Homochirality
Encyclopedia
Homochirality is a term used to refer to a group of molecules that possess the same sense of chirality
. Molecules involved are not necessarily the same compound, but similar groups are arranged in the same way around a central atom. In biology
homochirality is found in the chemical building blocks of life, the amino acids and sugars. All amino acids encoded by the genetic code have the same configuration about the chiral center, and are labeled
S, with the exception of cysteine, which is labeled R only because the sulfur in the side chain changes the priority of that group. Typically, the alternative form is inactive and sometimes even toxic to living things. The origin of this phenomenon is not clearly understood. It is unclear if homochirality has a purpose, however it appears to be a form of information storage.
One suggestion is that it reduces entropy
barriers in the formation of large organized molecules. It has been experimentally verified that amino acids form large aggregates in larger abundance from enantiopure substrates than from racemic
ones.
Homochirality is said to evolve in three distinct steps: mirror-symmetry breaking creates a minute enantiomeric imbalance and is key to homochirality, chiral amplification is a process of enantiomeric enrichment and chiral transmission allows the transfer of chirality of one set of molecules to another.
It is also entirely possible that homochirality is simply a result of the natural autoamplification process of life—that either the formation of life as preferring one chirality or the other was a chance rare event which happened to occur with the chiralities we observe, or that all chiralities of life emerged rapidly but due to catastrophic events and strong competition, the other unobserved chiral preferences were wiped out by the preponderance and metabolic, enantiomeric enrichment from the 'winning' chirality choices . The emergence of chirality consensus as a natural autoamplification process has been associated with the 2nd law of thermodynamics.
One supposition is that the discovery of an enantiomeric imbalance in molecules in the Murchison meteorite
supports an extraterrestrial origin of homochirality: there is evidence for the existence of circularly polarized light
originating from Mie scattering on aligned interstellar dust particles which may trigger the formation of optical isomers in space. Another speculation (the Vester-Ulbricht hypothesis) suggests that fundamental chirality of physical processes such as that of the beta decay (see Parity violation
) leads to slightly different half-lives of biologically relevant molecules. Homochirality may also result from spontaneous absolute asymmetric synthesis
.
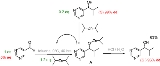
reaction systems the presence of a small amount of reaction product with enantiomeric excess at the start of the reaction can result in a much larger enantiomeric excess at the end of the reaction. In the Soai reaction
, pyrimidine-5-carbaldehyde
(Scheme 1) is alkylated by diisopropylzinc
to the corresponding pyrimidyl alcohol
. Because the initial reaction product is also an effective catalyst the reaction is autocatalytic. The presence of just 0.2 equivalent of the alcohol S-enantiomer
at the start of the reaction is sufficient to amplify the enantiomeric excess
to 93%.
 Another study concerns the proline
Another study concerns the proline
catalyzed aminoxylation of propionaldehyde
by nitrosobenzene
(scheme 2). In this system too the presence of enantioenriched catalyst drives the reaction towards one of the two possible optical isomers.
 Serine octamer cluster
Serine octamer cluster
s are also contenders. These clusters of 8 serine molecules appear in mass spectrometry with an unusual homochiral preference, however there is no evidence that such clusters exist under non-ionizing conditions and amino acid phase behavior is far more prebiotically relevant. The recent observation that partial sublimation of a 10% enantioenriched sample of leucine
results in up to 82% enrichment in the sublimate shows that enantioenrichment of amino acids could occur in space. Partial sublimation processes can take place on the surface of meteors where large variations in temperature exist. This finding may have consequences for the development of the Mars Organic Detector scheduled for launch in 2013 which aims to recover trace amounts of amino acids from the Mars surface exactly by a sublimation technique.
A high asymmetric amplification of the enantiomeric excess
of sugars are also present in the amino acid
catalyzed asymmetric formation of carbohydrates
One classic study involves an experiment that takes place in the laboratory. When sodium chlorate
is allowed to crystallize
from water and the collected crystals examined in a polarimeter
, each crystal turns out to be chiral and either the L form or the D form. In an ordinary experiment the amount of L crystals collected equals the amount of D crystals (corrected for statistical effects). However when the sodium chlorate solution is stirred during the crystallization process the crystals are either exclusively L or exclusively D. In 32 consecutive crystallization experiments 14 experiments deliver D-crystals and 18 others L-crystals. The explanation for this symmetry breaking is unclear but is related to autocatalysis
taking place in the nucleation
process.
In a related experiment, a crystal suspension of a racemic amino acid
derivative continuously stirred, results in a 100% crystal phase of one of the enantiomers because the enantiomeric pair is able to equilibrate in solution (compare with dynamic kinetic resolution)
of organic reactions by proline for example in Mannich reaction
s.
, which has a small methyl group, and phenylalanine
, which has a larger benzyl
group, a simple question is in what aspect, L-alanine resembles L-phenylalanine more than D-phenylalanine, and what kind of mechanism causes the selection of all L-amino acids. Because it might be possible that alanine was L and phenylalanine was D.
It was reported in 2004 that excess racemic D,L-asparagine
(Asn), which spontaneously forms crystals of either isomer during recrystallization, induces asymmetric resolution of a co-existing racemic amino acid such as arginine
(Arg), aspartic acid
(Asp), glutamine
(Gln), histidine
(His), leucine
(Leu), methionine
(Met), phenylalanine
(Phe), serine
(Ser), valine
(Val), tyrosine
(Tyr), and tryptophan
(Trp). The enantiomeric excess
{ee=100x(L-D)/(L+D)} of these amino acids was correlated almost linearly with that of the inducer, i.e., Asn. When recrystallizations from a mixture of 12 D,L-amino acids (Ala, Asp, Arg, Glu, Gln, His, Leu, Met, Ser, Val, Phe, and Tyr) and excess D,L-Asn were made, all amino acids with the same configuration with Asn were preferentially co-crystallized. It was incidental whether the enrichment took place in L- or D-Asn, however, once the selection was made, the co-existing amino acid with the same configuration at the α-carbon was preferentially involved because of thermodynamic stability in the crystal formation. The maximal ee was reported to be 100%. Based on these results, it is proposed that a mixture of racemic amino acids causes spontaneous and effective optical resolution, even if asymmetric synthesis of a single amino acid does not occur without an aid of an optically active molecule.
This is the first study elucidating reasonably the formation of chirality from racemic amino acids with experimental evidences.
in 1904, the year that published his Baltimore Lecture of 1884. Recently, however, homochiral has been used in the same sense as enantiomer
ically pure. This is permitted in some journals (but not encouraged), its meaning changing into the preference of a process or system for a single optical isomer in a pair of isomers in these journals.
of Dartmouth College
showed how proto-anti-codon RNAs or pacRNAs would require complementary homochiralities of amino acids (L-amino acids) and nucleotides (D-ribose sugar-based) in the ancestor of living organisms. The pacRNA model posits that homochirality of amino acids and homochirality of nucleotides were required in order for pacRNAs to auto-aminoacylate themselves. The pacRNA model predicts almost all of the features of the genetic code
, including the preference for L-amino acids. This marked the first time that the genetic code
was linked to the homochirality of life's molecules, and possibly also the first time that the homochirality of amino acids was linked to homochirality of nucleotides.
Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
. Molecules involved are not necessarily the same compound, but similar groups are arranged in the same way around a central atom. In biology
Biology
Biology is a natural science concerned with the study of life and living organisms, including their structure, function, growth, origin, evolution, distribution, and taxonomy. Biology is a vast subject containing many subdivisions, topics, and disciplines...
homochirality is found in the chemical building blocks of life, the amino acids and sugars. All amino acids encoded by the genetic code have the same configuration about the chiral center, and are labeled
S, with the exception of cysteine, which is labeled R only because the sulfur in the side chain changes the priority of that group. Typically, the alternative form is inactive and sometimes even toxic to living things. The origin of this phenomenon is not clearly understood. It is unclear if homochirality has a purpose, however it appears to be a form of information storage.
One suggestion is that it reduces entropy
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
barriers in the formation of large organized molecules. It has been experimentally verified that amino acids form large aggregates in larger abundance from enantiopure substrates than from racemic
Racemic
In chemistry, a racemic mixture, or racemate , is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule. The first known racemic mixture was "racemic acid", which Louis Pasteur found to be a mixture of the two enantiomeric isomers of tartaric acid.- Nomenclature :A...
ones.
Homochirality is said to evolve in three distinct steps: mirror-symmetry breaking creates a minute enantiomeric imbalance and is key to homochirality, chiral amplification is a process of enantiomeric enrichment and chiral transmission allows the transfer of chirality of one set of molecules to another.
