Cycloaddition
Encyclopedia
A cycloaddition is a pericyclic chemical reaction
, in which "two or more unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity." The resulting reaction is a cyclization reaction.
Cycloadditions are usually described by the backbone size of the participants. This would make the Diels-Alder reaction
a [4 + 2]cycloaddition, the 1,3-dipolar cycloaddition
a [3 + 2]cycloaddition and cyclopropanation of a carbene with an alkene a [2+1]cycloaddition. This type of reaction is non-polar addition reaction
.
, in which the orthogonal set of p orbitals allows the reaction to proceed via a crossed transition state
.
Cycloadditions in which 4n π electrons participate can also occur as a result of photochemical
activation. Here, one component has an electron promoted from the HOMO
(π bonding) to the LUMO
(π* antibonding
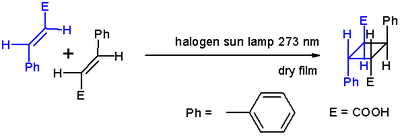
). Orbital symmetry is then such that the reaction can proceed in a suprafacial-suprafacial manner. An example is the DeMayo reaction
. Another example is shown below, the photochemical dimerization of cinnamic acid
.
Note that not all photochemical (2+2) cyclizations are cycloadditions; some are known to operate by radical mechanisms.
Some cycloadditions instead of π bonds operate through strained cyclopropane
rings; as these have significant π character. For example, an analog for the Diels-Alder reaction is the quadricyclane
-DMAD reaction:
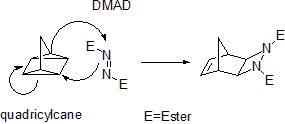 In the (i+j+...) cycloaddition notation i and j refer to the number of atoms involved in the cycloaddition. In this notation a Diels-Alder reaction is a (4+2)cycloaddition and a 1,3-dipolar addition such as the first step in ozonolysis
In the (i+j+...) cycloaddition notation i and j refer to the number of atoms involved in the cycloaddition. In this notation a Diels-Alder reaction is a (4+2)cycloaddition and a 1,3-dipolar addition such as the first step in ozonolysis
is a (3+2)cycloaddition. The IUPAC preferred notation however, with [i+j+...] takes electrons into account and not atoms. In this notation the DA reaction and the dipolar reaction both become a [4+2]cycloaddition. The reaction between norbornadiene
and an activated alkyne
is a [2+2+2]cycloaddition.
is a [4+2]cycloaddition reaction.
is a [3+2]cycloaddition.
analogs, however these are not strictly speaking pericyclic reactions. When in a cycloaddition charged or radical intermediates are involved or when the cycloaddition result is obtained in a series of reaction steps they are sometimes called formal cycloadditions to make the distinction with true pericyclic cycloadditions.
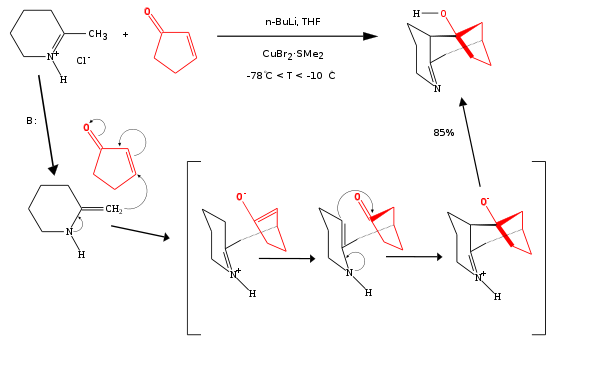
One example of a formal [3+3]cycloaddition between a cyclic enone
and an enamine
catalyzed by n-butyllithium
is a Stork enamine / 1,2-addition
cascade reaction
:
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
, in which "two or more unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity." The resulting reaction is a cyclization reaction.
Cycloadditions are usually described by the backbone size of the participants. This would make the Diels-Alder reaction
Diels-Alder reaction
The Diels–Alder reaction is an organic chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon...
a [4 + 2]cycloaddition, the 1,3-dipolar cycloaddition
1,3-dipolar cycloaddition
The 1,3-dipolar cycloaddition, also known as the Huisgen cycloaddition or Huisgen reaction, is an organic chemical reaction belonging to the larger class of concerted, pericyclic cycloadditions. It is the reaction between a 1,3-dipole and a dipolarophile, most of which are substituted alkenes, to...
a [3 + 2]cycloaddition and cyclopropanation of a carbene with an alkene a [2+1]cycloaddition. This type of reaction is non-polar addition reaction
Addition reaction
An addition reaction, in organic chemistry, is in its simplest terms an organic reaction where two or more molecules combine to form a larger one....
.
Reaction mechanism
Thermal cycloadditions usually have (4n + 2) π electrons participating in the starting material, for some integer n. These occur, for reasons of orbital symmetry, in a suprafacial-suprafacial or antarafacial-antarafacial manner (rare). There are a few examples of thermal cycloadditions which have 4n π electrons (for example the [2 + 2] cycloaddition); these proceed in a suprafacial-antarafacial sense, such as the dimerisation of keteneKetene
A ketene is an organic compound of the form R'RC=C=O. The term is also used specifically to mean ethenone, the simplest ketene, where R' and R are hydrogen atoms.Ketenes were first studied as a class by Hermann Staudinger.-Formation:...
, in which the orthogonal set of p orbitals allows the reaction to proceed via a crossed transition state
Transition state
The transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest energy along this reaction coordinate. At this point, assuming a perfectly irreversible reaction, colliding reactant molecules will always...
