
Rancidification
Encyclopedia
Rancidification is the chemical decomposition
of fat
s, oil
s and other lipid
s (this degradation also occurs in mechanical cutting fluid
s). When these processes occur in food, undesirable odors and flavors can result. In some cases, however, the flavors can be desirable (as in aged cheeses). In processed meats, these flavors are collectively known as "warmed over flavor
". Rancidification can also detract from the nutritional value of the food. Some vitamin
s are highly sensitive to degradation.
Hydrolytic
rancidity occurs when water splits fatty acid
chains away from the glycerol
backbone in triglyceride
s (fats). The chemical term is ester hydrolysis. Usually this hydrolysis process goes unnoticed, since most fatty acids are odorless and tasteless. When, however, the triglyceride is derived from short chain fatty acids, the released carboxylic acid can confer strong flavors and odors. A particular problem arises with butter, which contains triglycerides with a high content of butyric acid
derivatives.
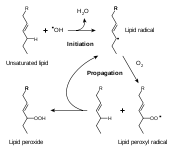
Oxidative rancidity is associated with the degradation by oxygen in the air. Via a free radical process, the double bond
s of an unsaturated fatty acid can undergo cleavage, releasing volatile aldehyde
s and ketone
s. This process can be suppressed by the exclusion of oxygen or by the addition of antioxidant
s. Oxidation primarily occurs with unsaturated fats.
Microbial
rancidity refers to a process in which microorganisms, such as bacteria, use their enzymes such as lipase
s to break down fat. This pathway can be prevented by sterilization.
 Antioxidant
Antioxidant
s are often added to fat-containing foods to delay the onset or slow the development of rancidity due to oxidation. Natural antioxidants include polyphenol
s (for instance flavonoids), ascorbic acid
(vitamin C) and tocopherol
s (vitamin E). Synthetic antioxidants include butylated hydroxyanisole
(BHA), butylated hydroxytoluene
(BHT), TBHQ, propyl gallate
and ethoxyquin. The natural antioxidants tend to be short-lived, so synthetic antioxidants are used when a longer shelf-life is preferred. The effectiveness of water-soluble antioxidants is limited in preventing direct oxidation within fats, but is valuable in intercepting free radical
s that travel through the watery parts of foods. A combination of water-soluble and fat-soluble antioxidants is ideal, usually in the ratio of fat to water.
In addition, rancidification can be decreased, but not completely eliminated, by storing fats and oils in a cool, dark place with little exposure to oxygen or free radicals, since heat and light accelerate the rate of reaction of fats with oxygen. The addition of antimicrobial agents can also delay or prevent rancidification by inhibiting the growth of bacteria or other micro-organisms.
The Rancimat Method is carried out using an air current at temperatures between 50 and 220 °C. The volatile oxidation products (largely formic acid
p.47) are carried by the air current into the measuring vessel, where they are absorbed (dissolve) in the measuring fluid (distilled water
). By continuous measurement of the conductivity of this solution, oxidation curves can be generated. The cusp point
of the oxidation curve (the point where a rapid rise in the conductivity starts) gives the induction time of the rancidification reaction,p.31 and can be taken as an indication of the oxidative stability of the sample.
The Rancimat method, the oxidative stability instrument (OSI) and the oxidograph were all developed as automatic versions of the more complicated AOM (active oxygen method), which is based on measuring peroxide valuesp.31, for determining the induction time of fats and oils. Over time, the rancimat method has become established, and it has been accepted into a number of national and international standards, for example AOCS Cd 12b-92 and ISO 6886.
Chemical decomposition
Chemical decomposition, analysis or breakdown is the separation of a chemical compound into elements or simpler compounds. It is sometimes defined as the exact opposite of a chemical synthesis. Chemical decomposition is often an undesired chemical reaction...
of fat
Fat
Fats consist of a wide group of compounds that are generally soluble in organic solvents and generally insoluble in water. Chemically, fats are triglycerides, triesters of glycerol and any of several fatty acids. Fats may be either solid or liquid at room temperature, depending on their structure...
s, oil
Oil
An oil is any substance that is liquid at ambient temperatures and does not mix with water but may mix with other oils and organic solvents. This general definition includes vegetable oils, volatile essential oils, petrochemical oils, and synthetic oils....
s and other lipid
Lipid
Lipids constitute a broad group of naturally occurring molecules that include fats, waxes, sterols, fat-soluble vitamins , monoglycerides, diglycerides, triglycerides, phospholipids, and others...
s (this degradation also occurs in mechanical cutting fluid
Cutting fluid
Cutting fluid is a type of coolant and lubricant designed specifically for metalworking and machining processes. There are various kinds of cutting fluids, which include oils, oil-water emulsions, pastes, gels, aerosols , and air or other gases. They may be made from petroleum distillates, animal...
s). When these processes occur in food, undesirable odors and flavors can result. In some cases, however, the flavors can be desirable (as in aged cheeses). In processed meats, these flavors are collectively known as "warmed over flavor
Warmed over flavor
Warmed-over flavor is an unpleasant flavor which develops in cooked meat which is subsequently refrigerated prior to reheating. As cooking and subsequent refrigeration is the case with most convenience foods containing meat, it is a significant challenge to the processed food industry...