It is also entirely possible that homochirality is simply a result of the natural autoamplification process of life—that either the formation of life as preferring one chirality or the other was a chance rare event which happened to occur with the chiralities we observe, or that all chiralities of life emerged rapidly but due to catastrophic events and strong competition, the other unobserved chiral preferences were wiped out by the preponderance and metabolic, enantiomeric enrichment from the 'winning' chirality choices . The emergence of chirality consensus as a natural autoamplification process has been associated with the 2nd law of thermodynamics.
Mirror-symmetry breaking
Explaining how an enantiomeric imbalance is created in the first place is a difficult question to answer, but there are some attempts to solve this problem.One supposition is that the discovery of an enantiomeric imbalance in molecules in the Murchison meteorite
Murchison meteorite
The Murchison meteorite is named after Murchison, Victoria, in Australia. It is one of the most studied meteorites due to its large mass , the fact that it was an observed fall, and it belongs to a group of meteorites rich in organic compounds....
supports an extraterrestrial origin of homochirality: there is evidence for the existence of circularly polarized light
Circular polarization
In electrodynamics, circular polarization of an electromagnetic wave is a polarization in which the electric field of the passing wave does not change strength but only changes direction in a rotary type manner....
originating from Mie scattering on aligned interstellar dust particles which may trigger the formation of optical isomers in space. Another speculation (the Vester-Ulbricht hypothesis) suggests that fundamental chirality of physical processes such as that of the beta decay (see Parity violation
Parity (physics)
In physics, a parity transformation is the flip in the sign of one spatial coordinate. In three dimensions, it is also commonly described by the simultaneous flip in the sign of all three spatial coordinates:...
) leads to slightly different half-lives of biologically relevant molecules. Homochirality may also result from spontaneous absolute asymmetric synthesis
Spontaneous absolute asymmetric synthesis
Spontaneous absolute asymmetric synthesis is a chemical phenomenon that stochastically generates chirality based on fluctuations and autocatalysis. In certain reactions which initially do not contain chiral information, stochastically distributed enantiomeric excess can be observed...
.
Chiral amplification
Laboratory experiments exist demonstrating how in certain autocatalyticAutocatalysis
A single chemical reaction is said to have undergone autocatalysis, or be autocatalytic, if the reaction product itself is the catalyst for that reaction....
reaction systems the presence of a small amount of reaction product with enantiomeric excess at the start of the reaction can result in a much larger enantiomeric excess at the end of the reaction. In the Soai reaction
Soai reaction
Soai reaction is alkylation of pyrimidine-5-carbaldehyde by diisopropylzinc. The product pyrimidyl alcohol acts as an autocatalyst, and with low enantiomeric excess yield product with very high enantiomeric excess....
, pyrimidine-5-carbaldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
(Scheme 1) is alkylated by diisopropylzinc
Diisopropylzinc
Diisopropylzinc is an organozinc compound with the chemical formulaZnC6H14....
to the corresponding pyrimidyl alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
. Because the initial reaction product is also an effective catalyst the reaction is autocatalytic. The presence of just 0.2 equivalent of the alcohol S-enantiomer
Enantiomer
In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable , much as one's left and right hands are the same except for opposite orientation. It can be clearly understood if you try to place your hands one over the other without...
at the start of the reaction is sufficient to amplify the enantiomeric excess
Enantiomeric excess
The enantiomeric excess of a substance is a measure of how pure it is. In this case, the impurity is the undesired enantiomer .-Definition:...
to 93%.

Proline
Proline is an α-amino acid, one of the twenty DNA-encoded amino acids. Its codons are CCU, CCC, CCA, and CCG. It is not an essential amino acid, which means that the human body can synthesize it. It is unique among the 20 protein-forming amino acids in that the α-amino group is secondary...
catalyzed aminoxylation of propionaldehyde
Propionaldehyde
Propionaldehyde or propanal is the organic compound with the formula CH3CH2CHO. It is a saturated 3-carbon aldehyde and is a structural isomer of acetone...
by nitrosobenzene
Nitrosobenzene
Nitrosobenzene is the organic compound with the formula C6H5NO. The compound can be viewed as hybrid of singlet O2 and azobenzene. This diamagnetic species exists in equilibrium with its dimer.-Preparation:...
(scheme 2). In this system too the presence of enantioenriched catalyst drives the reaction towards one of the two possible optical isomers.