.
Cycloadditions in which 4n π electrons participate can also occur as a result of photochemical
Photochemistry
Photochemistry, a sub-discipline of chemistry, is the study of chemical reactions that proceed with the absorption of light by atoms or molecules.. Everyday examples include photosynthesis, the degradation of plastics and the formation of vitamin D with sunlight.-Principles:Light is a type of...
activation. Here, one component has an electron promoted from the HOMO
Homo
Homo may refer to:*the Greek prefix ὅμο-, meaning "the same"*the Latin for man, human being*Homo, the taxonomical genus including modern humans...
(π bonding) to the LUMO
Lumo
Lumo is a 2007 documentary film about twenty-year-old Lumo Sinai, a woman who fell victim to "Africa's First World War." While returning home one day, Lumo and another woman were gang-raped by a group of soldiers fighting for control of the Democratic Republic of the Congo during the 1994 Rwandan...
(π* antibonding
Antibonding
Antibonding is a type of chemical bonding. An antibonding orbital is a form of molecular orbital that is located outside the region of two distinct nuclei...
). Orbital symmetry is then such that the reaction can proceed in a suprafacial-suprafacial manner. An example is the DeMayo reaction
DeMayo reaction
The DeMayo reaction is a photochemical reaction in which the enol of a 1,3-diketone reacts with an alkene and the resulting cyclobutane ring undergoes a retro-aldol reaction to yield a 1,5-diketone :...
. Another example is shown below, the photochemical dimerization of cinnamic acid
Cinnamic acid
Cinnamic acid is a white crystalline organic acid, which is slightly soluble in water.It is obtained from oil of cinnamon, or from balsams such as storax. It is also found in shea butter and is the best indication of its environmental history and post-extraction conditions...
.
Note that not all photochemical (2+2) cyclizations are cycloadditions; some are known to operate by radical mechanisms.
Some cycloadditions instead of π bonds operate through strained cyclopropane
Cyclopropane
Cyclopropane is a cycloalkane molecule with the molecular formula C3H6, consisting of three carbon atoms linked to each other to form a ring, with each carbon atom bearing two hydrogen atoms...
rings; as these have significant π character. For example, an analog for the Diels-Alder reaction is the quadricyclane
Quadricyclane
Quadricyclane is a strained, multi-cyclic hydrocarbon with potential uses as an additive for rocket propellants as well in solar energy conversion. These uses are limited, however, by the molecule's decomposition at relatively low temperatures .-Structure and properties:Quadricyclane is a highly...
-DMAD reaction:

Ozonolysis
Ozonolysis is the cleavage of an alkene or alkyne with ozone to form organic compounds in which the multiple carbon–carbon bond has been replaced by a double bond to oxygen...
is a (3+2)cycloaddition. The IUPAC preferred notation however, with [i+j+...] takes electrons into account and not atoms. In this notation the DA reaction and the dipolar reaction both become a [4+2]cycloaddition. The reaction between norbornadiene
Norbornadiene
Norbornadiene is an organic compound. This bicyclic hydrocarbon is the most stable diolefin derived from the norbornane and norbornene. Norbornadiene is primarily of interest as a ligand in homogeneous catalysis, but it has been heavily studied due to its high reactivity and distinctive...
and an activated alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
is a [2+2+2]cycloaddition.
Diels-Alder reactions
The Diels-Alder reactionDiels-Alder reaction
The Diels–Alder reaction is an organic chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon...
is a [4+2]cycloaddition reaction.
Huisgen cycloadditions
The Huisgen cycloaddition reaction is a [2+3]cycloaddition.Nitrone-olefin cycloaddition
The Nitrone-olefin cycloadditionNitrone-olefin 3+2 cycloaddition
The nitrone-olefin [3+2] cycloaddition reaction is the combination of a nitrone with an alkene or alkyne to generate an isoxazoline or isoxazolidine via a [3+2] cycloaddition process.-Introduction:...
is a [3+2]cycloaddition.
Formal cycloadditions
Cycloadditions often have metal-catalyzed and stepwise radicalRadical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
analogs, however these are not strictly speaking pericyclic reactions. When in a cycloaddition charged or radical intermediates are involved or when the cycloaddition result is obtained in a series of reaction steps they are sometimes called formal cycloadditions to make the distinction with true pericyclic cycloadditions.
One example of a formal [3+3]cycloaddition between a cyclic enone
Enone
An enone is an unsaturated chemical compound or functional group consisting of a conjugated system of an alkene and a ketone. The simplest enone is methyl vinyl ketone or CH2=CHCOCH3....
and an enamine
Enamine
An enamine is an unsaturated compound derived by the reaction of an aldehyde or ketone with a secondary amine followed by loss of H2O.The word "enamine" is derived from the affix en-, used as the suffix of alkene, and the root amine. This can be compared with enol, which is a functional group...
catalyzed by n-butyllithium
N-Butyllithium
n-Butyllithium is an organolithium reagent. It is widely used as a polymerization initiator in the production of elastomers such as polybutadiene or styrene-butadiene-styrene...
is a Stork enamine / 1,2-addition
Nucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where in a chemical compound a π bond is removed by the creation of two new covalent bonds by the addition of a nucleophile....
cascade reaction
Cascade reaction
A cascade reaction or tandem reaction or domino reaction is a consecutive series of intramolecular organic reactions which often proceed via highly reactive intermediates. It allows the organic synthesis of complex multinuclear molecules from a single acyclic precursor. The substrate contains many...
: