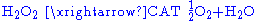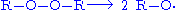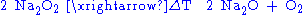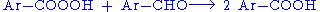Peroxide
Encyclopedia
A peroxide is a compound containing an oxygen
–oxygen single bond
or the peroxide anion ([O−O]2–).
The O−O group is called the peroxide group or peroxo group. In contrast to oxide ions
, the oxygen atoms in the peroxide ion have an oxidation state
of −1.
The simplest stable peroxide is hydrogen peroxide
. Superoxide
s, dioxygenyl
s, ozone
s and ozonide
s compound are considered separately. Peroxide compounds can be roughly classified into organic
and inorganic. Whereas the inorganic peroxides have an ionic, salt-like character, the organic peroxides are dominated by the covalent bonds. The oxygen-oxygen chemical bond
of peroxide is unstable and easily split into reactive radical
s via homolytic cleavage. For this reason, peroxides are found in nature only in small quantities, in water
, atmosphere
, plants, and animals.
Peroxides have a bleach
ing effect on organic substances and therefore are added to some detergent
s and hair colorants
. Other large-scale applications include medicine and chemical industry
, where peroxides are used in various synthesis reactions or occur as intermediate products. With an annual production of over 2 million tonne
s, hydrogen peroxide is the most economically important peroxide. Many peroxides are unstable and hazardous substances; they cannot be stored and therefore are synthesized in situ and used immediately.
, was synthesized by Alexander von Humboldt
in 1799 as a by-product of his attempts to decompose air. Nineteen years later Louis Jacques Thénard
recognized that this compound could be used for the preparation of a previously unknown compound, which he described as oxidized water – now known as hydrogen peroxide. Sodium peroxide
was synthesized in 1811 by Thénard and Joseph Louis Gay-Lussac
. The bleaching effect of peroxides and their salts on natural dye
s became known around that time, but early attempts of industrial production of peroxides failed, and the first plant producing hydrogen peroxide was built only in 1873 in Berlin
. The discovery of the synthesis of hydrogen peroxide by electrolysis
with sulfuric acid
had brought the more efficient electrochemical method. It was first implemented into industry in 1908 in Weißenstein
, Carinthia
, Germany. The anthraquinone process
, which is still used, was developed during the 1930s by the German chemical manufacturer IG Farben
in Ludwigshafen. The increased demand and improvements in the synthesis methods resulted in the rise of the annual production of hydrogen peroxide from 35,000 tonnes in 1950, to over 100,000 tonnes in 1960, to 300,000 tonnes by 1970, and by 1998, it reached 2.7 million tonnes.
and a peroxide derivative of prostaglandin
. Hydrogen peroxide occurs in surface water, groundwater and in the atmosphere
. It forms upon illumination or natural catalytic action by substances containing in water
. Sea water contains 0.5 to 14 mg/L of hydrogen peroxide, freshwater 1 to 30 mg/L and air 0.1 to 1 parts per billion.
to cells
. The toxicity is due to oxidation of protein
s, membrane lipids and DNA
by the peroxide ions. The class of biological enzyme
s called SOD (superoxide dismutase
) is developed in nearly all living cells as an important antioxidant
agent. They promote the disproportionation
of superoxide
into oxygen
and hydrogen peroxide
, which is then rapidly decomposed by the enzyme catalase
to oxygen and water.
Peroxisomes are organelles found in virtually all eukaryotic cells. They are involved in the catabolism
of very long chain fatty acid
s, branched chain fatty acids
, D-amino acids, polyamines, and biosynthesis of plasmalogens, etherphospholipids critical for the normal function of mammalian brains and lungs. Upon oxidation, they produce hydrogen peroxide in the following process:
Catalase
, which is another peroxisomal enzyme, uses this H2O2 to oxidize other substrates, including phenols
, formic acid
, formaldehyde
, and alcohol
, by means of the peroxidation reaction: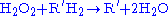 , thus eliminating the poisonous hydrogen peroxide in the process.
, thus eliminating the poisonous hydrogen peroxide in the process.
This reaction is important in liver and kidney cells, where the peroxisomes neutralize various toxic substances that enter the blood. Some of the ethanol
humans drink is oxidized to acetaldehyde
in this way. In addition, when excess H2O2 accumulates in the cell, catalase converts it to H2O through this reaction:
Another origin of hydrogen peroxide is the degradation of adenosine monophosphate
which yields hypoxanthine
. This is then oxidatively catabolized
first to xanthine
and then to uric acid
, and the reaction is catalyzed by the enzyme xanthine oxidase
:
 The degradation of guanosine monophosphate
The degradation of guanosine monophosphate
yields xanthine as an intermediate product which is then converted in the same way to uric acid with the formation of hydrogen peroxide.
Eggs of sea urchin
, shortly after fertilization by a sperm, produce hydrogen peroxide. It is then quickly dissociated to OH· radical
s. The radicals serve as initiator of radical polymerization
, which surrounds the eggs with a protective layer of polymer
.
The bombardier beetle
has a device which allows it to shoot corrosive and foul-smelling bubbles at its enemies. The beetle produces and stores hydroquinone
and hydrogen peroxide, in two separate reservoirs in the rear tip of its abdomen. When threatened, the beetle contracts muscles that force the two reactants through valved tubes into a mixing chamber containing water and a mixture of catalytic enzymes. When combined, the reactants undergo a violent exothermic
chemical reaction, raising the temperature to near the boiling point of water. The boiling, foul-smelling liquid partially becomes a gas (flash evaporation
) and is expelled through an outlet valve with a loud popping sound.
Furthermore, hydrogen peroxide is a signaling molecule
of plant defense against pathogens.
In firefly
, oxidation of luciferins
, which is catalyzed by luciferase
s, yields a peroxy compound 1,2-dioxetane. The dioxetane is unstable and decays spontaneously to carbon dioxide
and excited ketone
s, which release excess energy by emitting light (bioluminescence
).
of the peroxide ion, which predicts a doubly occupied antibonding π* orbital and a bond order of one. The bond length is 149 pm, which is larger than in the ground state (triplet oxygen
) of the oxygen molecule (3O2, 121 pm). This translates into the smaller force constant
of the bond (2.8 N/cm vs. 11.4 N/cm for 3O2) and the smaller frequency
of the molecular vibration (770 cm−1 vs. 1555 cm−1 for 3O2).
The peroxide ion can be compared with other molecular oxygen ions superoxide
O2− and ozonide
O3−, but contrary to them, the peroxide is not a radical and not paramagnetic
. Owing to the weak bonding between the oxygen atoms, peroxide easily undergoes homolytic cleavage
yielding two highly reactive radicals. This cleavage is accelerated by temperature, illumination or chemical reaction
s.
peroxides, which contain covalently bonded peroxide units. The first class mostly contains the peroxides of the alkali
and alkaline earth metal
s whereas the covalent peroxides are represented by such compounds as hydrogen peroxide and peroxymonosulfuric acid
(H2SO5). In contrast to the purely ionic character of alkali metal peroxides, peroxides of transition metal
s have a more covalent character.
can be synthesized directly by oxidation of the elements. Are shown with oxygen in the atmospheric pressure
. Lithium peroxide is synthesized by reacting lithium hydroxide
with hydrogen peroxide:
The historical production of barium peroxide used oxidation of barium oxide
at elevated temperature and pressure.
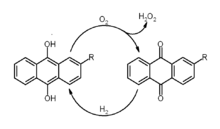 The most widely used synthesis method of hydrogen peroxide is the anthraquinone
The most widely used synthesis method of hydrogen peroxide is the anthraquinone
process. There, anthraquinone
is first catalyzed by palladium
with molecular hydrogen
. The resulting anthrahydroquinone is oxidized with molecular oxygen, reforming anthraquinone and releasing hydrogen peroxide. The overall reaction equation is
Direct synthesis of hydrogen peroxide from hydrogen and oxygen is rather inefficient and currently is not possible at industrial scale. Many peroxides of mineral acids, such as peroxodisulfate
s and percarbonates, can be obtained by anodic
oxidation of the respective acids. The anode material must be stable to the required high potentials of a few volts and therefore is either platinum or its alloys.
Peroxydisulfuric acid
was historically used for the production of hydrogen peroxide in a method developed in the early 20th century:
This process requires relatively high concentration of peroxydisulfuric acid as its more dilute solutions evolve oxygen gas instead of peroxide.
Upon heating, the reaction with water leads to the release of oxygen instead
The peroxide anion is a stronger nucleophile than hydroxide and displaces hydroxyl from oxyanions e.g. forming perborates and percarbonates. Sodium perborate
and sodium percarbonate
are important consumer and industrial bleaching agents; they stabilize hydrogen peroxide and limit side reactions (e.g. reduction and decomposition note below). The peroxide anion displaces the oxygen in urea
to form carbamide peroxide
. Peroxide forms bidendate complexes such as chromium(VI) oxide peroxide. The reaction of hydrogen peroxide with aqueous titanium(IV) gives a brightly colored peroxy complex that is a useful test for titanium
as well as hydrogen peroxide, it is a transition metal dioxygen complex. Molybdate
reacts in alkaline media with peroxide to form red peroxomolybdate {Mo(O2)4}2–.