Serine octamer cluster
The Serine octamer cluster in physical chemistry is an unusually stable cluster consisting of eight serine molecules implicated in the origin of homochirality. This cluster was first discovered in mass spectrometry experiments. Electrospray ionization of an aerosol of serine in methanol results in...
s are also contenders. These clusters of 8 serine molecules appear in mass spectrometry with an unusual homochiral preference, however there is no evidence that such clusters exist under non-ionizing conditions and amino acid phase behavior is far more prebiotically relevant. The recent observation that partial sublimation of a 10% enantioenriched sample of leucine
Leucine
Leucine is a branched-chain α-amino acid with the chemical formula HO2CCHCH2CH2. Leucine is classified as a hydrophobic amino acid due to its aliphatic isobutyl side chain. It is encoded by six codons and is a major component of the subunits in ferritin, astacin and other 'buffer' proteins...
results in up to 82% enrichment in the sublimate shows that enantioenrichment of amino acids could occur in space. Partial sublimation processes can take place on the surface of meteors where large variations in temperature exist. This finding may have consequences for the development of the Mars Organic Detector scheduled for launch in 2013 which aims to recover trace amounts of amino acids from the Mars surface exactly by a sublimation technique.
A high asymmetric amplification of the enantiomeric excess
Enantiomeric excess
The enantiomeric excess of a substance is a measure of how pure it is. In this case, the impurity is the undesired enantiomer .-Definition:...
of sugars are also present in the amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
catalyzed asymmetric formation of carbohydrates
One classic study involves an experiment that takes place in the laboratory. When sodium chlorate
Sodium chlorate
Sodium chlorate is a chemical compound with the chemical formula . When pure, it is a white crystalline powder that is readily soluble in water. It is hygroscopic. It decomposes above 250 °C to release oxygen and leave sodium chloride...
is allowed to crystallize
Crystallization
Crystallization is the process of formation of solid crystals precipitating from a solution, melt or more rarely deposited directly from a gas. Crystallization is also a chemical solid–liquid separation technique, in which mass transfer of a solute from the liquid solution to a pure solid...
from water and the collected crystals examined in a polarimeter
Polarimeter
A polarimeter is a scientific instrument used to measure the angle of rotation caused by passing polarized light through an optically active substance....
, each crystal turns out to be chiral and either the L form or the D form. In an ordinary experiment the amount of L crystals collected equals the amount of D crystals (corrected for statistical effects). However when the sodium chlorate solution is stirred during the crystallization process the crystals are either exclusively L or exclusively D. In 32 consecutive crystallization experiments 14 experiments deliver D-crystals and 18 others L-crystals. The explanation for this symmetry breaking is unclear but is related to autocatalysis
Autocatalysis
A single chemical reaction is said to have undergone autocatalysis, or be autocatalytic, if the reaction product itself is the catalyst for that reaction....
taking place in the nucleation
Nucleation
Nucleation is the extremely localized budding of a distinct thermodynamic phase. Some examples of phases that may form by way of nucleation in liquids are gaseous bubbles, crystals or glassy regions. Creation of liquid droplets in saturated vapor is also characterized by nucleation...
process.
In a related experiment, a crystal suspension of a racemic amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
derivative continuously stirred, results in a 100% crystal phase of one of the enantiomers because the enantiomeric pair is able to equilibrate in solution (compare with dynamic kinetic resolution)
Chiral transmission
Many strategies in asymmetric synthesis are built on chiral transmission. Especially important is the so-called organocatalysisOrganocatalysis
In organic chemistry, the term Organocatalysis refers to a form of catalysis, whereby the rate of a chemical reaction is increased by an organic catalyst referred to as an "organocatalyst" consisting of carbon, hydrogen, sulfur and other nonmetal elements found in organic compounds...
of organic reactions by proline for example in Mannich reaction
Mannich reaction
The Mannich reaction is an organic reaction which consists of an amino alkylation of an acidic proton placed next to a carbonyl functional group with formaldehyde and ammonia or any primary or secondary amine. The final product is a β-amino-carbonyl compound also known as a Mannich base...
s.
Optical resolution in racemic amino acids
There exists no theory elucidating correlations among L-amino acids. If one takes, for example, alanineAlanine
Alanine is an α-amino acid with the chemical formula CH3CHCOOH. The L-isomer is one of the 20 amino acids encoded by the genetic code. Its codons are GCU, GCC, GCA, and GCG. It is classified as a nonpolar amino acid...