". Rancidification can also detract from the nutritional value of the food. Some vitamin
Vitamin
A vitamin is an organic compound required as a nutrient in tiny amounts by an organism. In other words, an organic chemical compound is called a vitamin when it cannot be synthesized in sufficient quantities by an organism, and must be obtained from the diet. Thus, the term is conditional both on...
s are highly sensitive to degradation.
Rancidification pathways
Three pathways for rancidification are recognized.Hydrolytic
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
rancidity occurs when water splits fatty acid
Fatty acid
In chemistry, especially biochemistry, a fatty acid is a carboxylic acid with a long unbranched aliphatic tail , which is either saturated or unsaturated. Most naturally occurring fatty acids have a chain of an even number of carbon atoms, from 4 to 28. Fatty acids are usually derived from...
chains away from the glycerol
Glycerol
Glycerol is a simple polyol compound. It is a colorless, odorless, viscous liquid that is widely used in pharmaceutical formulations. Glycerol has three hydroxyl groups that are responsible for its solubility in water and its hygroscopic nature. The glycerol backbone is central to all lipids...
backbone in triglyceride
Triglyceride
A triglyceride is an ester derived from glycerol and three fatty acids. There are many triglycerides, depending on the oil source, some are highly unsaturated, some less so....
s (fats). The chemical term is ester hydrolysis. Usually this hydrolysis process goes unnoticed, since most fatty acids are odorless and tasteless. When, however, the triglyceride is derived from short chain fatty acids, the released carboxylic acid can confer strong flavors and odors. A particular problem arises with butter, which contains triglycerides with a high content of butyric acid
Butyric acid
Butyric acid , also known under the systematic name butanoic acid, is a carboxylic acid with the structural formula CH3CH2CH2-COOH. Salts and esters of butyric acid are known as butyrates or butanoates...
derivatives.
Oxidative rancidity is associated with the degradation by oxygen in the air. Via a free radical process, the double bond
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
s of an unsaturated fatty acid can undergo cleavage, releasing volatile aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s and ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s. This process can be suppressed by the exclusion of oxygen or by the addition of antioxidant
Antioxidant
An antioxidant is a molecule capable of inhibiting the oxidation of other molecules. Oxidation is a chemical reaction that transfers electrons or hydrogen from a substance to an oxidizing agent. Oxidation reactions can produce free radicals. In turn, these radicals can start chain reactions. When...
s. Oxidation primarily occurs with unsaturated fats.
Microbial
Microorganism
A microorganism or microbe is a microscopic organism that comprises either a single cell , cell clusters, or no cell at all...
rancidity refers to a process in which microorganisms, such as bacteria, use their enzymes such as lipase
Lipase
A lipase is an enzyme that catalyzes the formation or cleavage of fats . Lipases are a subclass of the esterases.Lipases perform essential roles in the digestion, transport and processing of dietary lipids in most, if not all, living organisms...
s to break down fat. This pathway can be prevented by sterilization.
Role of antioxidants

Antioxidant
An antioxidant is a molecule capable of inhibiting the oxidation of other molecules. Oxidation is a chemical reaction that transfers electrons or hydrogen from a substance to an oxidizing agent. Oxidation reactions can produce free radicals. In turn, these radicals can start chain reactions. When...
s are often added to fat-containing foods to delay the onset or slow the development of rancidity due to oxidation. Natural antioxidants include polyphenol
Polyphenol antioxidant
A polyphenol antioxidant is a type of antioxidant containing a polyphenolic substructure. Numbering over 4,000 distinct species, many of these compounds have antioxidant activity in vitro but are unlikely to have antioxidant roles in vivo...
s (for instance flavonoids), ascorbic acid
Ascorbic acid
Ascorbic acid is a naturally occurring organic compound with antioxidant properties. It is a white solid, but impure samples can appear yellowish. It dissolves well in water to give mildly acidic solutions. Ascorbic acid is one form of vitamin C. The name is derived from a- and scorbutus , the...
(vitamin C) and tocopherol
Tocopherol
Tocopherols are a class of chemical compounds of which many have vitamin E activity. It is a series of organic compounds consisting of various methylated phenols...
s (vitamin E). Synthetic antioxidants include butylated hydroxyanisole
Butylated hydroxyanisole
Butylated hydroxyanisole is an antioxidant consisting of a mixture of two isomeric organic compounds, 2-tert-butyl-4-hydroxyanisole and 3-tert-butyl-4-hydroxyanisole. It is prepared from 4-methoxyphenol and isobutylene. It is a waxy solid used as a food additive with the E number E320...
