula . It is an oxide of nitrogen. At room temperature, it is a colorless non-flammable gas, with a slightly sweet odor and taste. It is used in surgery and dentistry for its anesthetic and analgesic...
(N2O), an anaesthetic
General anaesthetic
A general anaesthetic is a drug that brings about a reversible loss of consciousness. These drugs are generally administered by an anaesthesia provider to induce or maintain general anaesthesia to facilitate surgery...
and greenhouse gas
Greenhouse gas
A greenhouse gas is a gas in an atmosphere that absorbs and emits radiation within the thermal infrared range. This process is the fundamental cause of the greenhouse effect. The primary greenhouse gases in the Earth's atmosphere are water vapor, carbon dioxide, methane, nitrous oxide, and ozone...
, or with nitrogen dioxide
Nitrogen dioxide
Nitrogen dioxide is the chemical compound with the formula it is one of several nitrogen oxides. is an intermediate in the industrial synthesis of nitric acid, millions of tons of which are produced each year. This reddish-brown toxic gas has a characteristic sharp, biting odor and is a prominent...
(NO2), a brown toxic gas and a major air pollutant. However, nitric oxide is rapidly oxidised in air to nitrogen dioxide. Humphrey Davy discovered this to his discomfort, when he inhaled the gas early in his career.
Despite being a simple molecule, NO is a fundamental component in the fields of neuroscience
Neuroscience
Neuroscience is the scientific study of the nervous system. Traditionally, neuroscience has been seen as a branch of biology. However, it is currently an interdisciplinary science that collaborates with other fields such as chemistry, computer science, engineering, linguistics, mathematics,...
, physiology
Physiology
Physiology is the science of the function of living systems. This includes how organisms, organ systems, organs, cells, and bio-molecules carry out the chemical or physical functions that exist in a living system. The highest honor awarded in physiology is the Nobel Prize in Physiology or...
, and immunology
Immunology
Immunology is a broad branch of biomedical science that covers the study of all aspects of the immune system in all organisms. It deals with the physiological functioning of the immune system in states of both health and diseases; malfunctions of the immune system in immunological disorders ; the...
, and was proclaimed “Molecule of the Year” in 1992.
Reactions
- When exposed to oxygenOxygenOxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
, NO is converted into nitrogen dioxideNitrogen dioxideNitrogen dioxide is the chemical compound with the formula it is one of several nitrogen oxides. is an intermediate in the industrial synthesis of nitric acid, millions of tons of which are produced each year. This reddish-brown toxic gas has a characteristic sharp, biting odor and is a prominent...
.
-
- 2 NO + O2 → 2 NO2
- This conversion has been speculated as occurring via the ONOONO intermediate. In water, NO reacts with oxygen and water to form HNO2 or nitrous acidNitrous acidNitrous acid is a weak and monobasic acid known only in solution and in the form of nitrite salts.Nitrous acid is used to make diazides from amines; this occurs by nucleophilic attack of the amine onto the nitrite, reprotonation by the surrounding solvent, and double-elimination of water...
. The reaction is thought to proceed via the following stoichiometryStoichiometryStoichiometry is a branch of chemistry that deals with the relative quantities of reactants and products in chemical reactions. In a balanced chemical reaction, the relations among quantities of reactants and products typically form a ratio of whole numbers...
:- 4 NO + O2 + 2 H2O → 4 HNO2
- NO will react with fluorineFluorineFluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
, chlorineChlorineChlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
, and bromineBromineBromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
to form the XNO species, known as the nitrosyl halides, such as nitrosyl chlorideNitrosyl chlorideNitrosyl chloride is the chemical compound NOCl. It is a yellow gas that is most commonly encountered as a decomposition product of aqua regia, a mixture of hydrochloric acid and nitric acid...
. Nitrosyl iodide can form but is an extremely short-lived species and tends to reform I2.
-
- 2 NO + Cl2 → 2 NOCl
- NitroxylNitroxylNitroxyl is the chemical compound HNO. It is well known in the gas phase . In aqueous solution it acts as an acid with the conjugate base NO−, . NO− is the reduced form of nitric oxide and is isoelectronic with dioxygen...
(HNO) is the reduced form of nitric oxide.
- Nitroxyl
- 2 NO + Cl2 → 2 NOCl
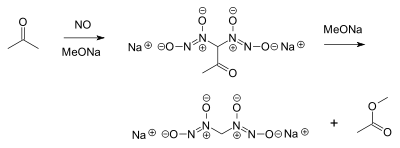
- Nitric oxide reacts with acetoneAcetoneAcetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
and an alkoxideAlkoxideAn alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They can be written as RO−, where R is the organic substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands...
to a diazeniumdiolate or nitrosohydroxylamine and methyl acetateMethyl acetateMethyl acetate, also known as MeOAc, acetic acid methyl ester or methyl ethanoate, is a carboxylate ester with the formula CH3COOCH3. It is normally a flammable liquid with a characteristic, pleasant smell like certain glues or nail polish removers. Methyl acetate has characteristics very similar...