, which has a small methyl group, and phenylalanine
Phenylalanine
Phenylalanine is an α-amino acid with the formula C6H5CH2CHCOOH. This essential amino acid is classified as nonpolar because of the hydrophobic nature of the benzyl side chain. L-Phenylalanine is an electrically neutral amino acid, one of the twenty common amino acids used to biochemically form...
, which has a larger benzyl
Benzyl
In organic chemistry, benzyl is the term used to describe the substituent or molecular fragment possessing the structure C6H5CH2-. Benzyl features a benzene ring attached to a CH2 group.-Nomenclature:...
group, a simple question is in what aspect, L-alanine resembles L-phenylalanine more than D-phenylalanine, and what kind of mechanism causes the selection of all L-amino acids. Because it might be possible that alanine was L and phenylalanine was D.
It was reported in 2004 that excess racemic D,L-asparagine
Asparagine
Asparagine is one of the 20 most common natural amino acids on Earth. It has carboxamide as the side-chain's functional group. It is not an essential amino acid...
(Asn), which spontaneously forms crystals of either isomer during recrystallization, induces asymmetric resolution of a co-existing racemic amino acid such as arginine
Arginine
Arginine is an α-amino acid. The L-form is one of the 20 most common natural amino acids. At the level of molecular genetics, in the structure of the messenger ribonucleic acid mRNA, CGU, CGC, CGA, CGG, AGA, and AGG, are the triplets of nucleotide bases or codons that codify for arginine during...
(Arg), aspartic acid
Aspartic acid
Aspartic acid is an α-amino acid with the chemical formula HOOCCHCH2COOH. The carboxylate anion, salt, or ester of aspartic acid is known as aspartate. The L-isomer of aspartate is one of the 20 proteinogenic amino acids, i.e., the building blocks of proteins...
(Asp), glutamine
Glutamine
Glutamine is one of the 20 amino acids encoded by the standard genetic code. It is not recognized as an essential amino acid but may become conditionally essential in certain situations, including intensive athletic training or certain gastrointestinal disorders...
(Gln), histidine
Histidine
Histidine Histidine, an essential amino acid, has a positively charged imidazole functional group. It is one of the 22 proteinogenic amino acids. Its codons are CAU and CAC. Histidine was first isolated by German physician Albrecht Kossel in 1896. Histidine is an essential amino acid in humans...
(His), leucine
Leucine
Leucine is a branched-chain α-amino acid with the chemical formula HO2CCHCH2CH2. Leucine is classified as a hydrophobic amino acid due to its aliphatic isobutyl side chain. It is encoded by six codons and is a major component of the subunits in ferritin, astacin and other 'buffer' proteins...
(Leu), methionine
Methionine
Methionine is an α-amino acid with the chemical formula HO2CCHCH2CH2SCH3. This essential amino acid is classified as nonpolar. This amino-acid is coded by the codon AUG, also known as the initiation codon, since it indicates mRNA's coding region where translation into protein...
(Met), phenylalanine
Phenylalanine
Phenylalanine is an α-amino acid with the formula C6H5CH2CHCOOH. This essential amino acid is classified as nonpolar because of the hydrophobic nature of the benzyl side chain. L-Phenylalanine is an electrically neutral amino acid, one of the twenty common amino acids used to biochemically form...
(Phe), serine
Serine
Serine is an amino acid with the formula HO2CCHCH2OH. It is one of the proteinogenic amino acids. By virtue of the hydroxyl group, serine is classified as a polar amino acid.-Occurrence and biosynthesis:...
(Ser), valine
Valine
Valine is an α-amino acid with the chemical formula HO2CCHCH2. L-Valine is one of 20 proteinogenic amino acids. Its codons are GUU, GUC, GUA, and GUG. This essential amino acid is classified as nonpolar...
(Val), tyrosine
Tyrosine
Tyrosine or 4-hydroxyphenylalanine, is one of the 22 amino acids that are used by cells to synthesize proteins. Its codons are UAC and UAU. It is a non-essential amino acid with a polar side group...
(Tyr), and tryptophan
Tryptophan
Tryptophan is one of the 20 standard amino acids, as well as an essential amino acid in the human diet. It is encoded in the standard genetic code as the codon UGG...
(Trp). The enantiomeric excess
Enantiomeric excess
The enantiomeric excess of a substance is a measure of how pure it is. In this case, the impurity is the undesired enantiomer .-Definition:...