(BHA), butylated hydroxytoluene
Butylated hydroxytoluene
Butylated hydroxytoluene , also known as butylhydroxytoluene, is a lipophilic organic compound that is primarily used as an antioxidant food additive as well as an antioxidant additive in cosmetics, pharmaceuticals, jet fuels, rubber, petroleum products, electrical transformer oil, and embalming...
(BHT), TBHQ, propyl gallate
Propyl gallate
Propyl gallate, or propyl 3,4,5-trihydroxybenzoate is an ester formed by the condensation of gallic acid and propanol. Since 1948, this antioxidant has been added to foods containing oils and fats to prevent oxidation. As a food additive, it is used under the E number E310.-Description:Propyl...
and ethoxyquin. The natural antioxidants tend to be short-lived, so synthetic antioxidants are used when a longer shelf-life is preferred. The effectiveness of water-soluble antioxidants is limited in preventing direct oxidation within fats, but is valuable in intercepting free radical
Radical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
s that travel through the watery parts of foods. A combination of water-soluble and fat-soluble antioxidants is ideal, usually in the ratio of fat to water.
In addition, rancidification can be decreased, but not completely eliminated, by storing fats and oils in a cool, dark place with little exposure to oxygen or free radicals, since heat and light accelerate the rate of reaction of fats with oxygen. The addition of antimicrobial agents can also delay or prevent rancidification by inhibiting the growth of bacteria or other micro-organisms.
Measurement of oxidative stability
Oxidative stability is a measure of an oil or fat's resistance to oxidation. Because the process takes place through a chain reaction, the oxidation reaction has a period when it is relatively slow, before it suddenly speeds up. The time for this to happen is called the "induction time", and it is repeatable under identical conditions (temperature, air flow, etc.). There are a number of ways to measure the progress of the oxidation reaction. One of the most popular methods currently in use is the Rancimat method.The Rancimat Method is carried out using an air current at temperatures between 50 and 220 °C. The volatile oxidation products (largely formic acid
Formic acid
Formic acid is the simplest carboxylic acid. Its chemical formula is HCOOH or HCO2H. It is an important intermediate in chemical synthesis and occurs naturally, most notably in the venom of bee and ant stings. In fact, its name comes from the Latin word for ant, formica, referring to its early...
p.47) are carried by the air current into the measuring vessel, where they are absorbed (dissolve) in the measuring fluid (distilled water
Distilled water
Distilled water is water that has many of its impurities removed through distillation. Distillation involves boiling the water and then condensing the steam into a clean container.-History:...
). By continuous measurement of the conductivity of this solution, oxidation curves can be generated. The cusp point
Cusp (singularity)
In the mathematical theory of singularities a cusp is a type of singular point of a curve. Cusps are local singularities in that they are not formed by self intersection points of the curve....
of the oxidation curve (the point where a rapid rise in the conductivity starts) gives the induction time of the rancidification reaction,p.31 and can be taken as an indication of the oxidative stability of the sample.
The Rancimat method, the oxidative stability instrument (OSI) and the oxidograph were all developed as automatic versions of the more complicated AOM (active oxygen method), which is based on measuring peroxide valuesp.31, for determining the induction time of fats and oils. Over time, the rancimat method has become established, and it has been accepted into a number of national and international standards, for example AOCS Cd 12b-92 and ISO 6886.
See also
- FermentationFermentation (food)Fermentation in food processing typically is the conversion of carbohydrates to alcohols and carbon dioxide or organic acids using yeasts, bacteria, or a combination thereof, under anaerobic conditions. Fermentation in simple terms is the chemical conversion of sugars into ethanol...
- Food preservationFood preservationFood preservation is the process of treating and handling food to stop or slow down spoilage and thus allow for longer storage....
- PreservativePreservativeA preservative is a naturally occurring or synthetically produced substance that is added to products such as foods, pharmaceuticals, paints, biological samples, wood, etc. to prevent decomposition by microbial growth or by undesirable chemical changes....
- PutrefactionPutrefactionPutrefaction is one of seven stages in the decomposition of the body of a dead animal. It can be viewed, in broad terms, as the decomposition of proteins, in a process that results in the eventual breakdown of cohesion between tissues and the liquefaction of most organs.-Description:In terms of...
- Lipid peroxidationLipid peroxidationLipid peroxidation refers to the oxidative degradation of lipids. It is the process in which free radicals "steal" electrons from the lipids in cell membranes, resulting in cell damage. This process proceeds by a free radical chain reaction mechanism...