:
- This is a very old reaction (1898) but of interest today in NO prodrugProdrugA prodrug is a pharmacological substance administered in an inactive form. Once administered, the prodrug is metabolised in vivo into an active metabolite, a process termed bioactivation. The rationale behind the use of a prodrug is generally for absorption, distribution, metabolism, and...
research. Nitric oxide can also react directly with sodium methoxide, forming sodium formateSodium formateSodium formate, HCOONa, is the sodium salt of formic acid, HCOOH. It usually appears as a white deliquescent powder.-Uses:Sodium formate is used in several fabric dyeing and printing processes...
and nitrous oxideNitrous oxideNitrous oxide, commonly known as laughing gas or sweet air, is a chemical compound with the formula . It is an oxide of nitrogen. At room temperature, it is a colorless non-flammable gas, with a slightly sweet odor and taste. It is used in surgery and dentistry for its anesthetic and analgesic...
.
Preparation
Commercially, NO is produced by the oxidation of ammoniaAmmonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
at 750 °C to 900 °C (normally at 850 °C) with platinum
Platinum
Platinum is a chemical element with the chemical symbol Pt and an atomic number of 78. Its name is derived from the Spanish term platina del Pinto, which is literally translated into "little silver of the Pinto River." It is a dense, malleable, ductile, precious, gray-white transition metal...
as catalyst:
- 4 NH3 + 5 O2 → 4 NO + 6 H2O
The uncatalyzed endothermic
Endothermic
In thermodynamics, the word endothermic describes a process or reaction in which the system absorbs energy from the surroundings in the form of heat. Its etymology stems from the prefix endo- and the Greek word thermasi,...
reaction of O2
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
and N2
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
, which is performed at high temperature (>2000 °C) by lightning has not been developed into a practical commercial synthesis (see Birkeland–Eyde process):
- N2 + O2 → 2 NO
In the laboratory, nitric oxide is conveniently generated by reduction of nitric acid
Nitric acid
Nitric acid , also known as aqua fortis and spirit of nitre, is a highly corrosive and toxic strong acid.Colorless when pure, older samples tend to acquire a yellow cast due to the accumulation of oxides of nitrogen. If the solution contains more than 86% nitric acid, it is referred to as fuming...
with copper
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
:
- 8 HNO3 + 3 Cu → 3 Cu(NO3)2 + 4 H2O + 2 NO
or by the reduction of nitrous acid in the form of sodium nitrite
Sodium nitrite
Sodium nitrite is the inorganic compound with the chemical formula NaNO2. It is a white to slight yellowish crystalline powder that is very soluble in water and is hygroscopic...
or potassium nitrite
Potassium nitrite
Potassium nitrite is a salt with chemical formula 2.It is a strong oxidizer and may accelerate the combustion of other materials. Like other nitrite salts such as sodium nitrite, potassium nitrite is toxic if swallowed, and laboratory tests suggest that it may be mutagenic or teratogenic...
:
- 2 NaNO2 + 2 NaI + 2 H2SO4 → I2 + 4 NaHSO4 + 2 NO
- 2 NaNO2 + 2 FeSO4 + 3 H2SO4 → Fe2(SO4)3 + 2 NaHSO4 + 2 H2O + 2 NO
- 3 KNO2 (l) + KNO3 (l) + Cr2O3(s) → 2 K2CrO4(s) + 4 NO (g)
The iron(II) sulfate route is simple and has been used in undergraduate laboratory experiments.
So-called NONOate
NONOate
In chemistry, a NONOate is a compound having the chemical formula R1R3N--N=O, where R1 and R2 are alkyl groups. One example for this is 1,1-diethyl-2-hydroxy-2-nitrosohydrazine, or diethylamine dinitric oxide. These compounds are unusual in having three sequential nitrogen atoms: an amine...
compounds are also used for NO generation.
Coordination chemistry
NO reacts with all transition metalTransition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
s to give complexes called metal nitrosyl
Metal nitrosyl
Metal nitrosyl complexes are complexes that contain nitric oxide, NO, bonded to a transition metal. Many kinds of nitrosyl complexes are known, which vary both in structure and coligand.-Bonding and structure:...
s. The most common bonding mode of NO is the terminal linear type (M-NO). The angle of the M-N-O group varies from 160° to 180° but is still termed "linear". In this case, the NO group is considered a 3-electron donor under the covalent (neutral) method of electron counting, or a 2-electron donor under the ionic method. In the case of a bent M-N-O conformation, the NO group can be considered a one-electron donor using neutral counting, or a 2-electron donor using ionic counting. One can view such complexes as derived from NO+, which is isoelectronic with CO.