{ee=100x(L-D)/(L+D)} of these amino acids was correlated almost linearly with that of the inducer, i.e., Asn. When recrystallizations from a mixture of 12 D,L-amino acids (Ala, Asp, Arg, Glu, Gln, His, Leu, Met, Ser, Val, Phe, and Tyr) and excess D,L-Asn were made, all amino acids with the same configuration with Asn were preferentially co-crystallized. It was incidental whether the enrichment took place in L- or D-Asn, however, once the selection was made, the co-existing amino acid with the same configuration at the α-carbon was preferentially involved because of thermodynamic stability in the crystal formation. The maximal ee was reported to be 100%. Based on these results, it is proposed that a mixture of racemic amino acids causes spontaneous and effective optical resolution, even if asymmetric synthesis of a single amino acid does not occur without an aid of an optically active molecule.
This is the first study elucidating reasonably the formation of chirality from racemic amino acids with experimental evidences.
History
This term was introduced by KelvinWilliam Thomson, 1st Baron Kelvin
William Thomson, 1st Baron Kelvin OM, GCVO, PC, PRS, PRSE, was a mathematical physicist and engineer. At the University of Glasgow he did important work in the mathematical analysis of electricity and formulation of the first and second laws of thermodynamics, and did much to unify the emerging...
in 1904, the year that published his Baltimore Lecture of 1884. Recently, however, homochiral has been used in the same sense as enantiomer
Enantiomer
In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable , much as one's left and right hands are the same except for opposite orientation. It can be clearly understood if you try to place your hands one over the other without...
ically pure. This is permitted in some journals (but not encouraged), its meaning changing into the preference of a process or system for a single optical isomer in a pair of isomers in these journals.
Connection of homochirality to genetic code
In 2011, Albert ErivesAlbert Erives
Albert Erives is a professor at Dartmouth College known for the pacRNA model for the joint origin of the genetic code and universal homochirality. He is also known for several findings in developmental biology and gene regulation....
of Dartmouth College
Dartmouth College
Dartmouth College is a private, Ivy League university in Hanover, New Hampshire, United States. The institution comprises a liberal arts college, Dartmouth Medical School, Thayer School of Engineering, and the Tuck School of Business, as well as 19 graduate programs in the arts and sciences...
showed how proto-anti-codon RNAs or pacRNAs would require complementary homochiralities of amino acids (L-amino acids) and nucleotides (D-ribose sugar-based) in the ancestor of living organisms. The pacRNA model posits that homochirality of amino acids and homochirality of nucleotides were required in order for pacRNAs to auto-aminoacylate themselves. The pacRNA model predicts almost all of the features of the genetic code
Genetic code
The genetic code is the set of rules by which information encoded in genetic material is translated into proteins by living cells....
, including the preference for L-amino acids. This marked the first time that the genetic code
Genetic code
The genetic code is the set of rules by which information encoded in genetic material is translated into proteins by living cells....
was linked to the homochirality of life's molecules, and possibly also the first time that the homochirality of amino acids was linked to homochirality of nucleotides.
See also
- StereochemistryStereochemistryStereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms within molecules. An important branch of stereochemistry is the study of chiral molecules....
- Unsolved problems in chemistryUnsolved problems in chemistryUnsolved problems in chemistry tend to be questions of the kind "Can we make X chemical compound?", "Can we analyse it?", "Can we purify it?" and are commonly solved rather quickly, but may just as well require considerable efforts to be solved. However, there are also some questions with deeper...
- CIP system
- Chirality (biology)
- Pfeiffer EffectPfeiffer EffectThe Pfeiffer Effect is an optical phenomenon whereby the presence of an optically active compound influences the optical rotation of a racemic mixture of a second compound....
External links
- On the Genesis of Homochirality A. Maureen Rouhi Chemical & Engineering NewsChemical & Engineering NewsChemical & Engineering News is a weekly magazine published by the American Chemical Society, providing professional and technical information in the fields of chemistry and chemical engineering...
June 17, 2004 Link - Observations Support Homochirality Theory Photonics TechnologyWorld November 1998 Link
- Scienceweek digest 1998 Link
- How left-handed amino acids got ahead: a demonstration of the evolution of biological homochirality in the lab Press release Imperial College London 2004 Link
- Origins of Homochirality conference in Nordita Stockholm, February 2008, talks available online http://agenda.albanova.se/conferenceDisplay.py?confId=322

