Singlet oxygen
Encyclopedia
Singlet oxygen is the common name used for the diamagnetic form of molecular oxygen
(O2), which is less stable than the normal triplet oxygen
. Because of its unusual properties, singlet oxygen can persist for over an hour at room temperature, depending on the environment. Because of differences in their electron shells, singlet and triplet oxygen differ in their chemical properties. Singlet oxygen is in the same quantum state as most molecules and thus reacts readily with them, thus making singlet oxygen highly reactive.
Singlet oxygen is usually generated with a photosensitizer pigment. The damaging effects of sunlight on many organic materials (polymers, etc.) are often attributed to the effects of singlet oxygen. In photodynamic therapy
, singlet oxygen is produced to kill cancer cells.
s. It can be generated in a photosensitized process by energy transfer from dye molecules such as rose bengal
, methylene blue
or porphyrins, or by chemical processes such as spontaneous decomposition of hydrogen trioxide in water or the reaction of hydrogen peroxide
with hypochlorite
. Singlet oxygen reacts with an alkene -C=C-CH- by abstraction of the allylic proton in an ene reaction
type reaction to the allyl hydroperoxide HO-O-R (R = alkyl), which can then be reduced to the allyl alcohol
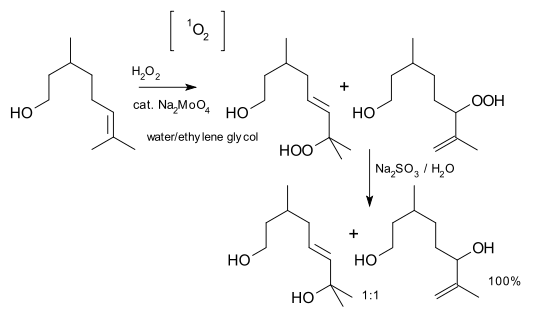
.This reaction is not actually an ene reaction, because it isn't concerted: singlet oxygen forms an exciplex that can be called an "epoxide oxide", which then abstracts the hydrogen. An example is an oxygenation of citronellol
:reagent: hydrogen peroxide
, catalyst: sodium molybdate
, reducing agent: sodium sulfite
}}
With some substrates 1,2-dioxetanes are formed and cyclic dienes such as 1,3-cyclohexadiene
form [4+2]cycloaddition
adducts.
, singlet oxygen can be produced from the light-harvesting chlorophyll
molecules. One of the roles of carotenoid
s in photosynthetic systems is to prevent damage caused by produced singlet oxygen by either removing excess light
energy from chlorophyll
molecules or quenching the singlet oxygen molecules directly.
In mammal
ian biology
, singlet oxygen is one of the reactive oxygen species
, which is linked to oxidation of LDL cholesterol
and resultant cardiovascular effects. Polyphenol antioxidant
s can scavenge and reduce concentrations of reactive oxygen species and may prevent such deleterious oxidative effects.
Ingestion of pigments capable of producing singlet oxygen with activation by light can produce severe photosensitivity
of skin. This is especially a concern in herbivorous animals (see Photosensitivity in animals
).
Singlet oxygen is the active species in photodynamic therapy
.
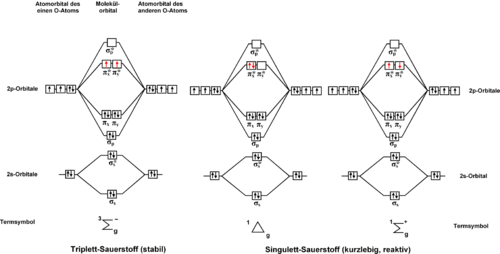
predicts two low-lying excited singlet states O2(a¹Δg) and O2(b¹Σg+) (for nomenclature see article on Molecular term symbol
). These electronic states differ only in the spin and the occupancy of oxygen's two degenerate antibonding
πg-orbitals (see degenerate energy level
). The O2(b¹Σg+)-state is very short lived and relaxes quickly to the lowest lying excited state
, O2(a¹Δg). Thus, the O2(a¹Δg)-state is commonly referred to as singlet oxygen. The energy difference between the lowest energy of O2 in the singlet state and the lowest energy in the triplet state is about 11340 kelvin (Te (a¹Δg <- X³Σg-) = 7882 cm−1, 94.3 kJ/mol, 0.98 eV) Molecular oxygen
differs from most molecules in having an open-shell triplet ground state, O2(X³Σg-).