Hydrogen peroxide is both an oxidizing agent and reducing agent. The oxidation of hydrogen peroxide by sodium hypochlorite
yields singlet oxygen
. The net reaction of a ferric ion with hydrogen peroxide is a ferrous ion and oxygen. This proceeds via single electron oxidation and hydroxyl radicals. This is used in some organic chemistry oxidations, e.g. in the Fenton's reagent
. Only catalytic quantities of iron ion is needed since peroxide also oxidizes ferrous to ferric ion. The net reaction of hydrogen peroxide
and permanganate
or manganese dioxide is manganous ion; however, until the peroxide is spent some manganous ions are reoxidized to make the reaction catalytic. This forms the basis for common monopropellant
rockets.
with sodium peroxide to dibenzoyl peroxide
.
Many inorganic peroxides are used for bleach
ing textiles and paper
and as a bleaching additive to detergents and cleaning products. The increasing environmental concerns resulted in the preference of peroxides over chlorine-based compounds and a sharp increase in the peroxide production. The past use of perborate
s as additives to detergents and cleaning products has been largely replaced by percarbonates
in order to decrease the emission of boron to the environment. Sodium percarbonate is used in such products as OxiClean
and Tide laundry detergent
. When dissolved in water, it releases hydrogen peroxide and soda ash (sodium carbonate):
The use of peroxide compounds in detergents is often reflected in their trade names, for example Persil
is a combination of the words perborate and silicate.
Some peroxide salts release oxygen upon reaction with carbon dioxide. This reaction is used in regeneration of oxygen from exhaled carbon dioxide on submarine
s and spaceships. Sodium or lithium peroxides are preferred in space applications because of their lower molar mass
and therefore higher oxygen yield per unit weight.
Barium peroxide has been historically used to produce pure oxygen from air. This process relies on the temperature-dependent chemical balance between barium oxide and peroxide: the reaction of barium oxide with air at 500 °C results in barium peroxide, which upon heating to above 700 °C in oxygen decomposes back to barium oxide releasing pure oxygen.
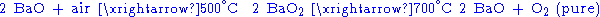
s are divided into two major classes, peroxy acid
s and organic hydroperoxides. The first class is derived from the carboxylic acid
and the second from ether
s or alcohol
s.
Another synthetic route employs acyl halide
s instead of the carboxylic acid. It is used primarily with aromatic compounds in basic
in order to neutralize the resulting hydrogen chloride
.
Aromatic aldehydes can be a auto-oxidized into peroxycarboxylic acids:
The products, however, react with the initial aldehyde forming the carboxylic acid:
Several synthesis routes are known for aliphatic
peroxides, such as the reaction of dialkylsulfates with alkaline hydrogen peroxide solution. In this method, the alkyl sulfate donates the alkyl group and the sulfate ion forms the leaving group
.
This method can also yield cyclic peroxides. The four-membered dioxetane
s can be obtained by 2+2 cycloaddition
of oxygen to alkene
s.
The selective synthesis of hydroperoxides can be carried out by free-radical oxidation of alkanes with oxygen. Here the active site formed by a radical initiator
reacts with oxygen to form a hydroperoxyl. The addition of oxygen results in a more active radical which can further extract hydrogen atoms and release the hydroperoxide, leaving a new radical. This process is used industrially for the synthesis of phenol
from benzene
and is called the Cumene process
or Hock process for its cumene
and cumene hydroperoxide
intermediates.
This auto-oxidation reaction can be used with common solvent
s from the group of ether
s, such as diethyl ether
, diisopropyl ether
, tetrahydrofuran
or 1,4-dioxane
. It yields a volatile hydroperoxide ether that upon heating can result in a serious explosion.
Peroxides are formed by living organisms through ene reaction
s or Diels–Alder reactions between alkene
s and oxygen. Unsaturated fatty acids can serve as the olefinic substrates
for the ene reaction and unsaturated amino acids like histidine
can be the reactant for the Diels-Alder cyclization. Rancidification
(decomposition) of fats is partly caused by the formation of peroxides.
s than the parent carboxylic acids. Like most peroxides, they are strong oxidants and tend to explode at high concentrations and higher temperatures.
Organic peracids are used in the synthesis of epoxies
via the Prilezhaev reaction
. Another important application is the synthesis of lactone
s of cyclic ketone
s in the Baeyer–Villiger oxidation process. In both cases, electron-poor peroxycarboxylic acids are especially efficient, such as meta-chloroperoxybenzoic acid
(mCPBA).
Tert-butyl hydroperoxide
is a common oxidant in the Sharpless epoxidation
, which is used for the stereoselective
synthesis of epoxides. Karl Barry Sharpless was awarded the 2001 Nobel prize in Chemistry for this reaction.
Peracetic acid is a popular disinfectant in the medical field and food industry. Various peroxide solutions are commercially produced for the cleaning and disinfection of contact lens
es.
 Dibenzoyl is used as a radical initiator
Dibenzoyl is used as a radical initiator
both in the laboratory research and in the industry. Its weak peroxide bond can be easily cleaved yielding reactive benzoyl radicals, which assist polymerization
of plastics like polyethylene
. One of the synthesis methods of the commercially important plastic caprolactam
—the precursor to Nylon 6
(polycaprolactam)—is a Baeyer-Villiger rearrangement of cyclohexanone
with peracetic acid. This yields caprolactone
, which is then converted to caprolactam by reacting it with ammonia
.
Industrial resins based on acrylic and/or methacrylic acid
esters are invariably produced by radical polymerization with organic peroxides at elevated temperatures. The polymerization rate is adjusted by suitable choice of temperature and type of peroxide.
Some peroxides are drug
s, whose action is based on the formation of radicals at desired locations in the organism. For example, artemisinin
and its derivatives, such as such artesunate
, possess the most rapid action of all current drugs against falciparum
malaria
. Artesunate is also efficient in reducing egg production in Schistosoma haematobium
infection.
Many organic peroxides can initiate explosive polymerization in materials with unsaturated chemical bonds, and specifically triacetone triperoxide
(TATP) and hexamethylene triperoxide diamine
(HMTD) are powerful explosives. TATP is an inexpensive compound and is relatively easy to make. Whereas most other potent explosives, such as trinitrotoluene (TNT) or RDX
(the major component of C4
mixtures), contain nitrogen, which is relatively easy to trace by sniffing techniques, TATP is nitrogen free and therefore is very difficult to detect by conventional screening methods. For this reason, it is an explosive favored by terrorists. TATP and HMTD were used in several executed or planned terrorist acts of the early 2000s, most notably in the 2001 shoe bomb plot and the 2005 London Underground bombings
. Several detection devices have been designed since those events. One, for example, releases a chemical mixture which changes color when interacting with traces of TATP.
. Here peroxides, hydroperoxides or peracids oxidize the added potassium iodide
into iodine
, which reacts with starch
producing a deep-blue color. Commercial paper indicators using this reaction are available. This method is also suitable for quantitative evaluation, but it can not distinguish between different types of peroxide compounds. Discoloration of various indigo dye
s in presence of peroxides is used instead for this purpose. For example, the loss of blue color in leuco-methylene blue
is selective for hydrogen peroxide.
Quantitative analysis of hydroperoxides is performed using potentiometric titration
with lithium aluminium hydride
. Another way to evaluate the content of peracids and peroxides is the volumetric titration with alkoxide
s such as sodium ethoxide
.
, and TATP, because of its high susceptibility to accidental detonation by shock, friction, or sparks, has earned the nickname "Mother of Satan" among certain Islamic militant groups. TATP can accidentally form as by-products in many commonly used reactions. These reactions range from synthesis of MDMA, where TATP is formed via isosafrole
oxidation in acetone, to industrial production of phenol
, where the second product of the cumene process
, acetone
, is partially oxidized to peroxide on the second reaction step. Accidental preparation of organic peroxides can occur by mixing ketone solvents (most commonly acetone) with waste materials containing hydrogen peroxide or other oxidizers and leaving the mixture standing for several hours. In addition, many liquid ether
s in the presence of air, light and metals (which act as catalysts) slowly – over a period of months – form highly unstable ether peroxides such as diethyl ether peroxide
. Therefore, ethers are often stored over potassium hydroxide, which not only destroys peroxides but also acts as a powerful desiccant
.
Peroxides are also strong oxidizers and easily react with skin, cotton and wood pulp. For safety reasons, peroxidic compounds are stored in a cool, opaque container, as heating and illumination accelerates their chemical reactions. Small amounts of peroxides, which emerge from storage or reaction vessels are neutralized using reducing agents such as iron(II) sulfate
. The safety measures in industrial plants producing large amounts of peroxides include the following. The equipment is located within reinforced concrete structures with foil windows, which would relieve pressure and not shatter in case of explosion. The products are bottled in small containers and are moved to a cold place promptly after the synthesis. The containers are made of non-reactive materials such as stainless steel, some aluminium alloys or dark glass.
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
–oxygen single bond
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
or the peroxide anion ([O−O]2–).
The O−O group is called the peroxide group or peroxo group. In contrast to oxide ions
Oxide
An oxide is a chemical compound that contains at least one oxygen atom in its chemical formula. Metal oxides typically contain an anion of oxygen in the oxidation state of −2....
, the oxygen atoms in the peroxide ion have an oxidation state
Oxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
of −1.
