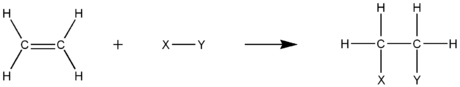Alkene
Encyclopedia

Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
, an alkene, olefin, or olefine is an unsaturated
Saturation (chemistry)
In chemistry, saturation has six different meanings, all based on reaching a maximum capacity...
chemical compound
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
containing at least one carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
-to-carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
double bond
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
. The simplest acyclic alkenes, with only one double bond and no other functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
s, form an homologous series
Homologous series
In chemistry, a homologous series is a series of compounds with a similar general formula, possessing similar chemical properties due to the presence of the same functional group, and showing a gradation in physical properties as a result of increase in molecular size and mass...
of hydrocarbon
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
s with the general formula CnH2n.
The simplest alkene is ethylene
Ethylene
Ethylene is a gaseous organic compound with the formula . It is the simplest alkene . Because it contains a carbon-carbon double bond, ethylene is classified as an unsaturated hydrocarbon. Ethylene is widely used in industry and is also a plant hormone...
(C2H4), which has the International Union of Pure and Applied Chemistry
International Union of Pure and Applied Chemistry
The International Union of Pure and Applied Chemistry is an international federation of National Adhering Organizations that represents chemists in individual countries. It is a member of the International Council for Science . The international headquarters of IUPAC is located in Zürich,...
(IUPAC) name ethene. Alkenes are also called olefins (an archaic synonym, widely used in the petrochemical
Petrochemical
Petrochemicals are chemical products derived from petroleum. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as corn or sugar cane....
industry). For bridged alkenes, the Bredt's rule
Bredt's Rule
Bredt's rule is an empirical observation in organic chemistry that states that a double bond cannot be placed at the bridgehead of a bridged ring system, unless the rings are large enough...
states that a double bond cannot be placed at the bridgehead of a bridged ring system, unless the rings are large enough. Aromatic compounds are often drawn as cyclic alkenes, but their structure and properties are different and they are not considered to be alkenes.
Bonding
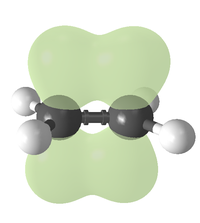
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
s, double bonds can be described in terms of overlapping atomic orbitals, except that, unlike a single bond (which consists of a single sigma bond
Sigma bond
In chemistry, sigma bonds are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most clearly defined for diatomic molecules using the language and tools of symmetry groups. In this formal approach, a σ-bond is...
), a carbon-carbon double bond consists of one sigma bond
Sigma bond
In chemistry, sigma bonds are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most clearly defined for diatomic molecules using the language and tools of symmetry groups. In this formal approach, a σ-bond is...
and one pi bond
Pi bond
In chemistry, pi bonds are covalent chemical bonds where two lobes of one involved atomic orbital overlap two lobes of the other involved atomic orbital...
. This double bond is stronger than a single covalent bond
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
(611 kJ/mol
Mole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
for C=C vs. 347 kJ/mol for C—C) and also shorter with an average bond length
Bond length
- Explanation :Bond length is related to bond order, when more electrons participate in bond formation the bond will get shorter. Bond length is also inversely related to bond strength and the bond dissociation energy, as a stronger bond will be shorter...
of 1.33 Angstrom
Ångström
The angstrom or ångström, is a unit of length equal to 1/10,000,000,000 of a meter . Its symbol is the Swedish letter Å....
s (133 pm
Picometre
A picometre is a unit of length in the metric system, equal to one trillionth, i.e. of a metre, which is the current SI base unit of length...
).
Each carbon of the double bond uses its three sp² hybrid orbitals to form sigma bonds to three atoms. The unhybridized 2p atomic orbitals, which lie perpendicular to the plane created by the axes of the three sp² hybrid orbitals, combine to form the pi bond. This bond lies outside the main C—C axis, with half of the bond on one side and half on the other.
Rotation about the carbon-carbon double bond is restricted because it involves breaking the pi bond, which requires a large amount of energy (264 kJ/mol in ethylene). As a consequence, substituted alkenes may exist as one of two isomer
Isomer
In chemistry, isomers are compounds with the same molecular formula but different structural formulas. Isomers do not necessarily share similar properties, unless they also have the same functional groups. There are many different classes of isomers, like stereoisomers, enantiomers, geometrical...
s, called cis or trans isomers. More complex alkenes may be named using the E-Z notation
E-Z notation
E-Z notation, or the E-Z convention, is the IUPAC preferred method of describing the stereochemistry of double bonds in organic chemistry...
, used to describe molecules having three or four different substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
s (side groups). For example, of the isomers of butene, the two methyl groups of (Z)-but-2-ene (aka cis-2-butene) face the same side of the double bond, and in (E)-but2-ene (aka trans-2-butene) the methyl groups face the opposite side. These two isomers of butene are slightly different in their chemical and physical properties.
It is certainly not impossible to twist a double bond. In fact, a 90° twist requires an energy approximately equal to half the strength of a pi bond
Pi bond
In chemistry, pi bonds are covalent chemical bonds where two lobes of one involved atomic orbital overlap two lobes of the other involved atomic orbital...
. The misalignment of the p orbitals is less than expected because pyramidalization takes place (See: pyramidal alkene
Pyramidal alkene
Pyramidal alkenes are alkenes in which the two carbon atoms making up the double bond are not coplanar with their four substituents . This deformation from a trigonal planar geometry to a tetrahedral molecular geometry is the result of angle strain induced in the molecule due to geometric constraints...