Although the three lowest energy states of oxygen can be described by the simple scheme in the figure below, this is a simplification. The excited states of oxygen are made up of combinations of electronic states. The second excited state involves states with the highest energy electrons paired in the same orbital, while the first excited state involves states with the electrons in separate degenerate orbitals, as might be expected from Hund's rule.
 The energy difference between ground state and singlet oxygen is 94.3 kJ/mol and corresponds to a transition in the near-infrared
The energy difference between ground state and singlet oxygen is 94.3 kJ/mol and corresponds to a transition in the near-infrared
at ~1270 nm. In the isolated molecule, the transition is strictly forbidden by spin, symmetry and parity selection rules, making it one of nature's most forbidden transitions. In other words, direct excitation of ground state oxygen by light to form singlet oxygen is very improbable. As a consequence, singlet oxygen in the gas phase is extremely long lived (72 minutes). Interaction with solvents, however, reduces the lifetime to microseconds or even nanoseconds.
Direct detection of singlet oxygen is possible using sensitive laser spectroscopy or through its extremely weak phosphorescence
at 1270 nm, which is not visible to the eye. However, at high singlet oxygen concentrations, the fluorescence
of the so-called singlet oxygen dimol (simultaneous emission from two singlet oxygen molecules upon collision) can be observed as a red glow at 634 nm.
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
(O2), which is less stable than the normal triplet oxygen
Triplet oxygen
Triplet oxygen is the ground state of the oxygen molecule. The electron configuration of the molecule has two unpaired electrons occupying two degenerate molecular orbitals...
. Because of its unusual properties, singlet oxygen can persist for over an hour at room temperature, depending on the environment. Because of differences in their electron shells, singlet and triplet oxygen differ in their chemical properties. Singlet oxygen is in the same quantum state as most molecules and thus reacts readily with them, thus making singlet oxygen highly reactive.
Singlet oxygen is usually generated with a photosensitizer pigment. The damaging effects of sunlight on many organic materials (polymers, etc.) are often attributed to the effects of singlet oxygen. In photodynamic therapy
Photodynamic therapy
Photodynamic therapy is used clinically to treat a wide range of medical conditions, including malignant cancers, and is recognised as a treatment strategy which is both minimally invasive and minimally toxic...
, singlet oxygen is produced to kill cancer cells.
Organic chemistry
The chemistry of singlet oxygen is different from that of ground state oxygen. For example, singlet oxygen can participate in Diels-Alder reactions and ene reactionEne reaction
The Ene reaction is a chemical reaction between an alkene with an allylic hydrogen and a compound containing a multiple bond , in order to form a new σ-bond with migration of the ene double bond and 1,5 hydrogen shift. The product is a substituted alkene with the double bond shifted to the...
s. It can be generated in a photosensitized process by energy transfer from dye molecules such as rose bengal
Rose bengal
Rose Bengal is a stain. Its sodium salt is commonly used in eye drops to stain damaged conjunctival and corneal cells and thereby identify damage to the eye...
, methylene blue
Methylene blue
Methylene blue is a heterocyclic aromatic chemical compound with the molecular formula C16H18N3SCl. It has many uses in a range of different fields, such as biology and chemistry. At room temperature it appears as a solid, odorless, dark green powder, that yields a blue solution when dissolved in...
or porphyrins, or by chemical processes such as spontaneous decomposition of hydrogen trioxide in water or the reaction of hydrogen peroxide
Hydrogen peroxide
Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
with hypochlorite
Hypochlorite
The hypochlorite ion, also known as chlorate anion is ClO−. A hypochlorite compound is a chemical compound containing this group, with chlorine in oxidation state +1.Hypochlorites are the salts of hypochlorous acid...
. Singlet oxygen reacts with an alkene -C=C-CH- by abstraction of the allylic proton in an ene reaction
Ene reaction
The Ene reaction is a chemical reaction between an alkene with an allylic hydrogen and a compound containing a multiple bond , in order to form a new σ-bond with migration of the ene double bond and 1,5 hydrogen shift. The product is a substituted alkene with the double bond shifted to the...
type reaction to the allyl hydroperoxide HO-O-R (R = alkyl), which can then be reduced to the allyl alcohol
Allyl alcohol
Allyl alcohol is an organic compound with the structural formula CH2=CHCH2OH. Like many alcohols,it is a water soluble, colourless liquid, but it is more toxic than typical small alcohols. Allyl alcohol is used as a raw material for the production of glycerol, but is used as a precursor to many...