The simplest stable peroxide is hydrogen peroxide
Hydrogen peroxide
Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
. Superoxide
Superoxide
A superoxide, also known by the obsolete name hyperoxide, is a compound that possesses the superoxide anion with the chemical formula O2−. The systematic name of the anion is dioxide. It is important as the product of the one-electron reduction of dioxygen O2, which occurs widely in nature...
s, dioxygenyl
Dioxygenyl
The dioxygenyl ion, O2+, is a rarely encountered oxycation in which both oxygen atoms have a formal oxidation state of +½. It is formally derived from oxygen by the removal of an electron:...
s, ozone
Ozone
Ozone , or trioxygen, is a triatomic molecule, consisting of three oxygen atoms. It is an allotrope of oxygen that is much less stable than the diatomic allotrope...
s and ozonide
Ozonide
Ozonide is an unstable, reactive polyatomic anion O3−, derived from ozone, or an organic compound similar to organic peroxide formed by a reaction of ozone with an unsaturated compound.-Inorganic ozonides:...
s compound are considered separately. Peroxide compounds can be roughly classified into organic
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
and inorganic. Whereas the inorganic peroxides have an ionic, salt-like character, the organic peroxides are dominated by the covalent bonds. The oxygen-oxygen chemical bond
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
of peroxide is unstable and easily split into reactive radical
Radical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
s via homolytic cleavage. For this reason, peroxides are found in nature only in small quantities, in water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
, atmosphere
Atmosphere
An atmosphere is a layer of gases that may surround a material body of sufficient mass, and that is held in place by the gravity of the body. An atmosphere may be retained for a longer duration, if the gravity is high and the atmosphere's temperature is low...
, plants, and animals.
Peroxides have a bleach
Bleach
Bleach refers to a number of chemicals that remove color, whiten, or disinfect, often via oxidation. Common chemical bleaches include household chlorine bleach , lye, oxygen bleach , and bleaching powder...
ing effect on organic substances and therefore are added to some detergent
Detergent
A detergent is a surfactant or a mixture of surfactants with "cleaning properties in dilute solutions." In common usage, "detergent" refers to alkylbenzenesulfonates, a family of compounds that are similar to soap but are less affected by hard water...
s and hair colorants
Hair coloring
Hair coloring is the practice of changing the color of hair. Common reasons are to cover gray hair, to change to a color regarded as more fashionable or desirable, and to restore the original hair color after it has been discolored by hairdressing processes or sun bleaching...
. Other large-scale applications include medicine and chemical industry
Chemical industry
The chemical industry comprises the companies that produce industrial chemicals. Central to the modern world economy, it converts raw materials into more than 70,000 different products.-Products:...
, where peroxides are used in various synthesis reactions or occur as intermediate products. With an annual production of over 2 million tonne
Tonne
The tonne, known as the metric ton in the US , often put pleonastically as "metric tonne" to avoid confusion with ton, is a metric system unit of mass equal to 1000 kilograms. The tonne is not an International System of Units unit, but is accepted for use with the SI...
s, hydrogen peroxide is the most economically important peroxide. Many peroxides are unstable and hazardous substances; they cannot be stored and therefore are synthesized in situ and used immediately.
History
One of the first synthetic peroxides, barium peroxideBarium peroxide
Barium peroxide is the chemical compound with the formula BaO2. This grey-white solid is one of the most common inorganic peroxides. Barium peroxide is an oxidizing agent, which is used for bleaching...
, was synthesized by Alexander von Humboldt
Alexander von Humboldt
Friedrich Wilhelm Heinrich Alexander Freiherr von Humboldt was a German naturalist and explorer, and the younger brother of the Prussian minister, philosopher and linguist Wilhelm von Humboldt...
in 1799 as a by-product of his attempts to decompose air. Nineteen years later Louis Jacques Thénard
Louis Jacques Thénard
Louis Jacques Thénard , was a French chemist.His father, a poor peasant, managed to have him educated at the academy of Sens, and sent him at the age of sixteen to study pharmacy in Paris. There he attended the lectures of Antoine François Fourcroy and Louis Nicolas Vauquelin...
recognized that this compound could be used for the preparation of a previously unknown compound, which he described as oxidized water – now known as hydrogen peroxide. Sodium peroxide
Sodium peroxide
Sodium peroxide is the inorganic compound with the formula Na2O2. This solid is the product when sodium is burned with oxygen. It is a strong base and a potent oxidizing agent. It exists in several hydrates and peroxyhydrates including Na2O2·2H2O2·4H2O, Na2O2·2H2O, Na2O2·2H2O2, and...
was synthesized in 1811 by Thénard and Joseph Louis Gay-Lussac
Joseph Louis Gay-Lussac
- External links :* from the American Chemical Society* from the Encyclopædia Britannica, 10th Edition * , Paris...
. The bleaching effect of peroxides and their salts on natural dye
Natural dye
Natural dyes are dyes or colorants derived from plants, invertebrates, or minerals. The majority of natural dyes are vegetable dyes from plant sources – roots, berries, bark, leaves, and wood — and other organic sources such as fungi and lichens....
s became known around that time, but early attempts of industrial production of peroxides failed, and the first plant producing hydrogen peroxide was built only in 1873 in Berlin
Berlin
Berlin is the capital city of Germany and is one of the 16 states of Germany. With a population of 3.45 million people, Berlin is Germany's largest city. It is the second most populous city proper and the seventh most populous urban area in the European Union...
. The discovery of the synthesis of hydrogen peroxide by electrolysis
Electrolysis
In chemistry and manufacturing, electrolysis is a method of using a direct electric current to drive an otherwise non-spontaneous chemical reaction...
with sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
had brought the more efficient electrochemical method. It was first implemented into industry in 1908 in Weißenstein
Weißenstein
Weißenstein is a town in the district of Villach-Land in Carinthia in Austria. The scenery around Weißenstein has been attracting artists for years, and their work is presented in the town's Wachaumuseum. The other main attraction is its Church of the Virgin Mary, on a hilltop to protect against...
, Carinthia
Carinthia (state)
Carinthia is the southernmost Austrian state or Land. Situated within the Eastern Alps it is chiefly noted for its mountains and lakes.The main language is German. Its regional dialects belong to the Southern Austro-Bavarian group...
, Germany. The anthraquinone process
Anthraquinone process
The anthraquinone process is a process for the production of hydrogen peroxide, which was developed by BASF. The technical production of hydrogen peroxide is based on the reduction of oxygen, as in the direct synthesis from the elements...
, which is still used, was developed during the 1930s by the German chemical manufacturer IG Farben
IG Farben
I.G. Farbenindustrie AG was a German chemical industry conglomerate. Its name is taken from Interessen-Gemeinschaft Farbenindustrie AG . The company was formed in 1925 from a number of major companies that had been working together closely since World War I...
in Ludwigshafen. The increased demand and improvements in the synthesis methods resulted in the rise of the annual production of hydrogen peroxide from 35,000 tonnes in 1950, to over 100,000 tonnes in 1960, to 300,000 tonnes by 1970, and by 1998, it reached 2.7 million tonnes.
In the environment
Peroxides are usually very reactive and thus occur in nature only in a few forms. These include, in addition to hydrogen peroxide, a few vegetable products such as ascaridoleAscaridole
Ascaridole is a natural organic compound classified as a bicyclic monoterpene that has an unusual bridging peroxide functional group. It is a colorless liquid with a pungent smell and taste that is soluble in most organic solvents. Like other low molecular weight organic peroxides, it is unstable...
and a peroxide derivative of prostaglandin
Prostaglandin
A prostaglandin is any member of a group of lipid compounds that are derived enzymatically from fatty acids and have important functions in the animal body. Every prostaglandin contains 20 carbon atoms, including a 5-carbon ring....
. Hydrogen peroxide occurs in surface water, groundwater and in the atmosphere
Atmosphere
An atmosphere is a layer of gases that may surround a material body of sufficient mass, and that is held in place by the gravity of the body. An atmosphere may be retained for a longer duration, if the gravity is high and the atmosphere's temperature is low...
. It forms upon illumination or natural catalytic action by substances containing in water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
. Sea water contains 0.5 to 14 mg/L of hydrogen peroxide, freshwater 1 to 30 mg/L and air 0.1 to 1 parts per billion.
In biochemical processes
Hydrogen peroxide is formed in human and animal organisms as a short-lived product in biochemical processes and is toxicToxicity
Toxicity is the degree to which a substance can damage a living or non-living organisms. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a substructure of the organism, such as a cell or an organ , such as the liver...
to cells
Cell (biology)
The cell is the basic structural and functional unit of all known living organisms. It is the smallest unit of life that is classified as a living thing, and is often called the building block of life. The Alberts text discusses how the "cellular building blocks" move to shape developing embryos....
. The toxicity is due to oxidation of protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s, membrane lipids and DNA
DNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
by the peroxide ions. The class of biological enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
s called SOD (superoxide dismutase
Superoxide dismutase
Superoxide dismutases are a class of enzymes that catalyze the dismutation of superoxide into oxygen and hydrogen peroxide. As such, they are an important antioxidant defense in nearly all cells exposed to oxygen...
) is developed in nearly all living cells as an important antioxidant
Antioxidant
An antioxidant is a molecule capable of inhibiting the oxidation of other molecules. Oxidation is a chemical reaction that transfers electrons or hydrogen from a substance to an oxidizing agent. Oxidation reactions can produce free radicals. In turn, these radicals can start chain reactions. When...
agent. They promote the disproportionation
Disproportionation
Disproportionation, also known as dismutation is used to describe a specific type of redox reaction in which a species is simultaneously reduced and oxidized so as to form two different products....
of superoxide
Superoxide
A superoxide, also known by the obsolete name hyperoxide, is a compound that possesses the superoxide anion with the chemical formula O2−. The systematic name of the anion is dioxide. It is important as the product of the one-electron reduction of dioxygen O2, which occurs widely in nature...
into oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
and hydrogen peroxide
Hydrogen peroxide
Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
, which is then rapidly decomposed by the enzyme catalase
Catalase
Catalase is a common enzyme found in nearly all living organisms that are exposed to oxygen, where it catalyzes the decomposition of hydrogen peroxide to water and oxygen...
to oxygen and water.