). trans-Cyclooctene
Cyclooctene
Cyclooctene is a cycloalkene with an eight-membered ring. It is notable because it is the smallest cycloalkene that can exist as either the cis- or trans-isomer with the cis-isomer more common...
is a stable strained alkene and the orbital misalignment is only 19° with a dihedral angle
Dihedral angle
In geometry, a dihedral or torsion angle is the angle between two planes.The dihedral angle of two planes can be seen by looking at the planes "edge on", i.e., along their line of intersection...
of 137° (normal 120°) and a degree of pyramidalization of 18°. This explains the dipole
Dipole
In physics, there are several kinds of dipoles:*An electric dipole is a separation of positive and negative charges. The simplest example of this is a pair of electric charges of equal magnitude but opposite sign, separated by some distance. A permanent electric dipole is called an electret.*A...
moment of 0.8 D
Debye
The debye is a CGS unit of electric dipole momentElectric dipole moment is defined as charge times displacement: Historically the debye was defined as the dipole moment resulting from two charges of opposite sign but an equal magnitude of 10-10 statcoulomb10-10 statcoulomb is approximately 0.2083...
for this compound (cis-isomer 0.4 D) where a value of zero is expected. The trans isomer of cycloheptene
Cycloheptene
Cycloheptene is a 7-membered cycloalkene with a flash point of -6 C°. It is a raw material in organic chemistry and a monomer in polymer synthesis. Cycloheptene can exist as either the cis- or the trans-isomer.- trans-Cycloheptene :...
is only stable at low temperatures.
Shape
As predicted by the VSEPRVSEPR theory
Valence shell electron pair repulsion theory is a model in chemistry used to predict the shape of individual molecules based upon the extent of electron-pair electrostatic repulsion. It is also named Gillespie–Nyholm theory after its two main developers...
model of electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
pair repulsion, the molecular geometry
Molecular geometry
Molecular geometry or molecular structure is the three-dimensional arrangement of the atoms that constitute a molecule. It determines several properties of a substance including its reactivity, polarity, phase of matter, color, magnetism, and biological activity.- Molecular geometry determination...
of alkenes includes bond angles about each carbon in a double bond of about 120°. The angle may vary because of steric strain introduced by nonbonded interactions created by functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
s attached to the carbons of the double bond. For example, the C-C-C bond angle in propylene
Propylene
Propene, also known as propylene or methylethylene, is an unsaturated organic compound having the chemical formula C3H6. It has one double bond, and is the second simplest member of the alkene class of hydrocarbons, and it is also second in natural abundance.-Properties:At room temperature and...
is 123.9°.
Physical properties
The physical properties of alkenes are comparable with those of alkaneAlkane
Alkanes are chemical compounds that consist only of hydrogen and carbon atoms and are bonded exclusively by single bonds without any cycles...
s. The main differences between the two are that the acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
ity levels of alkenes are much higher than the ones in alkanes. The physical state depends on molecular mass
Molecular mass
The molecular mass of a substance is the mass of one molecule of that substance, in unified atomic mass unit u...
(gases from ethene to butene - liquids from pentene onwards). The simplest alkenes, ethene, propene and butene are gases. Linear alkenes of approximately five to sixteen carbons are liquids, and higher alkenes are waxy solids.
Reactions
Alkenes are relatively stable compounds, but are more reactive than alkaneAlkane
Alkanes are chemical compounds that consist only of hydrogen and carbon atoms and are bonded exclusively by single bonds without any cycles...
s due to the presence of a carbon-carbon pi-bond. It is also attributed to the presence of pi-electrons in the molecule. The majority of the reactions of alkenes involve the rupture of this pi bond, forming new single bonds
Sigma bond
In chemistry, sigma bonds are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most clearly defined for diatomic molecules using the language and tools of symmetry groups. In this formal approach, a σ-bond is...
.
Alkenes serve as a feedstock for the petrochemical
Petrochemical
Petrochemicals are chemical products derived from petroleum. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as corn or sugar cane....
industry because they can participate in a wide variety of reactions.
Addition reactions
Alkenes react in many addition reactionAddition reaction
An addition reaction, in organic chemistry, is in its simplest terms an organic reaction where two or more molecules combine to form a larger one....
s, which occur by opening up the double-bond. Most addition reactions to alkenes follow the mechanism of electrophilic addition
Electrophilic addition
In organic chemistry, an electrophilic addition reaction is an addition reaction where, in a chemical compound, a π bond is broken and two new σ bonds are formed...
. Examples of addition reaction
Addition reaction
An addition reaction, in organic chemistry, is in its simplest terms an organic reaction where two or more molecules combine to form a larger one....
s are hydrohalogenation
Hydrohalogenation
A hydrohalogenation reaction is the electrophilic addition of hydrohalic acids like hydrogen chloride or hydrogen bromide to alkenes to yield the corresponding haloalkanes....
, halogenation
Halogenation
Halogenation is a chemical reaction that incorporates a halogen atom into a molecule in substitution of hydrogen atom. Halogenation takes place in the gas phase. There are four types of halogenation: fluorination, chlorination, bromination, and iodination...
, halohydrin formation, oxymercuration, hydroboration, dichlorocarbene addition, Simmons-Smith reaction
Simmons-Smith reaction
The Simmons–Smith reaction is an organic cheletropic reaction in which a carbenoid reacts with an alkene to form a cyclopropane. It is named after Howard Ensign Simmons, Jr. and R. D. Smith...
, catalytic hydrogenation, epoxidation, radical polymerization
Radical polymerization
Free radical polymerization is a method of polymerization by which a polymer forms by the successive addition of free radical building blocks. Free radicals can be formed via a number of different mechanisms usually involving separate initiator molecules...
and hydroxylation
Hydroxylation
Hydroxylation is a chemical process that introduces a hydroxyl group into an organic compound. In biochemistry, hydroxylation reactions are often facilitated by enzymes called hydroxylases. Hydroxylation is the first step in the oxidative degradation of organic compounds in air...
.
Hydrogenation
HydrogenationHydrogenation
Hydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
of alkenes produces the corresponding alkane
Alkane
Alkanes are chemical compounds that consist only of hydrogen and carbon atoms and are bonded exclusively by single bonds without any cycles...
s. The reaction is carried out under pressure at a temperature of 200 °C in the presence of a metallic catalyst. Common industrial catalysts are based on platinum
Platinum
Platinum is a chemical element with the chemical symbol Pt and an atomic number of 78. Its name is derived from the Spanish term platina del Pinto, which is literally translated into "little silver of the Pinto River." It is a dense, malleable, ductile, precious, gray-white transition metal...
, nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
or palladium
Palladium
Palladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired...
. For laboratory syntheses, Raney nickel
Raney nickel
Raney nickel is a solid catalyst composed of fine grains of a nickel-aluminium alloy, used in many industrial processes. It was developed in 1926 by American]] engineer Murray Raney as an alternative catalyst for the hydrogenation of vegetable oils in industrial processes...