Nitric oxide can serve as a one-electron pseudohalide. In such complexes, the M-N-O group is characterized by an angle between 120° and 140°.
The NO group can also bridge between metal centers through the nitrogen atom in a variety of geometries.
Measurement of nitric oxide concentration
_(white)_in_the_cytoplasm_(green)_of_clusters_of_conifer_cells_one_hour_after_mechanical_agitation.jpg)
Ozone
Ozone , or trioxygen, is a triatomic molecule, consisting of three oxygen atoms. It is an allotrope of oxygen that is much less stable than the diatomic allotrope...
: A sample containing nitric oxide is mixed with a large quantity of ozone. The nitric oxide reacts with the ozone to produce oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
and nitrogen dioxide
Nitrogen dioxide
Nitrogen dioxide is the chemical compound with the formula it is one of several nitrogen oxides. is an intermediate in the industrial synthesis of nitric acid, millions of tons of which are produced each year. This reddish-brown toxic gas has a characteristic sharp, biting odor and is a prominent...
. This reaction also produces light
Light
Light or visible light is electromagnetic radiation that is visible to the human eye, and is responsible for the sense of sight. Visible light has wavelength in a range from about 380 nanometres to about 740 nm, with a frequency range of about 405 THz to 790 THz...
(chemiluminescence), which can be measured with a photodetector
Photodetector
Photosensors or photodetectors are sensors of light or other electromagnetic energy. There are several varieties:*Active pixel sensors are image sensors consisting of an integrated circuit that contains an array of pixel sensors, each pixel containing a both a light sensor and an active amplifier...
. The amount of light produced is proportional to the amount of nitric oxide in the sample.
- NO + O3 → NO2 + O2 + light
Other methods of testing include electroanalysis
Electrochemistry
Electrochemistry is a branch of chemistry that studies chemical reactions which take place in a solution at the interface of an electron conductor and an ionic conductor , and which involve electron transfer between the electrode and the electrolyte or species in solution.If a chemical reaction is...
(amperometric approach), where NO reacts with an electrode to induce a current or voltage change. The detection of NO radicals in biological tissues is particularly difficult due to the short lifetime and concentration of these radicals in tissues. One of the few practical methods is spin trapping
Spin trapping
Spin trapping in chemistry is an analytical technique employed in the detection and identification of short-lived free radicals. Spin trapping involves the addition of radical to a nitrone spin trap resulting in the formation of a spin adduct, a nitroxide-based persistent radical, that can be...
of nitric oxide with iron-dithiocarbamate
Dithiocarbamate
A dithiocarbamate is a functional group in organic chemistry. It is the analog of a carbamate in which both oxygen atoms are replaced by sulfur atoms. Sodium diethyldithiocarbamate is a common ligand in inorganic chemistry....
complexes and subsequent detection of the mono-nitrosyl-iron complex with electron paramagnetic resonance
Electron paramagnetic resonance
Electron paramagnetic resonance or electron spin resonance spectroscopyis a technique for studying chemical species that have one or more unpaired electrons, such as organic and inorganic free radicals or inorganic complexes possessing a transition metal ion...
(EPR).
A group of fluorescent dye indicators that are also available in acetyl
Acetyl
In organic chemistry, acetyl is a functional group, the acyl with chemical formula COCH3. It is sometimes represented by the symbol Ac . The acetyl group contains a methyl group single-bonded to a carbonyl...
ated form for intracellular measurements exist. The most common compound is 4,5-diaminofluorescein (DAF-2).
Production
From a thermodynamic perspective, NO is unstable with respect to O2 and N2, although this conversion is very slow at ambient temperatures in the absence of a catalyst. Because the heat of formation of NO is endothermicEndothermic
In thermodynamics, the word endothermic describes a process or reaction in which the system absorbs energy from the surroundings in the form of heat. Its etymology stems from the prefix endo- and the Greek word thermasi,...
, its synthesis from molecular nitrogen and oxygen requires elevated temperatures above 1000 °C. A major natural source is lightning
Lightning
Lightning is an atmospheric electrostatic discharge accompanied by thunder, which typically occurs during thunderstorms, and sometimes during volcanic eruptions or dust storms...
. The use of internal combustion engine
Internal combustion engine
The internal combustion engine is an engine in which the combustion of a fuel occurs with an oxidizer in a combustion chamber. In an internal combustion engine, the expansion of the high-temperature and high -pressure gases produced by combustion apply direct force to some component of the engine...
s has drastically increased the presence of nitric oxide in the environment. One purpose of catalytic converter
Catalytic converter
A catalytic converter is a device used to convert toxic exhaust emissions from an internal combustion engine into non-toxic substances. Inside a catalytic converter, a catalyst stimulates a chemical reaction in which noxious byproducts of combustion are converted to less toxic substances by dint...
s in cars is to minimize NO emission by catalytic reversion to O2 and N2.
Environmental effects
Nitric oxide in the air may convert to nitric acidNitric acid
Nitric acid , also known as aqua fortis and spirit of nitre, is a highly corrosive and toxic strong acid.Colorless when pure, older samples tend to acquire a yellow cast due to the accumulation of oxides of nitrogen. If the solution contains more than 86% nitric acid, it is referred to as fuming...
, which has been implicated in acid rain
Acid rain
Acid rain is a rain or any other form of precipitation that is unusually acidic, meaning that it possesses elevated levels of hydrogen ions . It can have harmful effects on plants, aquatic animals, and infrastructure. Acid rain is caused by emissions of carbon dioxide, sulfur dioxide and nitrogen...
. Furthermore, both NO and NO2 participate in ozone layer depletion. Nitric oxide is a small highly diffusible gas and a ubiquitous bioactive molecule.
Technical applications
Although NO has relatively few direct uses, it is produced on a massive scale as an intermediate in the Ostwald processOstwald process
The Ostwald process is a chemical process for producing nitric acid, which was developed by Wilhelm Ostwald . It is a mainstay of the modern chemical industry. Historically and practically it is closely associated with the Haber process, which provides the requisite raw material,...
for the synthesis of nitric acid
Nitric acid
Nitric acid , also known as aqua fortis and spirit of nitre, is a highly corrosive and toxic strong acid.Colorless when pure, older samples tend to acquire a yellow cast due to the accumulation of oxides of nitrogen. If the solution contains more than 86% nitric acid, it is referred to as fuming...
from ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
. In 2005, the US alone produced 6 million metric tons of nitric acid. It finds use in the semiconductor
Semiconductor
A semiconductor is a material with electrical conductivity due to electron flow intermediate in magnitude between that of a conductor and an insulator. This means a conductivity roughly in the range of 103 to 10−8 siemens per centimeter...
industry for various processes. In one of its applications it is used along with nitrous oxide
Nitrous oxide
Nitrous oxide, commonly known as laughing gas or sweet air, is a chemical compound with the formula . It is an oxide of nitrogen. At room temperature, it is a colorless non-flammable gas, with a slightly sweet odor and taste. It is used in surgery and dentistry for its anesthetic and analgesic...
to form oxynitride
Silicon oxynitride
Silicon oxynitride is a ceramic material with the chemical formula SiOxNy. While in amorphous forms its composition can continuously vary between SiO2 and Si3N4 , the only known intermediate crystalline phase is Si2N2O...
gates in CMOS
CMOS
Complementary metal–oxide–semiconductor is a technology for constructing integrated circuits. CMOS technology is used in microprocessors, microcontrollers, static RAM, and other digital logic circuits...
devices.
Miscellaneous applications
Nitric oxide can be used for detecting surface radicals on polymers. Quenching of surface radicalsRadical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
with nitric oxide results in incorporation of nitrogen, which can be quantified by means of X-ray photoelectron spectroscopy
X-ray photoelectron spectroscopy
X-ray photoelectron spectroscopy is a quantitative spectroscopic technique that measures the elemental composition, empirical formula, chemical state and electronic state of the elements that exist within a material...
.
Biological functions
NO is one of the few gaseous signaling molecules known and is additionally exceptional due to the fact that it is a radical gas. It is a key vertebrateVertebrate
Vertebrates are animals that are members of the subphylum Vertebrata . Vertebrates are the largest group of chordates, with currently about 58,000 species described. Vertebrates include the jawless fishes, bony fishes, sharks and rays, amphibians, reptiles, mammals, and birds...
biological messenger
Signal transduction
Signal transduction occurs when an extracellular signaling molecule activates a cell surface receptor. In turn, this receptor alters intracellular molecules creating a response...
, playing a role in a variety of biological processes. Nitric oxide, known as the 'endothelium-derived relaxing factor
Endothelium-derived relaxing factor
Endothelium-derived relaxing factor is produced and released by the endothelium to promote smooth muscle relaxation. The best-characterized is nitric oxide . Some sources equate EDRF and nitric oxide....