-

- Formation of hydrogen peroxide by superoxide dismutase (SOD) .
Peroxisomes are organelles found in virtually all eukaryotic cells. They are involved in the catabolism
Catabolism
Catabolism is the set of metabolic pathways that break down molecules into smaller units and release energy. In catabolism, large molecules such as polysaccharides, lipids, nucleic acids and proteins are broken down into smaller units such as monosaccharides, fatty acids, nucleotides, and amino...
of very long chain fatty acid
Very long chain fatty acid
A very long chain fatty acid is a fatty acid with aliphatic tails longer than 22 carbons.Unlike most fatty acids, VLCFAs are too long to be metabolized in the mitochondria, and must be metabolized in peroxisomes....
s, branched chain fatty acids
Branched-chain-fatty-acid kinase
In enzymology, a branched-chain-fatty-acid kinase is an enzyme that catalyzes the chemical reactionThus, the two substrates of this enzyme are ATP and 2-methylpropanoate, whereas its two products are ADP and 2-methylpropanoyl phosphate....
, D-amino acids, polyamines, and biosynthesis of plasmalogens, etherphospholipids critical for the normal function of mammalian brains and lungs. Upon oxidation, they produce hydrogen peroxide in the following process:
-

- FAD = flavin adenine dinucleotide
Catalase
Catalase
Catalase is a common enzyme found in nearly all living organisms that are exposed to oxygen, where it catalyzes the decomposition of hydrogen peroxide to water and oxygen...
, which is another peroxisomal enzyme, uses this H2O2 to oxidize other substrates, including phenols
Phenols
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of a hydroxyl group bonded directly to an aromatic hydrocarbon group...
, formic acid
Formic acid
Formic acid is the simplest carboxylic acid. Its chemical formula is HCOOH or HCO2H. It is an important intermediate in chemical synthesis and occurs naturally, most notably in the venom of bee and ant stings. In fact, its name comes from the Latin word for ant, formica, referring to its early...
, formaldehyde
Formaldehyde
Formaldehyde is an organic compound with the formula CH2O. It is the simplest aldehyde, hence its systematic name methanal.Formaldehyde is a colorless gas with a characteristic pungent odor. It is an important precursor to many other chemical compounds, especially for polymers...
, and alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
, by means of the peroxidation reaction:
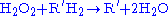 , thus eliminating the poisonous hydrogen peroxide in the process.
, thus eliminating the poisonous hydrogen peroxide in the process.This reaction is important in liver and kidney cells, where the peroxisomes neutralize various toxic substances that enter the blood. Some of the ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
humans drink is oxidized to acetaldehyde
Acetaldehyde
Acetaldehyde is an organic chemical compound with the formula CH3CHO or MeCHO. It is one of the most important aldehydes, occurring widely in nature and being produced on a large scale industrially. Acetaldehyde occurs naturally in coffee, bread, and ripe fruit, and is produced by plants as part...
in this way. In addition, when excess H2O2 accumulates in the cell, catalase converts it to H2O through this reaction:
Another origin of hydrogen peroxide is the degradation of adenosine monophosphate
Adenosine monophosphate
Adenosine monophosphate , also known as 5'-adenylic acid, is a nucleotide that is used as a monomer in RNA. It is an ester of phosphoric acid and the nucleoside adenosine. AMP consists of a phosphate group, the sugar ribose, and the nucleobase adenine...
which yields hypoxanthine
Hypoxanthine
Hypoxanthine is a naturally occurring purine derivative. It is occasionally found as a constituent of nucleic acids where it is present in the anticodon of tRNA in the form of its nucleoside inosine. It has a tautomer known as 6-Hydroxypurine. Hypoxanthine is a necessary additive in certain cell,...
. This is then oxidatively catabolized
Catabolism
Catabolism is the set of metabolic pathways that break down molecules into smaller units and release energy. In catabolism, large molecules such as polysaccharides, lipids, nucleic acids and proteins are broken down into smaller units such as monosaccharides, fatty acids, nucleotides, and amino...
first to xanthine
Xanthine
Xanthine , is a purine base found in most human body tissues and fluids and in other organisms. A number of stimulants are derived from xanthine, including caffeine and theobromine....
and then to uric acid
Uric acid
Uric acid is a heterocyclic compound of carbon, nitrogen, oxygen, and hydrogen with the formula C5H4N4O3. It forms ions and salts known as urates and acid urates such as ammonium acid urate. Uric acid is created when the body breaks down purine nucleotides. High blood concentrations of uric acid...
, and the reaction is catalyzed by the enzyme xanthine oxidase
Xanthine oxidase
Xanthine oxidase Xanthine oxidase Xanthine oxidase (XO (sometimes 'XAO'), a form of xanthine oxidoreductase that generates reactive oxygen species. Is an enzyme that catalyzes the oxidation of hypoxanthine to xanthine and can further catalyze the oxidation of xanthine to uric acid...
:

Guanosine monophosphate
Guanosine monophosphate, also known as 5'-guanidylic acid or guanylic acid and abbreviated GMP, is a nucleotide that is used as a monomer in RNA. It is an ester of phosphoric acid with the nucleoside guanosine. GMP consists of the phosphate group, the pentose sugar ribose, and the nucleobase...
yields xanthine as an intermediate product which is then converted in the same way to uric acid with the formation of hydrogen peroxide.
Eggs of sea urchin
Sea urchin
Sea urchins or urchins are small, spiny, globular animals which, with their close kin, such as sand dollars, constitute the class Echinoidea of the echinoderm phylum. They inhabit all oceans. Their shell, or "test", is round and spiny, typically from across. Common colors include black and dull...
, shortly after fertilization by a sperm, produce hydrogen peroxide. It is then quickly dissociated to OH· radical
Radical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
s. The radicals serve as initiator of radical polymerization
Radical polymerization
Free radical polymerization is a method of polymerization by which a polymer forms by the successive addition of free radical building blocks. Free radicals can be formed via a number of different mechanisms usually involving separate initiator molecules...
, which surrounds the eggs with a protective layer of polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
.
The bombardier beetle
Bombardier beetle
Bombardier beetles are ground beetles in the tribes Brachinini, Paussini, Ozaenini, or Metriini—more than 500 species altogether—which are most notable for the defense mechanism that gives them their name: When disturbed, the beetle ejects a noxious chemical spray in a rapid burst of pulses from...
has a device which allows it to shoot corrosive and foul-smelling bubbles at its enemies. The beetle produces and stores hydroquinone
Hydroquinone
Hydroquinone, also benzene-1,4-diol or quinol, is an aromatic organic compound that is a type of phenol, having the chemical formula C6H42. Its chemical structure, shown in the table at right, has two hydroxyl groups bonded to a benzene ring in a para position. It is a white granular solid...
and hydrogen peroxide, in two separate reservoirs in the rear tip of its abdomen. When threatened, the beetle contracts muscles that force the two reactants through valved tubes into a mixing chamber containing water and a mixture of catalytic enzymes. When combined, the reactants undergo a violent exothermic
Exothermic
In thermodynamics, the term exothermic describes a process or reaction that releases energy from the system, usually in the form of heat, but also in the form of light , electricity , or sound...
chemical reaction, raising the temperature to near the boiling point of water. The boiling, foul-smelling liquid partially becomes a gas (flash evaporation
Flash evaporation
Flash evaporation is the partial vapor that occurs when a saturated liquid stream undergoes a reduction in pressure by passing through a throttling valve or other throttling device. This process is one of the simplest unit operations...
) and is expelled through an outlet valve with a loud popping sound.
Furthermore, hydrogen peroxide is a signaling molecule
Signaling molecule
A signaling molecule is a chemical involved in transmitting information between cells. Such molecules are released from the cell sending the signal, cross over the gap between cells by diffusion, and interact with specific receptors in another cell, triggering a response in that cell by activating...
of plant defense against pathogens.
In firefly
Firefly
Lampyridae is a family of insects in the beetle order Coleoptera. They are winged beetles, and commonly called fireflies or lightning bugs for their conspicuous crepuscular use of bioluminescence to attract mates or prey. Fireflies produce a "cold light", with no infrared or ultraviolet frequencies...
, oxidation of luciferins
Firefly luciferin
Firefly luciferin is the luciferin, or light-emitting compound, found in many firefly species. It is the substrate of luciferase , which is responsible for the characteristic yellow light emission from many firefly species...
, which is catalyzed by luciferase
Luciferase
Luciferase is a generic term for the class of oxidative enzymes used in bioluminescence and is distinct from a photoprotein. One famous example is the firefly luciferase from the firefly Photinus pyralis. "Firefly luciferase" as a laboratory reagent usually refers to P...
s, yields a peroxy compound 1,2-dioxetane. The dioxetane is unstable and decays spontaneously to carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
and excited ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s, which release excess energy by emitting light (bioluminescence
Bioluminescence
Bioluminescence is the production and emission of light by a living organism. Its name is a hybrid word, originating from the Greek bios for "living" and the Latin lumen "light". Bioluminescence is a naturally occurring form of chemiluminescence where energy is released by a chemical reaction in...
).
Bonding
The peroxide ion is composed of two oxygen atoms, which are linked by a single bond. This is consistent with the molecular orbital diagramMolecular orbital theory
In chemistry, molecular orbital theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms, but are treated as moving under the influence of the nuclei in the whole molecule...
of the peroxide ion, which predicts a doubly occupied antibonding π* orbital and a bond order of one. The bond length is 149 pm, which is larger than in the ground state (triplet oxygen
Triplet oxygen
Triplet oxygen is the ground state of the oxygen molecule. The electron configuration of the molecule has two unpaired electrons occupying two degenerate molecular orbitals...
) of the oxygen molecule (3O2, 121 pm). This translates into the smaller force constant
Hooke's law
In mechanics, and physics, Hooke's law of elasticity is an approximation that states that the extension of a spring is in direct proportion with the load applied to it. Many materials obey this law as long as the load does not exceed the material's elastic limit. Materials for which Hooke's law...
of the bond (2.8 N/cm vs. 11.4 N/cm for 3O2) and the smaller frequency
Frequency
Frequency is the number of occurrences of a repeating event per unit time. It is also referred to as temporal frequency.The period is the duration of one cycle in a repeating event, so the period is the reciprocal of the frequency...
of the molecular vibration (770 cm−1 vs. 1555 cm−1 for 3O2).
The peroxide ion can be compared with other molecular oxygen ions superoxide
Superoxide
A superoxide, also known by the obsolete name hyperoxide, is a compound that possesses the superoxide anion with the chemical formula O2−. The systematic name of the anion is dioxide. It is important as the product of the one-electron reduction of dioxygen O2, which occurs widely in nature...
O2− and ozonide
Ozonide
Ozonide is an unstable, reactive polyatomic anion O3−, derived from ozone, or an organic compound similar to organic peroxide formed by a reaction of ozone with an unsaturated compound.-Inorganic ozonides:...
O3−, but contrary to them, the peroxide is not a radical and not paramagnetic
Paramagnetism
Paramagnetism is a form of magnetism whereby the paramagnetic material is only attracted when in the presence of an externally applied magnetic field. In contrast with this, diamagnetic materials are repulsive when placed in a magnetic field...
. Owing to the weak bonding between the oxygen atoms, peroxide easily undergoes homolytic cleavage
Homolysis
In general it means breakdown to equal pieces There are separate meanings for the word in chemistry and biology.-Homolysis in chemistry:...
yielding two highly reactive radicals. This cleavage is accelerated by temperature, illumination or chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
s.
Inorganic peroxides
Inorganic peroxides are divided into ionic peroxide salts and acidAcid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
peroxides, which contain covalently bonded peroxide units. The first class mostly contains the peroxides of the alkali
Alkali metal
The alkali metals are a series of chemical elements in the periodic table. In the modern IUPAC nomenclature, the alkali metals comprise the group 1 elements, along with hydrogen. The alkali metals are lithium , sodium , potassium , rubidium , caesium , and francium...
and alkaline earth metal
Alkaline earth metal
The alkaline earth metals are a group in the periodic table. In the modern IUPAC nomenclature, the alkaline earth metals are called the group 2 elements. Previously, they were called the Group IIA elements . The alkaline earth metals contain beryllium , magnesium , calcium , strontium , barium and...
s whereas the covalent peroxides are represented by such compounds as hydrogen peroxide and peroxymonosulfuric acid
Peroxymonosulfuric acid
Peroxymonosulfuric acid, also known as persulfuric acid, peroxysulfuric acid, or as Caro's acid, is H2SO5, a liquid at room temperature. In this acid, the S center adopts its characteristic tetrahedral geometry; the connectivity is indicated by the formula HO-O-S2-OH...
(H2SO5). In contrast to the purely ionic character of alkali metal peroxides, peroxides of transition metal
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
s have a more covalent character.
Synthesis of ionic peroxides
Alkali metal peroxides, with the exception of lithium peroxideLithium peroxide
Lithium peroxide is the inorganic compound with the formula Li2O2. This solid was deployed to remove CO2 from the atmosphere in the vehicles used in Apollo mission.-Preparation:...
can be synthesized directly by oxidation of the elements. Are shown with oxygen in the atmospheric pressure
Atmospheric pressure
Atmospheric pressure is the force per unit area exerted into a surface by the weight of air above that surface in the atmosphere of Earth . In most circumstances atmospheric pressure is closely approximated by the hydrostatic pressure caused by the weight of air above the measurement point...
. Lithium peroxide is synthesized by reacting lithium hydroxide
Lithium hydroxide
Lithium hydroxide is an inorganic compound with the formula LiOH. It is a white hygroscopic crystalline material. It is soluble in water and slightly soluble in ethanol...
with hydrogen peroxide:
The historical production of barium peroxide used oxidation of barium oxide
Barium oxide
Barium oxide, BaO, is a white hygroscopic compound formed by the burning of barium in oxygen, although it is often formed through the decomposition of other barium salts.It reacts with water to form barium hydroxide.-Uses:...
at elevated temperature and pressure.
Synthesis of covalent peroxides

Anthraquinone
Anthraquinone, also called anthracenedione or dioxoanthracene is an aromatic organic compound with formula . Several isomers are possible, each of which can be viewed as a quinone derivative...
process. There, anthraquinone
Anthraquinone
Anthraquinone, also called anthracenedione or dioxoanthracene is an aromatic organic compound with formula . Several isomers are possible, each of which can be viewed as a quinone derivative...
is first catalyzed by palladium
Palladium
Palladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired...
with molecular hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
. The resulting anthrahydroquinone is oxidized with molecular oxygen, reforming anthraquinone and releasing hydrogen peroxide. The overall reaction equation is
Direct synthesis of hydrogen peroxide from hydrogen and oxygen is rather inefficient and currently is not possible at industrial scale. Many peroxides of mineral acids, such as peroxodisulfate
Peroxodisulfate
The peroxodisulfate ion, S2O82−, is a sulfur oxoanion. It is commonly referred to as the persulfate ion, but this term also refers to the peroxomonosulfate ion, SO52−.-Compounds containing peroxodisulfate:* Na2S2O8* K2S2O8* 2S2O8...
s and percarbonates, can be obtained by anodic
Anode
An anode is an electrode through which electric current flows into a polarized electrical device. Mnemonic: ACID ....
oxidation of the respective acids. The anode material must be stable to the required high potentials of a few volts and therefore is either platinum or its alloys.
Peroxydisulfuric acid
Peroxydisulfuric acid
Peroxydisulfuric acid is a sulfur oxoacid with the chemical formula H2S2O8. It is also called Marshall's acid. In structural terms it can be written HO3SOOSO3H. It contains sulfur in its +6 oxidation state, but it also contains peroxide ions, which is why it appears to be in a higher oxidation...
was historically used for the production of hydrogen peroxide in a method developed in the early 20th century:
This process requires relatively high concentration of peroxydisulfuric acid as its more dilute solutions evolve oxygen gas instead of peroxide.
Properties
Few reactions are generally formulated for peroxide salt. In excess of dilute acids or water they release hydrogen peroxide.Upon heating, the reaction with water leads to the release of oxygen instead
The peroxide anion is a stronger nucleophile than hydroxide and displaces hydroxyl from oxyanions e.g. forming perborates and percarbonates. Sodium perborate
Sodium perborate
Sodium perborate is a white, odorless, water-soluble chemical compound with the chemical composition 3. It crystallizes as the monohydrate, NaBO3·H2O, trihydrate, NaBO3·3H2O and tetrahydrate, NaBO3·4H2O. The monohydrate and tetrahydrate are the commercially important forms...
and sodium percarbonate
Sodium percarbonate
Sodium percarbonate is a chemical, an adduct of sodium carbonate and hydrogen peroxide , with formula Na2CO3 · 1.5H2O2. It is a colorless, crystalline, hygroscopic and water-soluble solid...
are important consumer and industrial bleaching agents; they stabilize hydrogen peroxide and limit side reactions (e.g. reduction and decomposition note below). The peroxide anion displaces the oxygen in urea
Urea
Urea or carbamide is an organic compound with the chemical formula CO2. The molecule has two —NH2 groups joined by a carbonyl functional group....
to form carbamide peroxide
Carbamide peroxide
Carbamide peroxide, also called urea peroxide, urea hydrogen peroxide , and percarbamide, an adduct of hydrogen peroxide and urea. Like hydrogen peroxide, it is an oxidizer. This compound is a white crystalline solid which dissolves in water to give free hydrogen peroxide; the solubility of...
. Peroxide forms bidendate complexes such as chromium(VI) oxide peroxide. The reaction of hydrogen peroxide with aqueous titanium(IV) gives a brightly colored peroxy complex that is a useful test for titanium
Titanium
Titanium is a chemical element with the symbol Ti and atomic number 22. It has a low density and is a strong, lustrous, corrosion-resistant transition metal with a silver color....
as well as hydrogen peroxide, it is a transition metal dioxygen complex. Molybdate
Molybdate
In chemistry a molybdate is a compound containing an oxoanion with molybdenum in its highest oxidation state of 6. Molybdenum can form a very large range of such oxoanions which can be discrete structures or polymeric extended structures, although the latter are only found in the solid state.The...
reacts in alkaline media with peroxide to form red peroxomolybdate {Mo(O2)4}2–.
Hydrogen peroxide is both an oxidizing agent and reducing agent. The oxidation of hydrogen peroxide by sodium hypochlorite
Hypochlorite
The hypochlorite ion, also known as chlorate anion is ClO−. A hypochlorite compound is a chemical compound containing this group, with chlorine in oxidation state +1.Hypochlorites are the salts of hypochlorous acid...
yields singlet oxygen
Singlet oxygen
Singlet oxygen is the common name used for the diamagnetic form of molecular oxygen , which is less stable than the normal triplet oxygen. Because of its unusual properties, singlet oxygen can persist for over an hour at room temperature, depending on the environment...
. The net reaction of a ferric ion with hydrogen peroxide is a ferrous ion and oxygen. This proceeds via single electron oxidation and hydroxyl radicals. This is used in some organic chemistry oxidations, e.g. in the Fenton's reagent
Fenton's reagent
Fenton's reagent is a solution of hydrogen peroxide and an iron catalyst that is used to oxidize contaminants or waste waters. Fenton's reagent can be used to destroy organic compounds such as trichloroethylene and tetrachloroethylene ....
. Only catalytic quantities of iron ion is needed since peroxide also oxidizes ferrous to ferric ion. The net reaction of hydrogen peroxide
Hydrogen peroxide
Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
and permanganate
Permanganate
A permanganate is the general name for a chemical compound containing the manganate ion, . Because manganese is in the +7 oxidation state, the permanganate ion is a strong oxidizing agent. The ion has tetrahedral geometry...
or manganese dioxide is manganous ion; however, until the peroxide is spent some manganous ions are reoxidized to make the reaction catalytic. This forms the basis for common monopropellant
Monopropellant
Monopropellants are propellants composed of chemicals or mixtures of chemicals which can be stored in a single container with some degree of safety. While stable under defined storage conditions, they react very rapidly under certain other conditions to produce a large volume of energetic gases...
rockets.
Applications
Alkali metal peroxides can be used for the synthesis of organic peroxides. One example is the conversion of benzoyl chlorideBenzoyl chloride
Benzoyl chloride, also known as benzenecarbonyl chloride, is an organochlorine compound with the formula C6H5COCl. It is a colourless, fuming liquid with an irritating odour...
with sodium peroxide to dibenzoyl peroxide
Benzoyl peroxide
Benzoyl peroxide is an organic compound in the peroxide family. It consists of two benzoyl groups bridged by a peroxide link. Its structural formula is [C6H5C]2O2. It is one of the most important organic peroxides in terms of applications and the scale of its production...
.
Many inorganic peroxides are used for bleach
Bleach
Bleach refers to a number of chemicals that remove color, whiten, or disinfect, often via oxidation. Common chemical bleaches include household chlorine bleach , lye, oxygen bleach , and bleaching powder...
ing textiles and paper
Paper
Paper is a thin material mainly used for writing upon, printing upon, drawing or for packaging. It is produced by pressing together moist fibers, typically cellulose pulp derived from wood, rags or grasses, and drying them into flexible sheets....
and as a bleaching additive to detergents and cleaning products. The increasing environmental concerns resulted in the preference of peroxides over chlorine-based compounds and a sharp increase in the peroxide production. The past use of perborate
Sodium perborate
Sodium perborate is a white, odorless, water-soluble chemical compound with the chemical composition 3. It crystallizes as the monohydrate, NaBO3·H2O, trihydrate, NaBO3·3H2O and tetrahydrate, NaBO3·4H2O. The monohydrate and tetrahydrate are the commercially important forms...
s as additives to detergents and cleaning products has been largely replaced by percarbonates
Sodium percarbonate
Sodium percarbonate is a chemical, an adduct of sodium carbonate and hydrogen peroxide , with formula Na2CO3 · 1.5H2O2. It is a colorless, crystalline, hygroscopic and water-soluble solid...
in order to decrease the emission of boron to the environment. Sodium percarbonate is used in such products as OxiClean
OxiClean
-History:It was originally marketed through infomercials in the U.S. and Canada with Billy Mays, as a "miracle cleanser". Church and Dwight acquired the OxiClean brand through its acquisition of Orange Glo International in 2006. Endorsed by Mays until his death in 2009, the product is now seen...
and Tide laundry detergent
Tide (detergent)
Tide is the brand-name of a popular laundry detergent manufactured by Procter & Gamble and first introduced to the United States consumer in 1946. It is also marketed in Canada, Saudi Arabia, Morocco, India and several other countries...
. When dissolved in water, it releases hydrogen peroxide and soda ash (sodium carbonate):
- 2 Na2CO3·1.5H2O2 → 2 Na2CO3 + 3 H2O2
The use of peroxide compounds in detergents is often reflected in their trade names, for example Persil
Persil
Persil is a brand of laundry detergent currently and originally made by Henkel & Cie; but which is now also licensed for manufacture, distribution, and marketing in several countries by the Unilever Corporation. Henkel and Unilever both manufacture their own formulations...
is a combination of the words perborate and silicate.
Some peroxide salts release oxygen upon reaction with carbon dioxide. This reaction is used in regeneration of oxygen from exhaled carbon dioxide on submarine
Submarine
A submarine is a watercraft capable of independent operation below the surface of the water. It differs from a submersible, which has more limited underwater capability...
s and spaceships. Sodium or lithium peroxides are preferred in space applications because of their lower molar mass
Molar mass
Molar mass, symbol M, is a physical property of a given substance , namely its mass per amount of substance. The base SI unit for mass is the kilogram and that for amount of substance is the mole. Thus, the derived unit for molar mass is kg/mol...
and therefore higher oxygen yield per unit weight.
Barium peroxide has been historically used to produce pure oxygen from air. This process relies on the temperature-dependent chemical balance between barium oxide and peroxide: the reaction of barium oxide with air at 500 °C results in barium peroxide, which upon heating to above 700 °C in oxygen decomposes back to barium oxide releasing pure oxygen.
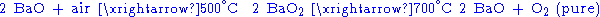
Organic peroxides
Organic peroxideOrganic peroxide
Organic peroxides are organic compounds containing the peroxide functional group . If the R' is hydrogen, the compound is called an organic hydroperoxide. Peresters have general structure RCOOR. The O-O bond easily breaks and forms free radicals of the form RO·...
s are divided into two major classes, peroxy acid
Peroxy acid
A peroxy acid is an acid which contains an acidic -OOH group. The two main classes are those derived from conventional mineral acids, especially sulfuric acid, and the organic derivatives of carboxylic acids...
s and organic hydroperoxides. The first class is derived from the carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
and the second from ether
Ether
Ethers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"...
s or alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s.
Synthesis
Most peroxy acids can be obtained by the reaction of hydrogen peroxide with the corresponding carboxylic acid:-

- R = organic group
Another synthetic route employs acyl halide
Acyl halide
An acyl halide is a chemical compound derived from an oxoacid by replacing a hydroxyl group with a halide group....
s instead of the carboxylic acid. It is used primarily with aromatic compounds in basic
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
in order to neutralize the resulting hydrogen chloride
Hydrogen chloride
The compound hydrogen chloride has the formula HCl. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric humidity. Hydrogen chloride gas and hydrochloric acid are important in technology and industry...
.
Aromatic aldehydes can be a auto-oxidized into peroxycarboxylic acids:
-

- Ar = arylArylIn the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
The products, however, react with the initial aldehyde forming the carboxylic acid:
Several synthesis routes are known for aliphatic
Aliphatic compound
In organic chemistry, aliphatic compounds are acyclic or cyclic, non-aromatic carbon compounds.Thus, aliphatic compounds are opposite to aromatic compounds.- Structure :...
peroxides, such as the reaction of dialkylsulfates with alkaline hydrogen peroxide solution. In this method, the alkyl sulfate donates the alkyl group and the sulfate ion forms the leaving group
Leaving group
In chemistry, a leaving group is a molecular fragment that departs with a pair of electrons in heterolytic bond cleavage. Leaving groups can be anions or neutral molecules. Common anionic leaving groups are halides such as Cl−, Br−, and I−, and sulfonate esters, such as para-toluenesulfonate...
.
This method can also yield cyclic peroxides. The four-membered dioxetane
Dioxetane
The chemical substance 1,2-dioxetane is an heterocyclic organic compound with formula C2O2H4, containing a ring of two adjacent oxygen atoms and two adjacent carbon atoms...
s can be obtained by 2+2 cycloaddition
Cycloaddition
A cycloaddition is a pericyclic chemical reaction, in which "two or more unsaturated molecules combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity." The resulting reaction is a cyclization reaction.Cycloadditions are usually described by the...
of oxygen to alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s.
The selective synthesis of hydroperoxides can be carried out by free-radical oxidation of alkanes with oxygen. Here the active site formed by a radical initiator
Radical initiator
In chemistry, radical initiators are substances that can produce radical species under mild conditions and promote radical reactions . These substances generally possess weak bonds—bonds that have small bond dissociation energies. Radical initiators are utilized in industrial processes such...
reacts with oxygen to form a hydroperoxyl. The addition of oxygen results in a more active radical which can further extract hydrogen atoms and release the hydroperoxide, leaving a new radical. This process is used industrially for the synthesis of phenol
Phenol
Phenol, also known as carbolic acid, phenic acid, is an organic compound with the chemical formula C6H5OH. It is a white crystalline solid. The molecule consists of a phenyl , bonded to a hydroxyl group. It is produced on a large scale as a precursor to many materials and useful compounds...
from benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
and is called the Cumene process
Cumene process
The Cumene process is an industrial process for developing phenol and acetone from benzene and propylene. The term stems from cumene , the intermediate material during the process. It was invented by Heinrich Hock in 1944 and independently by R. Ūdris and P...
or Hock process for its cumene
Cumene
Cumene is the common name for isopropylbenzene, an organic compound that is an aromatic hydrocarbon. It is a constituent of crude oil and refined fuels. It is a flammable colorless liquid that has a boiling point of 152 °C...
and cumene hydroperoxide
Cumene hydroperoxide
Cumene hydroperoxide is an intermediate in the cumene process for developing phenol and acetone from benzene and propylene. It is typically used as an oxidising agent. Products of decomposition of cumene hydroperoxide are methylstyrene, acetophenone and cumyl alcohol. Its formula is...
intermediates.