(an alloy
Alloy
An alloy is a mixture or metallic solid solution composed of two or more elements. Complete solid solution alloys give single solid phase microstructure, while partial solutions give two or more phases that may or may not be homogeneous in distribution, depending on thermal history...
of nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
and aluminium
Aluminium
Aluminium or aluminum is a silvery white member of the boron group of chemical elements. It has the symbol Al, and its atomic number is 13. It is not soluble in water under normal circumstances....
) is often employed. The simplest example of this reaction is the catalytic hydrogenation of ethylene
Ethylene
Ethylene is a gaseous organic compound with the formula . It is the simplest alkene . Because it contains a carbon-carbon double bond, ethylene is classified as an unsaturated hydrocarbon. Ethylene is widely used in industry and is also a plant hormone...
to yield ethane
Ethane
Ethane is a chemical compound with chemical formula C2H6. It is the only two-carbon alkane that is an aliphatic hydrocarbon. At standard temperature and pressure, ethane is a colorless, odorless gas....
:
- CH2=CH2 + H2 → CH3-CH3
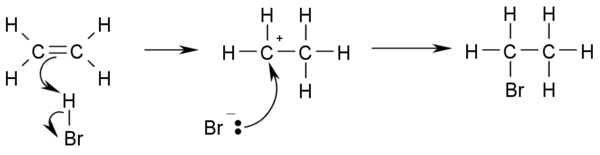
Halogenation
In electrophilic halogenationElectrophilic halogenation
In organic chemistry, an electrophilic aromatic halogenation is a type of electrophilic aromatic substitution. This organic reaction is typical of aromatic compounds and a very useful method for adding substituents to an aromatic system....
the addition of elemental bromine
Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
or chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
to alkenes yields vicinal
Vicinal (chemistry)
In chemistry vicinal stands for any two functional groups bonded to two adjacent carbon atoms. For example the molecule 2,3-dibromobutane carries two vicinal bromine atoms and 1,3-dibromobutane does not....
dibromo- and dichloroalkanes (1,2-dihalides or ethylene dihalides), respectively. The decoloration of a solution of bromine in water with dichloromethylene as catalyst is an analytical test for the presence of alkenes:
- CH2=CH2 + Br2 → BrCH2-CH2Br
It is also used as a quantitive test of unsaturation, expressed as the bromine number
Bromine number
Bromine number is the amount of bromine in grams absorbed by of a sample. The number indicates the degree of unsaturation.The Bromine Number is useful as a measure of aliphatic unsaturation in gasoline samples...
of a single compound or mixture. The reaction works because the high electron density at the double bond causes a temporary shift of electrons in the Br-Br bond causing a temporary induced dipole. This makes the Br closest to the double bond slightly positive and therefore an electrophile.
Hydrohalogenation
HydrohalogenationHydrohalogenation
A hydrohalogenation reaction is the electrophilic addition of hydrohalic acids like hydrogen chloride or hydrogen bromide to alkenes to yield the corresponding haloalkanes....
is the addition of hydrohalic acids such as HCl
Hydrogen chloride
The compound hydrogen chloride has the formula HCl. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric humidity. Hydrogen chloride gas and hydrochloric acid are important in technology and industry...
or HBr
Hydrogen bromide
Hydrogen bromide is the diatomic molecule HBr. HBr is a gas at standard conditions. Hydrobromic acid forms upon dissolving HBr in water. Conversely, HBr can be liberated from hydrobromic acid solutions with the addition of a dehydration agent, but not by distillation. Hydrogen bromide and...
to alkenes to yield the corresponding haloalkane
Haloalkane
The haloalkanes are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and...
s.
- CH3-CH=CH2 + HBr → CH3-CHBr-CH2-H
If the two carbon atoms at the double bond are linked to a different number of hydrogen atoms, the halogen is found preferentially at the carbon with fewer hydrogen substituents (Markovnikov's rule
Markovnikov's rule
In organic chemistry, Markovnikov's rule or Markownikoff's rule is an observation based on Zaitsev's rule. It was formulated by the Russian chemist Vladimir Vasilevich Markovnikov in 1870....
). But terminal olefin products don't yield by this method. For bromation an alternative method denominated Kharasch-Sosnovsky Reaction. is used for this purpose. It consists to add peroxides to hydrogen bromide or Copper bromide (II).
Halohydrin formation
Alkenes react with water and halogens to form halohydrinHalohydrin
A halohydrin or a haloalcohol is a type of organic compound or functional group in which one carbon atom has a halogen substituent, and an adjacent carbon atom has a hydroxyl substituent. They are derived from alcohols are therefore characterized by the presence of both the hydroxyl functional...
s by an addition reaction. Markovnikov regiochemistry
Markovnikov's rule
In organic chemistry, Markovnikov's rule or Markownikoff's rule is an observation based on Zaitsev's rule. It was formulated by the Russian chemist Vladimir Vasilevich Markovnikov in 1870....
and anti stereochemistry occur.
- CH2=CH2 + X2 + H2O → XCH2-CH2OH
Oxidation
Alkenes are oxidized with a large number of oxidizing agentOxidizing agent
An oxidizing agent can be defined as a substance that removes electrons from another reactant in a redox chemical reaction...
s. In the presence of oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
, alkenes burn with a bright flame to produce carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
and water. Catalytic oxidation with oxygen or the reaction with percarboxylic acids yields epoxide
Epoxide
An epoxide is a cyclic ether with three ring atoms. This ring approximately defines an equilateral triangle, which makes it highly strained. The strained ring makes epoxides more reactive than other ethers. Simple epoxides are named from the parent compound ethylene oxide or oxirane, such as in...
s.
Reaction with ozone in ozonolysis
Ozonolysis
Ozonolysis is the cleavage of an alkene or alkyne with ozone to form organic compounds in which the multiple carbon–carbon bond has been replaced by a double bond to oxygen...
leads to the breaking of the double bond, yielding two aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s or ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s. Reaction with concentrated, hot KMnO4 (or other oxidizing salts) in an acidic solution will yield ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s or carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
s.
- R1-CH=CH-R2 + O3 → R1-CHO + R2-CHO + H2O
This reaction can be used to determine the position of a double bond in an unknown alkene.