.This reaction is not actually an ene reaction, because it isn't concerted: singlet oxygen forms an exciplex that can be called an "epoxide oxide", which then abstracts the hydrogen. An example is an oxygenation of citronellol
Citronellol
Citronellol, or dihydrogeraniol, is a natural acyclic monoterpenoid. Both enantiomers occur in nature. -Citronellol, which is found in citronella oils, including Cymbopogon nardus , is the more common isomer. -Citronellol is found in the oils of rose and Pelargonium geraniums.Citronellol is...
:reagent: hydrogen peroxide
Hydrogen peroxide
Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
, catalyst: sodium molybdate
Sodium molybdate
Sodium molybdate, Na2MoO4, is useful as a source of molybdenum. It is often found as the dihydrate, Na2MoO4·2H2O.The molybdate anion is tetrahedral. Two sodium cations coordinate with every one anion.-History:...
, reducing agent: sodium sulfite
Sodium sulfite
Sodium sulfite is a soluble sodium salt of sulfurous acid. It is a product of sulfur dioxide scrubbing, a part of the flue gas desulfurization process...
}}
With some substrates 1,2-dioxetanes are formed and cyclic dienes such as 1,3-cyclohexadiene
1,3-Cyclohexadiene
1,3-Cyclohexadiene is a highly flammable cycloalkene that occurs as a colorless clear liquid. Its refractive index is 1.475 .It can be used as a hydrogen donor in transfer hydrogenation, since its conversion to benzene + hydrogen is in fact exothermic .Despite this apparent instability with respect...
form [4+2]cycloaddition
Cycloaddition
A cycloaddition is a pericyclic chemical reaction, in which "two or more unsaturated molecules combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity." The resulting reaction is a cyclization reaction.Cycloadditions are usually described by the...
adducts.
Biochemistry
In photosynthesisPhotosynthesis
Photosynthesis is a chemical process that converts carbon dioxide into organic compounds, especially sugars, using the energy from sunlight. Photosynthesis occurs in plants, algae, and many species of bacteria, but not in archaea. Photosynthetic organisms are called photoautotrophs, since they can...
, singlet oxygen can be produced from the light-harvesting chlorophyll
Chlorophyll
Chlorophyll is a green pigment found in almost all plants, algae, and cyanobacteria. Its name is derived from the Greek words χλωρος, chloros and φύλλον, phyllon . Chlorophyll is an extremely important biomolecule, critical in photosynthesis, which allows plants to obtain energy from light...
molecules. One of the roles of carotenoid
Carotenoid
Carotenoids are tetraterpenoid organic pigments that are naturally occurring in the chloroplasts and chromoplasts of plants and some other photosynthetic organisms like algae, some bacteria, and some types of fungus. Carotenoids can be synthesized fats and other basic organic metabolic building...
s in photosynthetic systems is to prevent damage caused by produced singlet oxygen by either removing excess light
Light
Light or visible light is electromagnetic radiation that is visible to the human eye, and is responsible for the sense of sight. Visible light has wavelength in a range from about 380 nanometres to about 740 nm, with a frequency range of about 405 THz to 790 THz...
energy from chlorophyll
Chlorophyll
Chlorophyll is a green pigment found in almost all plants, algae, and cyanobacteria. Its name is derived from the Greek words χλωρος, chloros and φύλλον, phyllon . Chlorophyll is an extremely important biomolecule, critical in photosynthesis, which allows plants to obtain energy from light...
molecules or quenching the singlet oxygen molecules directly.
In mammal
Mammal
Mammals are members of a class of air-breathing vertebrate animals characterised by the possession of endothermy, hair, three middle ear bones, and mammary glands functional in mothers with young...
ian biology
Biology
Biology is a natural science concerned with the study of life and living organisms, including their structure, function, growth, origin, evolution, distribution, and taxonomy. Biology is a vast subject containing many subdivisions, topics, and disciplines...
, singlet oxygen is one of the reactive oxygen species
Reactive oxygen species
Reactive oxygen species are chemically reactive molecules containing oxygen. Examples include oxygen ions and peroxides. Reactive oxygen species are highly reactive due to the presence of unpaired valence shell electrons....
, which is linked to oxidation of LDL cholesterol
Cholesterol
Cholesterol is a complex isoprenoid. Specifically, it is a waxy steroid of fat that is produced in the liver or intestines. It is used to produce hormones and cell membranes and is transported in the blood plasma of all mammals. It is an essential structural component of mammalian cell membranes...
and resultant cardiovascular effects. Polyphenol antioxidant
Polyphenol antioxidant
A polyphenol antioxidant is a type of antioxidant containing a polyphenolic substructure. Numbering over 4,000 distinct species, many of these compounds have antioxidant activity in vitro but are unlikely to have antioxidant roles in vivo...
s can scavenge and reduce concentrations of reactive oxygen species and may prevent such deleterious oxidative effects.