This auto-oxidation reaction can be used with common solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
s from the group of ether
Ether
Ethers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"...
s, such as diethyl ether
Diethyl ether
Diethyl ether, also known as ethyl ether, simply ether, or ethoxyethane, is an organic compound in the ether class with the formula . It is a colorless, highly volatile flammable liquid with a characteristic odor...
, diisopropyl ether
Diisopropyl ether
Diisopropyl ether is secondary ether that is used as a solvent. It is a colorless liquid that is slightly soluble in water, but miscible with most organic solvents...
, tetrahydrofuran
Tetrahydrofuran
Tetrahydrofuran is a colorless, water-miscible organic liquid with low viscosity at standard temperature and pressure. This heterocyclic compound has the chemical formula 4O. As one of the most polar ethers with a wide liquid range, it is a useful solvent. Its main use, however, is as a precursor...
or 1,4-dioxane
1,4-Dioxane
1,4-Dioxane, often called dioxane because the other isomers of dioxane are rare, is a heterocyclic organic compound. It is a colorless liquid with a faint sweet odor similar to that of diethyl ether. It is classified as an ether. This colorless liquid is mainly used as a stabilizer for the solvent...
. It yields a volatile hydroperoxide ether that upon heating can result in a serious explosion.
Peroxides are formed by living organisms through ene reaction
Ene reaction
The Ene reaction is a chemical reaction between an alkene with an allylic hydrogen and a compound containing a multiple bond , in order to form a new σ-bond with migration of the ene double bond and 1,5 hydrogen shift. The product is a substituted alkene with the double bond shifted to the...
s or Diels–Alder reactions between alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s and oxygen. Unsaturated fatty acids can serve as the olefinic substrates
Substrate (chemistry)
In chemistry, a substrate is the chemical species being observed, which reacts with a reagent. This term is highly context-dependent. In particular, in biochemistry, an enzyme substrate is the material upon which an enzyme acts....
for the ene reaction and unsaturated amino acids like histidine
Histidine
Histidine Histidine, an essential amino acid, has a positively charged imidazole functional group. It is one of the 22 proteinogenic amino acids. Its codons are CAU and CAC. Histidine was first isolated by German physician Albrecht Kossel in 1896. Histidine is an essential amino acid in humans...
can be the reactant for the Diels-Alder cyclization. Rancidification
Rancidification
Rancidification is the chemical decomposition of fats, oils and other lipids . When these processes occur in food, undesirable odors and flavors can result. In some cases, however, the flavors can be desirable . In processed meats, these flavors are collectively known as "warmed over flavor"...
(decomposition) of fats is partly caused by the formation of peroxides.
Properties and applications
Peroxycarboxylic acids are generally weaker acidAcid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
s than the parent carboxylic acids. Like most peroxides, they are strong oxidants and tend to explode at high concentrations and higher temperatures.
Organic peracids are used in the synthesis of epoxies
Epoxy
Epoxy, also known as polyepoxide, is a thermosetting polymer formed from reaction of an epoxide "resin" with polyamine "hardener". Epoxy has a wide range of applications, including fiber-reinforced plastic materials and general purpose adhesives....
via the Prilezhaev reaction
Epoxide
An epoxide is a cyclic ether with three ring atoms. This ring approximately defines an equilateral triangle, which makes it highly strained. The strained ring makes epoxides more reactive than other ethers. Simple epoxides are named from the parent compound ethylene oxide or oxirane, such as in...
. Another important application is the synthesis of lactone
Lactone
In chemistry, a lactone is a cyclic ester which can be seen as the condensation product of an alcohol group -OH and a carboxylic acid group -COOH in the same molecule...
s of cyclic ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s in the Baeyer–Villiger oxidation process. In both cases, electron-poor peroxycarboxylic acids are especially efficient, such as meta-chloroperoxybenzoic acid
Meta-Chloroperoxybenzoic acid
meta-Chloroperoxybenzoic acid is a peroxycarboxylic acid used widely as an oxidant in organic synthesis. mCPBA is often preferred to other peroxy acids because of its relative ease of handling...
(mCPBA).
Tert-butyl hydroperoxide
Tert-Butyl hydroperoxide
tert-Butyl hydroperoxide is an organic peroxide widely used in a variety of oxidation processes, for example Sharpless epoxidation...
is a common oxidant in the Sharpless epoxidation
Sharpless epoxidation
The Sharpless Epoxidation reaction is an enantioselective chemical reaction to prepare 2,3-epoxyalcohols from primary and secondary allylic alcohols....
, which is used for the stereoselective
Enantiomer
In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable , much as one's left and right hands are the same except for opposite orientation. It can be clearly understood if you try to place your hands one over the other without...
synthesis of epoxides. Karl Barry Sharpless was awarded the 2001 Nobel prize in Chemistry for this reaction.
Peracetic acid is a popular disinfectant in the medical field and food industry. Various peroxide solutions are commercially produced for the cleaning and disinfection of contact lens
Contact lens
A contact lens, or simply contact, is a lens placed on the eye. They are considered medical devices and can be worn to correct vision, for cosmetic or therapeutic reasons. In 2004, it was estimated that 125 million people use contact lenses worldwide, including 28 to 38 million in the United...
es.

Radical initiator
In chemistry, radical initiators are substances that can produce radical species under mild conditions and promote radical reactions . These substances generally possess weak bonds—bonds that have small bond dissociation energies. Radical initiators are utilized in industrial processes such...
both in the laboratory research and in the industry. Its weak peroxide bond can be easily cleaved yielding reactive benzoyl radicals, which assist polymerization
Polymerization
In polymer chemistry, polymerization is a process of reacting monomer molecules together in a chemical reaction to form three-dimensional networks or polymer chains...
of plastics like polyethylene
Polyethylene
Polyethylene or polythene is the most widely used plastic, with an annual production of approximately 80 million metric tons...
. One of the synthesis methods of the commercially important plastic caprolactam
Caprolactam
Caprolactam is an organic compound with the formula 5CNH. This colourless solid is a lactam or a cyclic amide of caproic acid. Approximately 2 billion kilograms are produced annually...
—the precursor to Nylon 6
Nylon 6
Nylon 6 or polycaprolactam is a polymer developed by Paul Schlack at IG Farben to reproduce the properties of nylon 6,6 without violating the patent on its production. Unlike most other nylons, nylon 6 is not a condensation polymer, but instead is formed by ring-opening polymerization. This makes...
(polycaprolactam)—is a Baeyer-Villiger rearrangement of cyclohexanone
Cyclohexanone
Cyclohexanone is the organic compound with the formula 5CO. The molecule consists of six-carbon cyclic molecule with a ketone functional group. This colorless oil has an odor reminiscent of peardrop sweets as well as acetone. Over time, samples assume a yellow color due to oxidation...
with peracetic acid. This yields caprolactone
Caprolactone
ε-Caprolactone or simply caprolactone is a cyclic ester, a member of the lactone family, with a seven-membered ring with the formula 5CO2. This colorless liquid is miscible with most organic solvents...
, which is then converted to caprolactam by reacting it with ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
.
Industrial resins based on acrylic and/or methacrylic acid
Methacrylic acid
Methacrylic acid, abbreviated MAA, is an organic compound. This colourless, viscous liquid is a carboxylic acid with an acrid unpleasant odor. It is soluble in warm water and miscible with most organic solvents. Methacrylic acid is produced industrially on a large scale as a precursor to its...
esters are invariably produced by radical polymerization with organic peroxides at elevated temperatures. The polymerization rate is adjusted by suitable choice of temperature and type of peroxide.
Some peroxides are drug
Drug
A drug, broadly speaking, is any substance that, when absorbed into the body of a living organism, alters normal bodily function. There is no single, precise definition, as there are different meanings in drug control law, government regulations, medicine, and colloquial usage.In pharmacology, a...
s, whose action is based on the formation of radicals at desired locations in the organism. For example, artemisinin
Artemisinin
Artemisinin , also known as Qinghaosu , and its derivatives are a group of drugs that possess the most rapid action of all current drugs against falciparum malaria. Treatments containing an artemisinin derivative are now standard treatment worldwide for falciparum malaria...
and its derivatives, such as such artesunate
Artesunate
Artesunate is part of the artemisinin group of drugs that treat malaria. It is a semi-synthetic derivative of artemisinin that is water-soluble and may therefore be given by injection...
, possess the most rapid action of all current drugs against falciparum
Plasmodium falciparum
Plasmodium falciparum is a protozoan parasite, one of the species of Plasmodium that cause malaria in humans. It is transmitted by the female Anopheles mosquito. Malaria caused by this species is the most dangerous form of malaria, with the highest rates of complications and mortality...
malaria
Malaria
Malaria is a mosquito-borne infectious disease of humans and other animals caused by eukaryotic protists of the genus Plasmodium. The disease results from the multiplication of Plasmodium parasites within red blood cells, causing symptoms that typically include fever and headache, in severe cases...
