
Leaving group
Encyclopedia
In chemistry
, a leaving group is a molecular fragment that departs with a pair of electrons in heterolytic bond cleavage. Leaving groups can be anions or neutral molecules. Common anionic leaving groups are halides such as Cl−, Br−, and I−, and sulfonate
esters, such as para-toluenesulfonate
("tosylate", TsO−). Common neutral molecule leaving groups are water (H2O), ammonia (NH3), and alcohols (ROH)
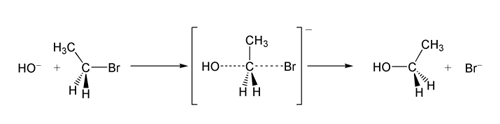 The ability of a leaving group to depart is correlated with the pKa
The ability of a leaving group to depart is correlated with the pKa
of the conjugate acid
, with lower pKa being associated with better leaving group ability. The correlation is not perfect because leaving group ability is a kinetic phenomenon, relating to a reaction's rate, whereas pKa is a thermodynamic phenomenon, describing the position of an equilibrium. Nevertheless, it is a general rule that more highly stabilized anions act as better leaving groups. Consistent with this rule, strong bases such as alkoxide (RO−), hydroxide (HO−), and amide (R2N−) are poor leaving groups.
It is uncommon for groups such as H- (hydride
s), R3C- (alkyl anions, R=alkyl or H), or R2N- (amides, R=alkyl or H) to depart with a pair of electrons because of the instability of these bases.
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
, a leaving group is a molecular fragment that departs with a pair of electrons in heterolytic bond cleavage. Leaving groups can be anions or neutral molecules. Common anionic leaving groups are halides such as Cl−, Br−, and I−, and sulfonate
Sulfonate
A sulfonate is a salt or ester of a sulfonic acid. It contains the functional group R-SO2O-.- Sulfonate salts:Anions with the general formula RSO2O− are called sulfonates. They are the conjugate bases of sulfonic acids with formula RSO2OH. As sulfonic acids tend to be strong acids, the...
esters, such as para-toluenesulfonate
Tosyl
A tosyl group is CH3C6H4SO2. This group is usually derived from the compound 4-toluenesulfonyl chloride, CH3C6H4SO2Cl, which forms esters and amides of toluenesulfonic or tosylic acid...
("tosylate", TsO−). Common neutral molecule leaving groups are water (H2O), ammonia (NH3), and alcohols (ROH)

Acid dissociation constant
An acid dissociation constant, Ka, is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction known as dissociation in the context of acid-base reactions...
of the conjugate acid
Conjugate acid
Within the Brønsted–Lowry acid-base theory , a conjugate acid is the acid member, HX, of a pair of two compounds that transform into each other by gain or loss of a proton. A conjugate acid can also be seen as the chemical substance that releases, or donates, a proton in the forward chemical...
, with lower pKa being associated with better leaving group ability. The correlation is not perfect because leaving group ability is a kinetic phenomenon, relating to a reaction's rate, whereas pKa is a thermodynamic phenomenon, describing the position of an equilibrium. Nevertheless, it is a general rule that more highly stabilized anions act as better leaving groups. Consistent with this rule, strong bases such as alkoxide (RO−), hydroxide (HO−), and amide (R2N−) are poor leaving groups.
| Leaving groups ordered approximately in decreasing ability to leave | |
|---|---|
| *R-N2+ | diazonium salts |
| R-OR'2+ | oxonium ion Oxonium ion The oxonium ion in chemistry is any oxygen cation with three bonds. The simplest oxonium ion is the hydronium ion H3O+. Another oxonium ion frequently encountered in organic chemistry is obtained by protonation or alkylation of a carbonyl group e.g... s |
| R-OSO2C4F9 | nonaflates |
| R-OSO2CF3 | triflates |
| R-OSO2F | fluorosulfonates |
| R-OTs, R-OMs, etc. | tosylates Tosyl A tosyl group is CH3C6H4SO2. This group is usually derived from the compound 4-toluenesulfonyl chloride, CH3C6H4SO2Cl, which forms esters and amides of toluenesulfonic or tosylic acid... , mesylate Mesylate In chemistry, a mesylate is any salt or ester of methanesulfonic acid . In salts, the mesylate is present as the CH3SO3− anion. When modifying the International Nonproprietary Name of a pharmaceutical substance containing the group or anion, the correct spelling is mesilate .Mesylate esters are a... s, and similar |
| R-I | iodides |
| R-Br | bromides |
| R-OH2+ | (Conjugate acid of an alcohol) |
| R-Cl | chlorides, and acyl chloride Acyl chloride In organic chemistry, an acyl chloride is an organic compound with the functional group -CO-Cl. Their formula is usually written RCOCl, where R is a side chain. They are usually considered to be reactive derivatives of carboxylic acids. A specific example of an acyl chloride is acetyl chloride,... when attached to carbonyl carbon |
| R-OHR'+ | Conjugate acid of an ether |
| R-ONO2, R-OPO(OH)2 | nitrates, phosphates, and other inorganic esters |
| R-SR'2+ | |
| R-NR'3+ | tetraalkylammonium Salts Quaternary ammonium cation Quaternary ammonium cations, also known as quats, are positively charged polyatomic ions of the structure NR4+, R being an alkyl group or an aryl group. Unlike the ammonium ion and the primary, secondary, or tertiary ammonium cations, the quaternary ammonium cations are permanently charged,... |
| R-F | fluorides |
| R-OCOR | esters, and acid anhydrides when attached to carbonyl carbon |
| R-NH3+ | ammonium salts Ammonium The ammonium cation is a positively charged polyatomic cation with the chemical formula NH. It is formed by the protonation of ammonia... |
| R-OAr | phenoxides Phenol Phenol, also known as carbolic acid, phenic acid, is an organic compound with the chemical formula C6H5OH. It is a white crystalline solid. The molecule consists of a phenyl , bonded to a hydroxyl group. It is produced on a large scale as a precursor to many materials and useful compounds... |
| R-OH | alcohol Alcohol In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms.... s, and carboxylic acids when attached to carbonyl carbon |
| R-OR | ethers, and esters when attached to carbonyl carbon |
It is uncommon for groups such as H- (hydride
Hydride
In chemistry, a hydride is the anion of hydrogen, H−, or, more commonly, a compound in which one or more hydrogen centres have nucleophilic, reducing, or basic properties. In compounds that are regarded as hydrides, hydrogen is bonded to a more electropositive element or group...
s), R3C- (alkyl anions, R=alkyl or H), or R2N- (amides, R=alkyl or H) to depart with a pair of electrons because of the instability of these bases.