Oxymercuration
HydrationHydration reaction
In organic chemistry, a hydration reaction is a chemical reaction in which a hydroxyl group and a hydrogen cation are added to the two carbon atoms bonded together in the carbon-carbon double bond which makes up an alkene functional group. The reaction usually runs in a strong acidic, aqueous...
of alkenes via oxymercuration
Oxymercuration reaction
The oxymercuration reaction is an electrophilic addition organic reaction that transforms an alkene into a neutral alcohol. In oxymercuration, the alkene reacts with mercuric acetate in aqueous solution to yield the addition of an acetoxymercuri group and a hydroxy group across the double bond...
to produces alcohols. Reaction takes place on treatment of alkenes with strong acid as catalyst.
- CH2=CH2 + H2O → CH3-CH2OH
Polymerization
PolymerizationPolymerization
In polymer chemistry, polymerization is a process of reacting monomer molecules together in a chemical reaction to form three-dimensional networks or polymer chains...
of alkenes is a reaction that yields polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
s of high industrial value at great economy, such as the plastics polyethylene
Polyethylene
Polyethylene or polythene is the most widely used plastic, with an annual production of approximately 80 million metric tons...
and polypropylene
Polypropylene
Polypropylene , also known as polypropene, is a thermoplastic polymer used in a wide variety of applications including packaging, textiles , stationery, plastic parts and reusable containers of various types, laboratory equipment, loudspeakers, automotive components, and polymer banknotes...
. Polymers from alkene monomer
Monomer
A monomer is an atom or a small molecule that may bind chemically to other monomers to form a polymer; the term "monomeric protein" may also be used to describe one of the proteins making up a multiprotein complex...
s are referred to in a general way as polyolefin
Polyolefin
A polyolefin is a polymer produced from a simple olefin as a monomer. For example, polyethylene is the polyolefin produced by polymerizing the olefin ethylene. An equivalent term is polyalkene; this is a more modern term, although polyolefin is still used in the petrochemical industry...
s or in rare instances as polyalkenes. A polymer from alpha-olefin
Alpha-olefin
Alpha-olefins are a family of organic compounds which are olefins or alkenes with a chemical formula CxH2x, distinguished by having a double bond at the primary or alpha position...
s is called a polyalphaolefin (PAO). Polymerization can proceed via either a free-radical
Radical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
or an ionic mechanism, converting the double to a single bond and forming single bonds to join the other monomers. Polymerization of conjugated
Conjugated system
In chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds, which in general may lower the overall energy of the molecule and increase stability. Lone pairs, radicals or carbenium ions may be part of the...
diene
Diene
In organic chemistry a diene or diolefin is a hydrocarbon that contains two carbon double bonds.Conjugated dienes are functional groups, with a general formula of CnH2n-2. Dienes and alkynes are functional isomers...
s such as buta-1,3-diene or isoprene
Isoprene
Isoprene , or 2-methyl-1,3-butadiene, is a common organic compound with the formula CH2=CCH=CH2. Under standard conditions it is a colorless liquid...
(2-methylbuta-1,3-diene) results in largely 1,4-addition with possibly some 1,2-addition of the diene monomer to a growing polymer chain. For details, see "Polybutadiene
Polybutadiene
Polybutadiene is a synthetic rubber that is a polymer formed from the polymerization process of the monomer 1,3-butadiene.It has a high resistance to wear and is used especially in the manufacture of tires, which consumes about 70% of the production...
".
Reaction overview
| Reaction name | Product | | Comment |
|---|---|---|
| Hydrogenation Hydrogenation Hydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically... |
alkanes | addition of hydrogen |
| Hydroalkenylation | alkenes | hydrometalation / insertion / beta elimination by metal catalyst |
| Halogen addition reaction Halogen addition reaction A halogen addition reaction is a simple organic reaction where a halogen molecule is added to the carbon–carbon double bond of an alkene functional group.The general chemical formula of the halogen addition reaction is:... |
1,2-dihalide | electrophilic addition of halogens |
| Hydrohalogenation Hydrohalogenation A hydrohalogenation reaction is the electrophilic addition of hydrohalic acids like hydrogen chloride or hydrogen bromide to alkenes to yield the corresponding haloalkanes.... (Markovnikov) |
haloalkanes | addition of hydrohalic acids |
| Kharasch-Sosnovsky Reaction (Antimarkovnikov Hydrohalogenation Hydrohalogenation A hydrohalogenation reaction is the electrophilic addition of hydrohalic acids like hydrogen chloride or hydrogen bromide to alkenes to yield the corresponding haloalkanes.... ) |
haloalkanes | free radicals mediated addition of hydrohalic acids |
| Hydroamination Hydroamination The hydroamination reaction is the addition of an N-H bond across the C=C or C≡C bonds of an alkene or alkyne. This is a highly atom economical method of preparing substituted amines that are attractive targets for organic synthesis and the pharmaceutical industry.The hydroamination reaction is... |
amines | addition of N-H bond across C-C double bond |
| Hydroformylation Hydroformylation Hydroformylation, also known as oxo synthesis or oxo process, is an important industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group and a hydrogen atom to a carbon-carbon double bond... |
aldehydes | industrial process, addition of CO and H2 |
| Sharpless bishydroxylation | diols | oxidation, reagent: osmium tetroxide, chiral ligand |
| Woodward cis-hydroxylation Woodward cis-hydroxylation The Woodward cis-hydroxylation is the chemical reaction of alkenes with iodine and silver acetate in wet acetic acid to form cis-diols. The reaction is named after its discoverer, Robert Burns Woodward.... |
diols | oxidation, reagents: iodine, silver acetate |
| ozonolysis Ozonolysis Ozonolysis is the cleavage of an alkene or alkyne with ozone to form organic compounds in which the multiple carbon–carbon bond has been replaced by a double bond to oxygen... |
aldehydes or ketones | reagent: ozone |
| Olefin metathesis Olefin metathesis Olefin metathesis or transalkylidenation is an organic reaction that entails redistribution of alkylene fragments by the scission of carbon - carbon double bonds in olefins . Its advantages include the creation of fewer sideproducts and hazardous wastes. Yves Chauvin, Robert H. Grubbs, and Richard R... |