. Artesunate is also efficient in reducing egg production in Schistosoma haematobium
Schistosoma haematobium
Schistosoma haematobium is an important digenetic trematode, and is found in the Middle East, India, Portugal and Africa. It is a major agent of schistosomiasis; more specifically, it is associated with urinary schistosomiasis....
infection.
Many organic peroxides can initiate explosive polymerization in materials with unsaturated chemical bonds, and specifically triacetone triperoxide
Acetone peroxide
Acetone peroxide is an organic peroxide and a primary high explosive. It takes the form of a white crystalline powder with a distinctive bleach-like odor....
(TATP) and hexamethylene triperoxide diamine
Hexamethylene triperoxide diamine
Hexamethylene triperoxide diamine is ahigh explosive organic compound, first synthesised in 1885 by Legler. The theorised structure lent itself well to acting as an initiating, or primary explosive...
(HMTD) are powerful explosives. TATP is an inexpensive compound and is relatively easy to make. Whereas most other potent explosives, such as trinitrotoluene (TNT) or RDX
RDX
RDX, an initialism for Research Department Explosive, is an explosive nitroamine widely used in military and industrial applications. It was developed as an explosive which was more powerful than TNT, and it saw wide use in WWII. RDX is also known as cyclonite, hexogen , and T4...
(the major component of C4
C-4 (explosive)
C4 or Composition C4 is a common variety of the plastic explosive known as Composition C.-Composition and manufacture:C4 is made up of explosives, plastic binder, plasticizer and usually marker or odorizing taggant chemicals such as 2,3-dimethyl-2,3-dinitrobutane to help detect the explosive and...
mixtures), contain nitrogen, which is relatively easy to trace by sniffing techniques, TATP is nitrogen free and therefore is very difficult to detect by conventional screening methods. For this reason, it is an explosive favored by terrorists. TATP and HMTD were used in several executed or planned terrorist acts of the early 2000s, most notably in the 2001 shoe bomb plot and the 2005 London Underground bombings
7 July 2005 London bombings
The 7 July 2005 London bombings were a series of co-ordinated suicide attacks in the United Kingdom, targeting civilians using London's public transport system during the morning rush hour....
. Several detection devices have been designed since those events. One, for example, releases a chemical mixture which changes color when interacting with traces of TATP.
Laboratory identification
Several analytical methods are used for qualitative and quantitative determination of peroxides. A simple qualitative detection of peroxides is carried out with the iodine-starch reactionIodine test
The Iodine test is used to test for the presence of starch. Iodine solution — iodine dissolved in an aqueous solution of potassium iodide — reacts with the starch producing a purple black color. The colour can be detected visually with concentrations of iodine as low as 0.00002M at 20°C...
. Here peroxides, hydroperoxides or peracids oxidize the added potassium iodide
Potassium iodide
Potassium iodide is an inorganic compound with the chemical formula KI. This white salt is the most commercially significant iodide compound, with approximately 37,000 tons produced in 1985. It is less hygroscopic than sodium iodide, making it easier to work with...
into iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
, which reacts with starch
Starch
Starch or amylum is a carbohydrate consisting of a large number of glucose units joined together by glycosidic bonds. This polysaccharide is produced by all green plants as an energy store...
producing a deep-blue color. Commercial paper indicators using this reaction are available. This method is also suitable for quantitative evaluation, but it can not distinguish between different types of peroxide compounds. Discoloration of various indigo dye
Indigo dye
Indigo dye is an organic compound with a distinctive blue color . Historically, indigo was a natural dye extracted from plants, and this process was important economically because blue dyes were once rare. Nearly all indigo dye produced today — several thousand tons each year — is synthetic...
s in presence of peroxides is used instead for this purpose. For example, the loss of blue color in leuco-methylene blue
Methylene blue
Methylene blue is a heterocyclic aromatic chemical compound with the molecular formula C16H18N3SCl. It has many uses in a range of different fields, such as biology and chemistry. At room temperature it appears as a solid, odorless, dark green powder, that yields a blue solution when dissolved in...
is selective for hydrogen peroxide.
Quantitative analysis of hydroperoxides is performed using potentiometric titration
Titration
Titration, also known as titrimetry, is a common laboratory method of quantitative chemical analysis that is used to determine the unknown concentration of an identified analyte. Because volume measurements play a key role in titration, it is also known as volumetric analysis. A reagent, called the...
with lithium aluminium hydride
Lithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH or known as LithAl, is an inorganic compound with the chemical formula LiAlH4. It was discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters,...
. Another way to evaluate the content of peracids and peroxides is the volumetric titration with alkoxide
Alkoxide
An alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They can be written as RO−, where R is the organic substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands...
s such as sodium ethoxide
Sodium ethoxide
Sodium ethoxide is an alkoxide salt with the chemical formula C2H5ONa.-Preparation:It is commercially available as a white solid, or as a solution in ethanol. It is easily prepared in the laboratory by reacting sodium metal with ethanol:...
.
Safety
Organic peroxides can accidentally initiate explosive polymerization in materials with unsaturated chemical bonds. Most notably, TATP and HMTD are high explosivesExplosive material
An explosive material, also called an explosive, is a reactive substance that contains a great amount of potential energy that can produce an explosion if released suddenly, usually accompanied by the production of light, heat, sound, and pressure...
, and TATP, because of its high susceptibility to accidental detonation by shock, friction, or sparks, has earned the nickname "Mother of Satan" among certain Islamic militant groups. TATP can accidentally form as by-products in many commonly used reactions. These reactions range from synthesis of MDMA, where TATP is formed via isosafrole
Safrole
Safrole, also known as shikimol, is a phenylpropene. It is a colorless or slightly yellow oily liquid. It is typically extracted from the root-bark or the fruit of sassafras plants in the form of sassafras oil , or synthesized from other related methylenedioxy...
oxidation in acetone, to industrial production of phenol
Phenol
Phenol, also known as carbolic acid, phenic acid, is an organic compound with the chemical formula C6H5OH. It is a white crystalline solid. The molecule consists of a phenyl , bonded to a hydroxyl group. It is produced on a large scale as a precursor to many materials and useful compounds...
, where the second product of the cumene process
Cumene process
The Cumene process is an industrial process for developing phenol and acetone from benzene and propylene. The term stems from cumene , the intermediate material during the process. It was invented by Heinrich Hock in 1944 and independently by R. Ūdris and P...
, acetone
Acetone
Acetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
, is partially oxidized to peroxide on the second reaction step. Accidental preparation of organic peroxides can occur by mixing ketone solvents (most commonly acetone) with waste materials containing hydrogen peroxide or other oxidizers and leaving the mixture standing for several hours. In addition, many liquid ether
Ether
Ethers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"...
s in the presence of air, light and metals (which act as catalysts) slowly – over a period of months – form highly unstable ether peroxides such as diethyl ether peroxide
Diethyl ether peroxide
Diethyl ether peroxides are a class of organic peroxides that slowly form in diethyl ether upon storage under air, light, or in the presence of metal by autoxidation.- Diethyl ether hydroperoxide :...
. Therefore, ethers are often stored over potassium hydroxide, which not only destroys peroxides but also acts as a powerful desiccant
Desiccation
Desiccation is the state of extreme dryness, or the process of extreme drying. A desiccant is a hygroscopic substance that induces or sustains such a state in its local vicinity in a moderately sealed container.-Science:...
.
Peroxides are also strong oxidizers and easily react with skin, cotton and wood pulp. For safety reasons, peroxidic compounds are stored in a cool, opaque container, as heating and illumination accelerates their chemical reactions. Small amounts of peroxides, which emerge from storage or reaction vessels are neutralized using reducing agents such as iron(II) sulfate
Iron(II) sulfate
Iron sulfate or ferrous sulfate is the chemical compound with the formula FeSO4. Known since ancient times as copperas and as green vitriol, the blue-green heptahydrate is the most common form of this material...
. The safety measures in industrial plants producing large amounts of peroxides include the following. The equipment is located within reinforced concrete structures with foil windows, which would relieve pressure and not shatter in case of explosion. The products are bottled in small containers and are moved to a cold place promptly after the synthesis. The containers are made of non-reactive materials such as stainless steel, some aluminium alloys or dark glass.
See also
- Peroxidases
- Peroxide fusionPeroxide fusionPeroxide fusion is used to prepare samples for inductively coupled plasma , atomic absorption analysis and wet chemistry. Sodium peroxide is used to oxidize the sample that becomes soluble in a diluted acid solution...
- OzoneOzoneOzone , or trioxygen, is a triatomic molecule, consisting of three oxygen atoms. It is an allotrope of oxygen that is much less stable than the diatomic allotrope...
- OzonideOzonideOzonide is an unstable, reactive polyatomic anion O3−, derived from ozone, or an organic compound similar to organic peroxide formed by a reaction of ozone with an unsaturated compound.-Inorganic ozonides:...
, O3– - SuperoxideSuperoxideA superoxide, also known by the obsolete name hyperoxide, is a compound that possesses the superoxide anion with the chemical formula O2−. The systematic name of the anion is dioxide. It is important as the product of the one-electron reduction of dioxygen O2, which occurs widely in nature...
, O2– - DioxygenylDioxygenylThe dioxygenyl ion, O2+, is a rarely encountered oxycation in which both oxygen atoms have a formal oxidation state of +½. It is formally derived from oxygen by the removal of an electron:...
, O2+