Ingestion of pigments capable of producing singlet oxygen with activation by light can produce severe photosensitivity
Photosensitivity
Photosensitivity is the amount to which an object reacts upon receiving photons, especially visible light.- Human medicine :Sensitivity of the skin to a light source can take various forms. People with particular skin types are more sensitive to sunburn...
of skin. This is especially a concern in herbivorous animals (see Photosensitivity in animals
Photosensitivity in animals
Photosensitivity is an abnormal skin reaction to direct sunlight exposure. It is unrelated to a sunburn. These reactions are due to photosensitization, the accumulation of photosensitive compounds beneath the skin. In some cases, the photodynamic substances come from ingested plants or drugs, after...
).
Singlet oxygen is the active species in photodynamic therapy
Photodynamic therapy
Photodynamic therapy is used clinically to treat a wide range of medical conditions, including malignant cancers, and is recognised as a treatment strategy which is both minimally invasive and minimally toxic...
.
Orbital states
Molecular orbital theoryMolecular orbital theory
In chemistry, molecular orbital theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms, but are treated as moving under the influence of the nuclei in the whole molecule...
predicts two low-lying excited singlet states O2(a¹Δg) and O2(b¹Σg+) (for nomenclature see article on Molecular term symbol
Molecular term symbol
In molecular physics, the molecular term symbol is a shorthand expression of the group representation and angular momenta that characterize the state of a molecule, i.e. its electronic quantum state which is an eigenstate of the electronic molecular Hamiltonian. It is the equivalent of the term...
). These electronic states differ only in the spin and the occupancy of oxygen's two degenerate antibonding
Antibonding
Antibonding is a type of chemical bonding. An antibonding orbital is a form of molecular orbital that is located outside the region of two distinct nuclei...
πg-orbitals (see degenerate energy level
Degenerate energy level
In physics, two or more different quantum states are said to be degenerate if they are all at the same energy level. Statistically this means that they are all equally probable of being filled, and in Quantum Mechanics it is represented mathematically by the Hamiltonian for the system having more...
). The O2(b¹Σg+)-state is very short lived and relaxes quickly to the lowest lying excited state
Excited state
Excitation is an elevation in energy level above an arbitrary baseline energy state. In physics there is a specific technical definition for energy level which is often associated with an atom being excited to an excited state....
, O2(a¹Δg). Thus, the O2(a¹Δg)-state is commonly referred to as singlet oxygen. The energy difference between the lowest energy of O2 in the singlet state and the lowest energy in the triplet state is about 11340 kelvin (Te (a¹Δg <- X³Σg-) = 7882 cm−1, 94.3 kJ/mol, 0.98 eV) Molecular oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
differs from most molecules in having an open-shell triplet ground state, O2(X³Σg-).
Although the three lowest energy states of oxygen can be described by the simple scheme in the figure below, this is a simplification. The excited states of oxygen are made up of combinations of electronic states. The second excited state involves states with the highest energy electrons paired in the same orbital, while the first excited state involves states with the electrons in separate degenerate orbitals, as might be expected from Hund's rule.
Chemistry

Infrared
Infrared light is electromagnetic radiation with a wavelength longer than that of visible light, measured from the nominal edge of visible red light at 0.74 micrometres , and extending conventionally to 300 µm...
at ~1270 nm. In the isolated molecule, the transition is strictly forbidden by spin, symmetry and parity selection rules, making it one of nature's most forbidden transitions. In other words, direct excitation of ground state oxygen by light to form singlet oxygen is very improbable. As a consequence, singlet oxygen in the gas phase is extremely long lived (72 minutes). Interaction with solvents, however, reduces the lifetime to microseconds or even nanoseconds.
Direct detection of singlet oxygen is possible using sensitive laser spectroscopy or through its extremely weak phosphorescence
Phosphorescence
Phosphorescence is a specific type of photoluminescence related to fluorescence. Unlike fluorescence, a phosphorescent material does not immediately re-emit the radiation it absorbs. The slower time scales of the re-emission are associated with "forbidden" energy state transitions in quantum...
at 1270 nm, which is not visible to the eye. However, at high singlet oxygen concentrations, the fluorescence
Fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation of a different wavelength. It is a form of luminescence. In most cases, emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation...
of the so-called singlet oxygen dimol (simultaneous emission from two singlet oxygen molecules upon collision) can be observed as a red glow at 634 nm.