', or 'EDRF', is biosynthesized endogenously from L-arginine, oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
and NADPH by various nitric oxide synthase
Nitric oxide synthase
Nitric oxide synthases are a family of enzymes that catalyze the production of nitric oxide from L-arginine. NO is an important cellular signaling molecule, having a vital role in many biological processes...
(NOS) enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
s. Reduction of inorganic nitrate may also serve to make nitric oxide. The endothelium
Endothelium
The endothelium is the thin layer of cells that lines the interior surface of blood vessels, forming an interface between circulating blood in the lumen and the rest of the vessel wall. These cells are called endothelial cells. Endothelial cells line the entire circulatory system, from the heart...
(inner lining) of blood vessel
Blood vessel
The blood vessels are the part of the circulatory system that transports blood throughout the body. There are three major types of blood vessels: the arteries, which carry the blood away from the heart; the capillaries, which enable the actual exchange of water and chemicals between the blood and...
s uses nitric oxide to signal the surrounding smooth muscle
Smooth muscle
Smooth muscle is an involuntary non-striated muscle. It is divided into two sub-groups; the single-unit and multiunit smooth muscle. Within single-unit smooth muscle tissues, the autonomic nervous system innervates a single cell within a sheet or bundle and the action potential is propagated by...
to relax, thus resulting in vasodilation
Vasodilation
Vasodilation refers to the widening of blood vessels resulting from relaxation of smooth muscle cells within the vessel walls, particularly in the large arteries, smaller arterioles and large veins. The process is essentially the opposite of vasoconstriction, or the narrowing of blood vessels. When...
and increasing blood flow. Nitric oxide is highly reactive (having a lifetime of a few seconds), yet diffuses freely across membranes. These attributes make nitric oxide ideal for a transient paracrine (between adjacent cells) and autocrine (within a single cell) signaling molecule.
The production of nitric oxide is elevated in populations living at high altitudes, which helps these people avoid hypoxia
Hypoxia (medical)
Hypoxia, or hypoxiation, is a pathological condition in which the body as a whole or a region of the body is deprived of adequate oxygen supply. Variations in arterial oxygen concentrations can be part of the normal physiology, for example, during strenuous physical exercise...
by aiding in pulmonary vasculature vasodilation
Vasodilation
Vasodilation refers to the widening of blood vessels resulting from relaxation of smooth muscle cells within the vessel walls, particularly in the large arteries, smaller arterioles and large veins. The process is essentially the opposite of vasoconstriction, or the narrowing of blood vessels. When...
. Effects include vasodilatation, neurotransmission
Neurotransmitter
Neurotransmitters are endogenous chemicals that transmit signals from a neuron to a target cell across a synapse. Neurotransmitters are packaged into synaptic vesicles clustered beneath the membrane on the presynaptic side of a synapse, and are released into the synaptic cleft, where they bind to...
(see gasotransmitters
Gasotransmitters
Gasotransmitters are gaseous molecules synthesized in the body. They include nitric oxide, hydrogen sulfide, carbon monoxide, and possibly nitrous oxide.-Overview:...
), modulation of the hair cycle
Hair
Hair is a filamentous biomaterial, that grows from follicles found in the dermis. Found exclusively in mammals, hair is one of the defining characteristics of the mammalian class....
, production of reactive nitrogen intermediates and penile erections
Erection
Penile erection is a physiological phenomenon where the penis becomes enlarged and firm. Penile erection is the result of a complex interaction of psychological, neural, vascular and endocrine factors, and is usually, though not exclusively, associated with sexual arousal...
(through its ability to vasodilate
Vascular resistance
Vascular resistance is a term used to define the resistance to flow that must be overcome to push blood through the circulatory system. The resistance offered by the peripheral circulation is known as the systemic vascular resistance , while the resistance offered by the vasculature of the lungs...
). Nitroglycerin and amyl nitrite
Amyl nitrite
Amyl nitrite is the chemical compound with the formula C5H11ONO. A variety of isomers are known, but they all feature an amyl group attached to the nitrito functional group. The alkyl group is unreactive and the chemical and biological properties are mainly due to the nitrite group...
serve as vasodilators because they are converted to nitric oxide in the body. The vasodialating antihypertensive drug minoxidil
Minoxidil
Minoxidil is an antihypertensive vasodilator medication which also slows or stops hair loss and promotes hair regrowth. Now off-patent, it is available over-the-counter for the treatment of androgenic alopecia. Minoxidil must be used indefinitely for continued support of existing hair follicles and...
contains an NO moity and may act as an NO agonist. Similarly, Sildenafil citrate
Sildenafil
Sildenafil citrate, sold as Viagra, Revatio and under various other trade names, is a drug used to treat erectile dysfunction and pulmonary arterial hypertension . It was originally developed by British scientists and then brought to market by the US-based pharmaceutical company Pfizer...