alkenes | two alkenes rearrange to form two new alkenes |
| Diels-Alder reaction Diels-Alder reaction The Diels–Alder reaction is an organic chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon... |
cyclohexenes | cycloaddition with a diene |
| Pauson-Khand reaction | cyclopentenones | cycloaddition with an alkyne and CO |
| Hydroboration–oxidation | alcohols | reagents: borane, then a peroxide |
| oxymercuration-reduction | alcohols | electrophilic addition of mercuric acetate, then reduction |
| Prins reaction Prins reaction The Prins reaction is an organic reaction consisting of an electrophilic addition of an aldehyde or ketone to an alkene or alkyne followed by capture of a nucleophile. The outcome of the reaction depends on reaction conditions . With water and a protic acid such as sulfuric acid as the reaction... |
1,3-diols | electrophilic addition with aldehyde or ketone |
| Paterno–Büchi reaction | oxetanes | photochemical reaction with aldehyde or ketone |
| Epoxidation | epoxide | electrophilic addition of a peroxide |
| Cyclopropanation | cyclopropanes | addition of carbenes or carbenoids |
| Hydroacylation Hydroacylation Hydroacylation is a type of organic reaction in which an aldehyde is added over an alkene or alkyne bond. The reaction product is a ketone. The reaction requires a metal catalyst and intramolecular reaction is favored over a intermolecular one. With alkynes the reaction product is an... |
ketones | oxidative addition / reductive elimination by metal catalyst |
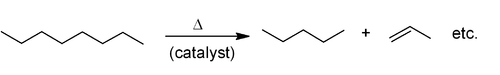
Industrial methods
Alkenes are produced by hydrocarbon crackingCracking (chemistry)
In petroleum geology and chemistry, cracking is the process whereby complex organic molecules such as kerogens or heavy hydrocarbons are broken down into simpler molecules such as light hydrocarbons, by the breaking of carbon-carbon bonds in the precursors. The rate of cracking and the end products...
. Raw materials are mostly natural gas condensate
Natural gas condensate
Natural-gas condensate is a low-density mixture of hydrocarbon liquids that are present as gaseous components in the raw natural gas produced from many natural gas fields....
components (principally ethane and propane) in the US and Mideast and naphtha
Naphtha
Naphtha normally refers to a number of different flammable liquid mixtures of hydrocarbons, i.e., a component of natural gas condensate or a distillation product from petroleum, coal tar or peat boiling in a certain range and containing certain hydrocarbons. It is a broad term covering among the...
in Europe and Asia. Alkanes are broken apart at high temperatures, often in the presence of a zeolite
Zeolite
Zeolites are microporous, aluminosilicate minerals commonly used as commercial adsorbents. The term zeolite was originally coined in 1756 by Swedish mineralogist Axel Fredrik Cronstedt, who observed that upon rapidly heating the material stilbite, it produced large amounts of steam from water that...
catalyst, to produce a mixture of primarily aliphatic alkenes and lower molecular weight alkanes. The mixture is feedstock dependent and separated by fractional distillation. This is mainly used for the manufacture of small alkenes (up to six carbons).
Dehydrogenation
Dehydrogenation is a chemical reaction that involves the elimination of hydrogen . It is the reverse process of hydrogenation. Dehydrogenation reactions may be either large scale industrial processes or smaller scale laboratory procedures....
, where an alkane loses hydrogen at high temperatures to produce a corresponding alkene. This is the reverse of the catalytic hydrogenation of alkenes.
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
(the TΔS portion of the equation ΔG = ΔH – TΔS dominates for high T).
Catalytic synthesis of higher α-alkenes (of the type RCH=CH2) can also be achieved by a reaction of ethylene with the organometallic compound triethylaluminium
Triethylaluminium
Triethylaluminium is an organoaluminium compound. This volatile, colorless liquid is highly pyrophoric, igniting immediately upon exposure to air. It is normally stored in stainless steel containers either as a pure liquid or as a solution in hydrocarbon solvents such as hexane, heptane, or ...
in the presence of nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
, cobalt
Cobalt
Cobalt is a chemical element with symbol Co and atomic number 27. It is found naturally only in chemically combined form. The free element, produced by reductive smelting, is a hard, lustrous, silver-gray metal....
, or platinum
Platinum
Platinum is a chemical element with the chemical symbol Pt and an atomic number of 78. Its name is derived from the Spanish term platina del Pinto, which is literally translated into "little silver of the Pinto River." It is a dense, malleable, ductile, precious, gray-white transition metal...
.
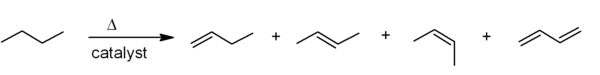
Elimination reactions
One of the principal methods for alkene synthesis in the laboratory is the eliminationElimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
of alkyl halides, alcohols, and similar compounds. Most common is the β-elimination via the E2 or E1 mechanism, but α-eliminations are also known.
The E2 mechanism provides a more reliable β-elimination method than E1 for most alkene syntheses. Most E2 eliminations start with an alkyl halide or alkyl sulfonate ester (such as a tosylate or triflate
Triflate
Trifluoromethanesulfonate, also known by the trivial name triflate, is a functional group with the formula CF3SO3-. The triflate group is often represented by -OTf, as opposed to -Tf...
). When an alkyl halide is used, the reaction is called a dehydrohalogenation
Dehydrohalogenation
Dehydrohalogenation is an organic reaction from which an alkene is obtained from an alkyl halide . It is also called a β-Elimination reaction and is a type of elimination reaction....
. For unsymmetrical products, the more substituted alkenes (those with fewer hydrogens attached to the C=C) tend to predominate (see Zaitsev's rule
Zaitsev's rule
In chemistry, Zaitsev's rule, Saytzeff's rule or Saytsev's rule named after Alexander Mikhailovich Zaitsev is a rule that states that if more than one alkene can be formed during dehalogenation by an elimination reaction, the more stable alkene is the major product...
). Two common methods of elimination reactions are dehydrohalogenation of alkyl halides and dehydration of alcohols. A typical example is shown below; note that if possible, the H is anti to the leaving group, even though this leads to the less stable Z-isomer.
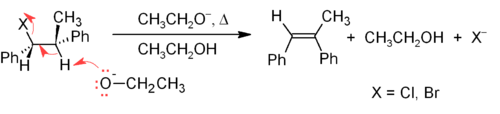
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s via dehydration
Dehydration reaction
In chemistry and the biological sciences, a dehydration reaction is usually defined as a chemical reaction that involves the loss of water from the reacting molecule. Dehydration reactions are a subset of elimination reactions...
, in which case water is lost via the E1 mechanism. For example, the dehydration of ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
produces ethene:
- CH3CH2OHEthanolEthanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
+ H2SO4Sulfuric acidSulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
→ H2C=CH2 + H3O+ + HSO4−
An alcohol may also be converted to a better leaving group (e.g., xanthate
Xanthate
Xanthate usually refers to a salt with the formula ROCS2-M+ . The name xanthates is derived from Greek ξανθός , meaning “yellowish, golden”, and indeed most xanthate salts are yellow...
), so as to allow a milder syn-elimination such as the Chugaev elimination
Chugaev elimination
The Chugaev elimination is a chemical reaction that involves the elimination of water from alcohols to produce alkenes. The intermediate is a xanthate. It is named for its discoverer, the Russian chemist Lev Aleksandrovich Chugaev....
and the Grieco elimination
Grieco elimination
The Grieco elimination is an organic reaction describing the elimination reaction of an aliphatic primary alcohol through a selenide to a terminal alkene ....
. Related reactions include eliminations by β-haloethers (the Boord olefin synthesis
Boord olefin synthesis
The Boord olefin synthesis is an organic reaction forming alkenes from ethers carrying a halogen atom 2 carbons removed from the oxygen atom catalyzed by a metal such as magnesium or zinc. The reaction, discovered by Cecil E...
) and esters (ester pyrolysis
Ester pyrolysis
Ester pyrolysis in organic chemistry is a vacuum pyrolysis reaction converting esters containing a β-hydrogen atom into the corresponding carboxylic acid and the alkene...
).
Alkenes can be prepared indirectly from alkyl amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s. The amine or ammonia is not a suitable leaving group, so the amine is first either alkylated
Alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion or a carbene . Alkylating agents are widely used in chemistry because the alkyl group is probably the most common group encountered in...
(as in the Hofmann elimination
Hofmann elimination
Hofmann elimination is a process where an amine is reacted to create a tertiary amine and an alkene by treatment with excess methyl iodide followed by treatment with silver oxide, water, and heat.After the first step, a quaternary ammonium iodide salt is created...
) or oxidized to an amine oxide
Amine oxide
An amine oxide, also known as amine-N-oxide and N-oxide, is a chemical compound that contains the functional group R3N+-O−, an N-O bond with three additional hydrogen and/or hydrocarbon side chains attached to N. Sometimes it is written as R3N→O or, wrongly, as R3N=O.In the strict sense the...
(the Cope reaction
Cope reaction
The Cope reaction or Cope elimination, developed by Arthur C. Cope, is an elimination reaction of an amine oxide to form an alkene and a hydroxylamine. The reaction mechanism involves an intramolcular 5-membered cyclic transition state, leading to a syn elimination product...
) to render a smooth elimination possible. Hofmann elimination is unusual in that the less substituted (non-Saytseff) alkene is usually the major product. The Cope reaction is a syn-elimination that occurs at or below 150 °C, for example:
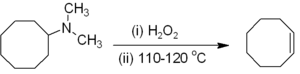
Sulfone
A sulfone is a chemical compound containing a sulfonyl functional group attached to two carbon atoms. The central hexavalent sulfur atom is double bonded to each of two oxygen atoms and has a single bond to each of two carbon atoms, usually in two separate hydrocarbon substituents.-IUPAC name and...
s in the Ramberg-Bäcklund reaction
Ramberg-Bäcklund reaction
The Ramberg-Bäcklund Reaction is an organic reaction converting an α-halo sulfone into an alkene in presence of a base with extrusion of sulfur dioxide. The reaction is named after the two Swedish chemists Ludwig Ramberg and Birger Bäcklund. The carbanion formed by deprotonation gives an unstable...
, via a three-membered ring sulfone intermediate.
Synthesis from carbonyl compounds
Another important method for alkene synthesis involves construction of a new carbon-carbon double bond by coupling of a carbonyl compound (such as an aldehydeAldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
or ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
) to a carbanion
Carbanion
A carbanion is an anion in which carbon has an unshared pair of electrons and bears a negative charge usually with three substituents for a total of eight valence electrons. The carbanion exists in a trigonal pyramidal geometry. Formally a carbanion is the conjugate base of a carbon acid.where B...
equivalent. Such reactions are sometimes called olefinations. The most well-known of these methods is the Wittig reaction
Wittig reaction
The Wittig reaction is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide to give an alkene and triphenylphosphine oxide....
, but other related methods are known.
The Wittig reaction involves reaction of an aldehyde or ketone with a Wittig reagent (or phosphorane) of the type Ph3P=CHR to produce an alkene and Ph3P=O
Triphenylphosphine oxide
Triphenylphosphine oxide is the chemical compound with the formula OP3. Often chemists abbreviate the formula by writing Ph3PO or PPh3O . This white crystalline compound is a common side product in reactions involving triphenylphosphine...
. The Wittig reagent is itself prepared easily from triphenylphosphine
Triphenylphosphine
Triphenylphosphine is a common organophosphorus compound with the formula P3 - often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature...
and an alkyl halide. The reaction is quite general and many functional groups are tolerated, even esters, as in this example:
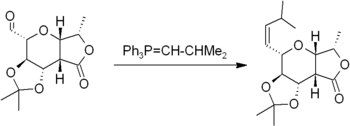
Peterson olefination
The Peterson olefination is the chemical reaction of α-silyl carbanions 1 with ketones to form a β-hydroxysilane 2 which eliminates to form alkenes 3.Several reviews have been published....
. This uses a less accessible silicon-based reagent in place of the phosphorane, but it allows for the selection of E or Z products. If an E-product is desired, another alternative is the Julia olefination
Julia olefination
The Julia olefination is the chemical reaction of phenyl sulfones with aldehydes to give alkenes after alcohol functionalization and reductive elimination using sodium amalgam or SmI2...