, popularly known by the trade name Viagra, stimulates erections primarily by enhancing signaling through the nitric oxide pathway in the penis.
Nitric oxide (NO) contributes to vessel homeostasis by inhibiting vascular smooth muscle contraction and growth, platelet aggregation, and leukocyte adhesion to
the endothelium. Humans with atherosclerosis
Atherosclerosis
Atherosclerosis is a condition in which an artery wall thickens as a result of the accumulation of fatty materials such as cholesterol...
, diabetes, or hypertension
Hypertension
Hypertension or high blood pressure is a cardiac chronic medical condition in which the systemic arterial blood pressure is elevated. What that means is that the heart is having to work harder than it should to pump the blood around the body. Blood pressure involves two measurements, systolic and...
often show impaired NO pathways. A high salt intake was demonstrated to attenuate NO production, although bioavailability remains unregulated.
Nitric oxide is also generated by phagocytes (monocyte
Monocyte
Monocytes are a type of white blood cell and are part of the innate immune system of vertebrates including all mammals , birds, reptiles, and fish. Monocytes play multiple roles in immune function...
s, macrophage
Macrophage
Macrophages are cells produced by the differentiation of monocytes in tissues. Human macrophages are about in diameter. Monocytes and macrophages are phagocytes. Macrophages function in both non-specific defense as well as help initiate specific defense mechanisms of vertebrate animals...
s, and neutrophils) as part of the human immune response. Phagocytes are armed with inducible nitric oxide synthase (iNOS), which is activated by interferon-gamma
Interferon-gamma
Interferon-gamma is a dimerized soluble cytokine that is the only member of the type II class of interferons. This interferon was originally called macrophage-activating factor, a term now used to describe a larger family of proteins to which IFN-γ belongs...
(IFN-γ) as a single signal or by tumor necrosis factor
Tumor necrosis factor
Tumor necrosis factor is a cytokine involved in systemic inflammation and is a member of a group of cytokines that stimulate the acute phase reaction...
(TNF) along with a second signal. On the other hand, transforming growth factor-beta (TGF-β) provides a strong inhibitory signal to iNOS, whereas interleukin
Interleukin
Interleukins are a group of cytokines that were first seen to be expressed by white blood cells . The term interleukin derives from "as a means of communication", and "deriving from the fact that many of these proteins are produced by leukocytes and act on leukocytes"...
-4 (IL-4) and IL-10 provide weak inhibitory signals. In this way the immune system may regulate the armamentarium of phagocytes that play a role in inflammation and immune responses. Nitric oxide secreted as an immune response is as free radicals and is toxic to bacteria; the mechanism for this includes DNA damage and degradation of iron sulfur centers into iron ions and iron-nitrosyl
Metal nitrosyl
Metal nitrosyl complexes are complexes that contain nitric oxide, NO, bonded to a transition metal. Many kinds of nitrosyl complexes are known, which vary both in structure and coligand.-Bonding and structure:...
compounds. In response, however, many bacterial pathogens have evolved mechanisms for nitric oxide resistance. Because nitric oxide might serve as an inflammometer in conditions like asthma
Asthma
Asthma is the common chronic inflammatory disease of the airways characterized by variable and recurring symptoms, reversible airflow obstruction, and bronchospasm. Symptoms include wheezing, coughing, chest tightness, and shortness of breath...
, there has been increasing interest in the use of exhaled nitric oxide
Exhaled nitric oxide
In medicine, exhaled nitric oxide can be measured in a breath test for asthma or other conditions characterized by airway inflammation. Nitric oxide is a gaseous molecule produced by certain cell types in an inflammatory response. The fraction of exhaled NO is a promising biomarker for the...
as a breath test
Breath test
A breath test is a type of test performed on air generated from the act of exhalation.Types include:*Breathalyzer - By far the most common usage of this term relates to the legal breath test to determine if a person is driving under the influence of alcohol.*Hydrogen breath test - it is becoming...
in diseases with airway
Airway
The pulmonary airway comprises those parts of the respiratory system through which air flows, conceptually beginning at the nose and mouth, and terminating in the alveoli...
inflammation. Reduced levels of exhaled NO have been associated with exposure to air pollution.
Nitric oxide can contribute to reperfusion injury
Reperfusion injury
Reperfusion injury is the tissue damage caused when blood supply returns to the tissue after a period of ischemia or lack of oxygen. The absence of oxygen and nutrients from blood during the ischemic period creates a condition in which the restoration of circulation results in inflammation and...
when an excessive amount produced during reperfusion (following a period of ischemia
Ischemia
In medicine, ischemia is a restriction in blood supply, generally due to factors in the blood vessels, with resultant damage or dysfunction of tissue. It may also be spelled ischaemia or ischæmia...
) reacts with superoxide
Superoxide
A superoxide, also known by the obsolete name hyperoxide, is a compound that possesses the superoxide anion with the chemical formula O2−. The systematic name of the anion is dioxide. It is important as the product of the one-electron reduction of dioxygen O2, which occurs widely in nature...
to produce the damaging oxidant peroxynitrite
Peroxynitrite
Peroxynitrite is the anion with the formula ONOO−. It is an unstable structural isomer of nitrate, NO3−, which has the same formula but a different structure. Although peroxynitrous acid is highly reactive, its conjugate base peroxynitrite is stable in basic solution...
. In contrast, inhaled nitric oxide has been shown to help survival and recovery from paraquat
Paraquat
Paraquat is the trade name for N,N′-dimethyl-4,4′-bipyridinium dichloride, one of the most widely used herbicides in the world. Paraquat, a viologen, is quick-acting and non-selective, killing green plant tissue on contact. It is also toxic to human beings and animals...
poisoning, which produces lung tissue–damaging superoxide and hinders NOS metabolism.
In plants, nitric oxide can be produced by any of four routes: (i) L-arginine-dependent nitric oxide synthase, (although the existence of animal NOS homologs in plants is debated), (ii) by plasma membrane-bound nitrate reductase
Nitrate reductase
Nitrate reductases are molybdoenzymes that reduce nitrate to nitrite .* Eukaryotic nitrate reductases are part of the sulfite oxidase family of molybdoenzymes....
, (iii) by mitochondrial electron transport chain, or (iv) by non-enzymatic reactions. It is a signaling molecule, acts mainly against oxidative stress and also plays a role in plant pathogen interactions. Treating cut flowers and other plants with nitric oxide has been shown to lengthen the time before wilting.
An important biological reaction of nitric oxide is S-nitrosylation
Nitrosylation
Nitrosylation is a protein modification in which a nitrosyl group is post-translationally added to a protein.There is a range of enzymes that produce nitric oxide, and the frequent consequence of this production is nitrosylation....
, the conversion of thiol
Thiol
In organic chemistry, a thiol is an organosulfur compound that contains a carbon-bonded sulfhydryl group...
groups, including cysteine
Cysteine
Cysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid...
residues in proteins, to form S-nitrosothiols (RSNOs). S-Nitrosylation
Nitrosylation
Nitrosylation is a protein modification in which a nitrosyl group is post-translationally added to a protein.There is a range of enzymes that produce nitric oxide, and the frequent consequence of this production is nitrosylation....
is a mechanism for dynamic, post-translational regulation of most or all major classes of protein.
Mechanism of action
There are several mechanisms by which NO has been demonstrated to affect the biology of living cells. These include oxidation of iron-containing proteins such as ribonucleotide reductaseRibonucleotide reductase
Ribonucleotide reductase is an enzyme that catalyzes the formation of deoxyribonucleotides from ribonucleotides. Deoxyribonucleotides in turn are used in the synthesis of DNA. The reaction catalyzed by RNR is strictly conserved in all living organisms...
and aconitase
Aconitase
Aconitase is an enzyme that catalyses the stereo-specific isomerization of citrate to isocitrate via cis-aconitate in the tricarboxylic acid cycle, a non-redox-active process.- Function :...
, activation of the soluble guanylate cyclase
Guanylate cyclase
-Reaction:Guanylate cyclase catalyzes the reaction of guanosine triphosphate to 3',5'-cyclic guanosine monophosphate and pyrophosphate:-Types:...
, ADP ribosylation of proteins, protein sulfhydryl group nitrosylation
Nitrosylation
Nitrosylation is a protein modification in which a nitrosyl group is post-translationally added to a protein.There is a range of enzymes that produce nitric oxide, and the frequent consequence of this production is nitrosylation....
, and iron regulatory factor activation. NO has been demonstrated to activate NF-κB in peripheral blood mononuclear cells, an important transcription factor in iNOS gene expression in response to inflammation. It was found that NO acts through the stimulation of the soluble guanylate cyclase, which is a heterodimeric enzyme with subsequent formation of cyclic GMP. Cyclic GMP activates protein kinase G, which causes phosphorylation of myosin light chain phosphatase, and therefore inactivation of myosin light-chain kinase
Myosin light-chain kinase
Myosin light-chain kinase also known as MYLK or MLCK is a serine/threonine-specific protein kinase that phosphorylates the regulatory light chain of myosin II.- Isoforms :Four different MLCK isoforms exist:* MYLK – smooth muscle...