, which uses the carbanion generated from a phenyl sulfone
Sulfone
A sulfone is a chemical compound containing a sulfonyl functional group attached to two carbon atoms. The central hexavalent sulfur atom is double bonded to each of two oxygen atoms and has a single bond to each of two carbon atoms, usually in two separate hydrocarbon substituents.-IUPAC name and...
. The Takai olefination
Takai olefination
Takai olefination in organic chemistry describes the organic reaction of an aldehyde with a diorganochromium compound to form an alkene. In the original 1986 publication the aldehyde is benzaldehyde and the organochromium species is generated from iodoform or bromoform and an excess of chromium...
based on an organochromium intermediate also delivers E-products. A titanium compound, Tebbe's reagent
Tebbe's reagent
The Tebbe reagent is the organometallic compound with the formula 2TiCH2ClAl2. It used in the methylenation of carbonyl compounds, that is it converts organic compounds containing the R2C=O group into the related R2C=CH2 derivative...
, is useful for the synthesis of methylene compounds; in this case, even esters and amides react.
A pair of carbonyl compounds can also be reductively coupled together (with reduction) to generate an alkene. Symmetrical alkenes can be prepared from a single aldehyde or ketone coupling with itself, using Ti metal reduction (the McMurry reaction
McMurry reaction
The McMurry reaction is an organic reaction in which two ketone or aldehyde groups are coupled to an alkene using titanium chloride compound such as titanium chloride and a reducing agent. The reaction is named after its co-discoverer, John E. McMurry. The McMurry reaction originally involved the...
). If two different ketones are to be coupled, a more complex, indirect method such as the Barton-Kellogg reaction
Barton-Kellogg reaction
The Barton-Kellogg reaction is a coupling reaction between a ketone and a thioketone through a diazo intermediate forming an alkene.This reaction has been pioneered by Hermann Staudinger and therefore the reaction also goes by the name Staudinger type diazo-thioketone coupling.- Reaction mechanism...
may be used.
A single ketone can also be converted to the corresponding alkene via its tosylhydrazone, using sodium methoxide
Sodium methoxide
Sodium methoxide is a chemical compound, with formula CH3ONa. This colourless solid, which is formed by the deprotonation of methanol, is a widely used reagent in industry and the laboratory...
(the Bamford-Stevens reaction
Bamford-Stevens reaction
The Bamford–Stevens reaction is a chemical reaction whereby treatment of tosylhydrazones with strong base gives alkenes. It is named for the British chemist William Randall Bamford and the Scottish chemist Thomas Stevens Stevens...
) or an alkyllithium (the Shapiro reaction
Shapiro reaction
The Shapiro reaction or tosylhydrazone decomposition is an organic reaction in which a ketone or aldehyde is converted to an alkene through an intermediate hydrazone in the presence of 2 equivalents of strong base. The reaction was discovered by Robert H. Shapiro in 1975...
).
Synthesis from alkenes: Olefin metathesis and hydrovinylation
Alkenes can be prepared by exchange with other alkenes, in a reaction known as olefin metathesisOlefin metathesis
Olefin metathesis or transalkylidenation is an organic reaction that entails redistribution of alkylene fragments by the scission of carbon - carbon double bonds in olefins . Its advantages include the creation of fewer sideproducts and hazardous wastes. Yves Chauvin, Robert H. Grubbs, and Richard R...
. Frequently, loss of ethene gas is used to drive the reaction towards a desired product. In many cases, a mixture of geometric isomers is obtained, but the reaction tolerates many functional groups. The method is particularly effective for the preparation of cyclic alkenes, as in this synthesis of muscone
Muscone
Muscone is an organic compound that is the primary contributor to the odor of musk.The chemical structure of muscone was first elucidated by Lavoslav Ružička. It consists of a 15-membered ring ketone with one methyl substituent in the 3-position. It is an oily liquid that is found naturally as...
:
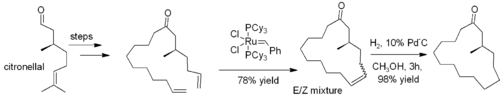
From alkynes
Reduction of alkyneAlkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
s is a useful method for the stereoselective
Stereoselectivity
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during the non-stereospecific creation of a new stereocenter or during the non-stereospecific transformation of a pre-existing one...
synthesis of disubstituted alkenes. If the cis-alkene is desired, hydrogenation
Hydrogenation
Hydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
in the presence of Lindlar's catalyst(-heterogeneous catalyst that consists of palladium deposited on calcium carbonate and treated with various forms of lead) is commonly used, though hydroboration followed by hydrolysis provides an alternative approach. Reduction of the alkyne by sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
metal in liquid ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
gives the trans-alkene.
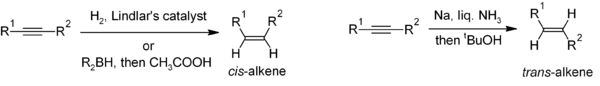
Carbometalation
Carbometalation is an organometallic reaction involving the nucleophilic addition to alkenes and alkynes of a diverse range of organometallic reagents such as organolithium compounds, organocopper compounds and Grignard reagents according to the following general alkyne scheme:The addition can...
of alkynes can give rise to a large variety of alkene derivatives.
Rearrangements and related reactions
Alkenes can be synthesized from other alkenes via rearrangement reactionRearrangement reaction
A rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule...
s. Besides olefin metathesis
Olefin metathesis
Olefin metathesis or transalkylidenation is an organic reaction that entails redistribution of alkylene fragments by the scission of carbon - carbon double bonds in olefins . Its advantages include the creation of fewer sideproducts and hazardous wastes. Yves Chauvin, Robert H. Grubbs, and Richard R...
(described above), a large number of pericyclic reaction
Pericyclic reaction
In organic chemistry, a pericyclic reaction is a type of organic reaction wherein the transition state of the molecule has a cyclic geometry, and the reaction progresses in a concerted fashion. Pericyclic reactions are usually rearrangement reactions...
s can be used such as the ene reaction
Ene reaction
The Ene reaction is a chemical reaction between an alkene with an allylic hydrogen and a compound containing a multiple bond , in order to form a new σ-bond with migration of the ene double bond and 1,5 hydrogen shift. The product is a substituted alkene with the double bond shifted to the...
and the Cope rearrangement
Cope rearrangement
The Cope rearrangement is an extensively studied organic reaction involving the [3,3]-sigmatropic rearrangement of 1,5-dienes. It was developed by Arthur C. Cope...
.
Diels-Alder reaction
The Diels–Alder reaction is an organic chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon...
, a cyclohexene
Cyclohexene
Cyclohexene is a hydrocarbon with the formula C6H10. This cycloalkene is a colorless liquid with a sharp smell. It is an intermediate in various industrial processes...
derivative is prepared from a diene and a reactive or electron-deficient alkene.
IUPAC Names
To form the root of the IUPACIUPAC nomenclature
A chemical nomenclature is a set of rules to generate systematic names for chemical compounds. The nomenclature used most frequently worldwide is the one created and developed by the International Union of Pure and Applied Chemistry ....
names for alkenes, simply change the -an- infix of the parent to -en-. For example, CH3-CH3 is the alkane
Alkane
Alkanes are chemical compounds that consist only of hydrogen and carbon atoms and are bonded exclusively by single bonds without any cycles...
ethANe. The name of CH2=CH2 is therefore ethENe.
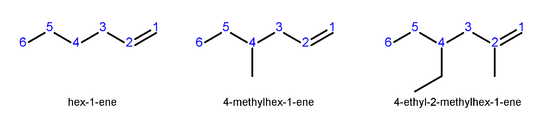
In higher alkenes, where isomers exist that differ in location of the double bond, the following numbering system is used:
- Number the longest carbon chain that contains the double bond in the direction that gives the carbon atoms of the double bond the lowest possible numbers.
- Indicate the location of the double bond by the location of its first carbon.
- Name branched or substituted alkenes in a manner similar to alkaneAlkaneAlkanes are chemical compounds that consist only of hydrogen and carbon atoms and are bonded exclusively by single bonds without any cycles...
s. - Number the carbon atoms, locate and name substituent groups, locate the double bond, and name the main chain.
Cis-Trans notation
In the specific case of disubstituted alkenes where the two carbons have one substituent each, Cis-trans notation may be used. If both substituents are on the same side of the bond, it is defined as (cis-). If the substituents are on either side of the bond, it is defined as (trans-).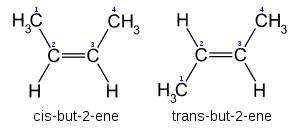
E,Z notation
When an alkene has more than one substituent (especially necessary with 3 or 4 substituents), the double bond geometry is described using the labels E and Z. These labels come from the German words "entgegen," meaning "opposite," and "zusammen," meaning "together." Alkenes with the higher priority groups (as determined by CIP rulesCahn-Ingold-Prelog priority rule
The Cahn–Ingold–Prelog priority rules, CIP system or CIP conventions are a set of rules used in organic chemistry to name the stereoisomers of a molecule. A molecule may contain any number of stereocenters and any number of double bonds, and each gives rise to two possible configurations...
) on the same side of the double bond have these groups together and are designated Z. Alkenes with the higher priority groups on opposite sides are designated E. A mnemonic to remember this: Z notation has the higher priority groups on "ze zame zide."

Groups containing C=C double bonds
IUPAC recognizes two names for hydrocarbon groups containing carbon-carbon double bonds, the vinylVinyl
A vinyl compound is any organic compound that contains a vinyl group ,which are derivatives of ethene, CH2=CH2, with one hydrogen atom replaced with some other group...
group and the allyl
Allyl
An allyl group is a substituent with the structural formula H2C=CH-CH2R, where R is the connection to the rest of the molecule. It is made up of a methylene , attached to a vinyl group . The name is derived from the Latin word for garlic, Allium sativum. Theodor Wertheim isolated an allyl...
group. .

See also
- Alpha-olefinAlpha-olefinAlpha-olefins are a family of organic compounds which are olefins or alkenes with a chemical formula CxH2x, distinguished by having a double bond at the primary or alpha position...
- CycloalkeneCycloalkeneA cycloalkene or cycloolefin is a type of alkene hydrocarbon which contains a closed ring of carbon atoms, but has no aromatic character. Some cycloalkenes, such as cyclobutene and cyclopentene, can be used as monomers to produce polymer chains. Unless the rings are very large, cycloalkenes are...
- ArenesAromatic hydrocarbonAn aromatic hydrocarbon or arene is a hydrocarbon with alternating double and single bonds between carbon atoms. The term 'aromatic' was assigned before the physical mechanism determining aromaticity was discovered, and was derived from the fact that many of the compounds have a sweet scent...
- DieneDieneIn organic chemistry a diene or diolefin is a hydrocarbon that contains two carbon double bonds.Conjugated dienes are functional groups, with a general formula of CnH2n-2. Dienes and alkynes are functional isomers...
- Dendralene
- RadialeneRadialene[n]Radialenes are alicyclic organic compounds containing n cross-conjugated exocyclic double bonds. The double bonds are commonly alkene groups but those with a carbonyl group are also called radialenes...
- AnnuleneAnnuleneAnnulenes are completely conjugated monocyclic hydrocarbons. They have the general formula CnHn or CnHn+1...
- PolyenePolyenePolyenes are poly-unsaturated organic compounds that contain one or more sequences of alternating double and single carbon-carbon bonds. These double carbon-carbon bonds interact in a process known as conjugation, which results in an overall lower energy state of the molecule.Organic compounds with...
Nomenclature links
- Rule A-3. Unsaturated Compounds and Univalent Radicals http://www.acdlabs.com/iupac/nomenclature/79/r79_53.htm IUPAC Blue Book.
- Rule A-4. Bivalent and Multivalent Radicals http://www.acdlabs.com/iupac/nomenclature/79/r79_78.htm IUPAC Blue Book.
- Rules A-11.3, A-11.4, A-11.5 Unsaturated monocyclic hydrocarbons and substituents http://www.acdlabs.com/iupac/nomenclature/79/r79_60.htm IUPAC Blue Book.
- Rule A-23. Hydrogenated Compounds of Fused Polycyclic Hydrocarbons http://www.acdlabs.com/iupac/nomenclature/79/r79_73.htm IUPAC Blue Book.