, and leads ultimately to the dephosphorylation of the myosin light chain, causing smooth muscle relaxation.
Neonatal and pediatric use
Nitric oxide/oxygen blends are used in critical care to promote capillary and pulmonary dilation to treat primary pulmonary hypertensionPulmonary hypertension
In medicine, pulmonary hypertension is an increase in blood pressure in the pulmonary artery, pulmonary vein, or pulmonary capillaries, together known as the lung vasculature, leading to shortness of breath, dizziness, fainting, and other symptoms, all of which are exacerbated by exertion...
in neonatal patients post-meconium aspiration and related to birth defects. These are often a last-resort gas mixture before the use of extracorporeal membrane oxygenation
Extracorporeal membrane oxygenation
In intensive care medicine, extracorporeal membrane oxygenation is an extracorporeal technique of providing both cardiac and respiratory support oxygen to patients whose heart and lungs are so severely diseased or damaged that they can no longer serve their function...
(ECMO). Nitric oxide therapy has the potential to significantly increase the quality of life and, in some cases, save the lives of infants at risk for pulmonary vascular disease.
Pulmonary embolism
Nitric oxide is also administered as salvage therapySalvage therapy
Salvage therapy is a form of treatment given after an ailment does not respond to standard treatment. The most common diseases that require salvage therapy are HIV and various tumors...
in patients with acute right ventricular failure secondary to pulmonary embolism
Pulmonary embolism
Pulmonary embolism is a blockage of the main artery of the lung or one of its branches by a substance that has travelled from elsewhere in the body through the bloodstream . Usually this is due to embolism of a thrombus from the deep veins in the legs, a process termed venous thromboembolism...
.
Pharmacology
Nitric oxide is considered an antiAntianginal
An antianginal is any drug used in the treatment of angina pectoris, a symptom of ischaemic heart disease.-Nitrates:Nitrates cause vasodilation of the venous capacitance vessels by stimulating the endothelium-derived relaxing factor...
anginal drug: it causes vasodilation
Vasodilation
Vasodilation refers to the widening of blood vessels resulting from relaxation of smooth muscle cells within the vessel walls, particularly in the large arteries, smaller arterioles and large veins. The process is essentially the opposite of vasoconstriction, or the narrowing of blood vessels. When...
, which can help with ischemic pain, known as angina, by decreasing the cardiac workload. By dilating (expanding) the veins, nitric oxide drugs lower arterial pressure and left ventricular filling pressure. This vasodilation does not decrease the volume of blood the heart pumps, but rather it decreases the force the heart muscle must exert to pump the same volume of blood. Nitroglycerin pills, taken sublingually (under the tongue), are used to prevent or treat acute chest pain. The nitroglycerin reacts with a sulfhydryl
Thiol
In organic chemistry, a thiol is an organosulfur compound that contains a carbon-bonded sulfhydryl group...
group (–SH) to produce nitric oxide, which eases the pain by causing vasodilation. Recent evidence suggests that nitrates may be beneficial for treatment of angina due to reduced myocardial oxygen consumption both by decreasing preload and afterload and by some direct vasodilation of coronary vessels.
Mainly for its utility as a vasodilator, NO has entered the fitness industry as an array of supplements geared towards accelerated muscle growth and prolonged stamina during endurance activities.
Further reading
- Butler A. and Nicholson R.; "Life, death and NO." Cambridge 2003. ISBN 978-0-85404-686-7.
- van Faassen, E. E.; Vanin, A. F. (eds); "Radicals for life: The various forms of Nitric Oxide." Elsevier, Amsterdam 2007. ISBN 978-0-444-52236-8.
- Ignarro, L. J. (ed.); "Nitric oxide:biology and pathobiology." Academic Press, San Diego 2000. ISBN 0-12-370420-0.
External links
- International Chemical Safety Card 1311
- National Pollutant Inventory – Oxides of nitrogen Fact Sheet
- 1998 Nobel Prize in Physiology/Medicine for discovery of NO's role in cardiovascular regulation
- Nitric Oxide and its Role in Diabetes, Wound Healing and Peripheral Neuropathy
- Microscale Gas Chemistry: Experiments with Nitrogen Oxides
- Your Brain Boots Up Like a Computer – new insights about the biological role of nitric oxide.
- Assessing The Potential of Nitric Oxide in the Diabetic Foot
- New Discoveries About Nitric Oxide Can Provide Drugs For Schizophrenia
- Nitric Oxide at the Chemical Database