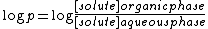Equilibrium chemistry
Encyclopedia
Equilibrium chemistry is a concerned with systems in chemical equilibrium
. The unifying principle is that the free energy
of a system at equilibrium is the minimum possible, so that the slope of the free energy with respect to the reaction coordinate
is zero. This principle, applied to mixtures at equilibrium provides a definition of an equilibrium constant. Applications include acid-base
, host-guest
, metal-complex
, solubility
, partition
, chromatography
and redox
equilibria.
state. The system at chemical equilibrium will be at a constant temperature, pressure (or volume) and composition. It will be insulated from exchange of heat with the surroundings, that is, it is a closed system
. A change of temperature, pressure (or volume) constitutes an external influence and the equilibrium quantities will change as a result of such a change. If there is a possibility that the composition might change, but the rate of change is negligibly slow, the system is said to be in a metastable state. The equation of chemical equilibrium can be expressed symbolically as
The sign means "are in equilibrium with". This definition refers to macroscopic
properties. Changes do occur at the microscopic level of atoms and molecules, but to such a minute extent that they are not measurable and in a balanced way so that the macroscopic quantities do not change. Chemical equilibrium is a dynamic state in which forward and backward reactions proceed at such rates that the macroscopic composition of the mixture is constant. Thus, equilibrium sign symbolizes the fact that reactions occur in both forward and backward
and backward  directions.
directions.
 A steady state
A steady state
, on the other hand, is not necessarily an equilibrium state in the chemical sense. For example, in a radioactive decay chain
the concentrations of intermediate isotopes are constant because the rate of production is equal to the rate of decay. It is not a chemical equilibrium because the decay process occurs in one direction only.
Thermodynamic equilibrium is characterized by the free energy for the whole (closed) system being a minimum. For systems at constant volume the Helmholtz free energy
is minimum and for systems at constant pressure the Gibbs free energy
is minimum. Thus a metastable state is one for which the free energy change between reactants and products is not minimal even though the composition does not change in time.
The existence of this minimum is due to the free energy of mixing of reactants and products being always negative. For ideal solution
s the enthalpy
of mixing is zero, so the minimum exists because the entropy
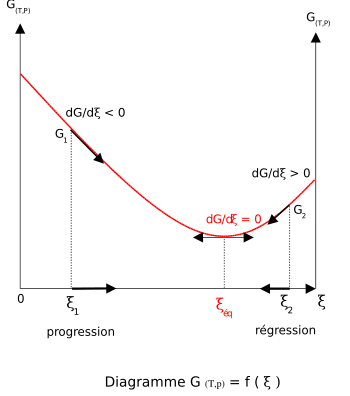
of mixing is always positive. The slope of the reaction free energy, δGr with respect to the reaction coordinate
, ξ, is zero when the free energy is at its minimum value.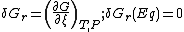
is the partial molar free energy. The potential, μi, of the ith species in a chemical reaction is the partial derivative of the free energy with respect to the number of moles of that species, Ni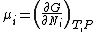
A general chemical equilibrium can be written asThe general expression is not used much in chemistry. To help understand the notation consider the equilibrium
for this reaction n1=1, n2=2,m1=1 and m2=2, Reactant1=H2SO4, Reactant2=OH-, Product1=SO42- and Product2=H2O.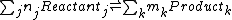
nj are the stoichiometric coefficients of the reactants in the equilibrium equation, and mj are the coefficients of the products. The value of δGr for these reactions is a function of the chemical potentials of all the species.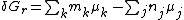
The chemical potential, μi, of the ith species can be calculated in terms of its activity
, ai.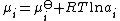
μi is the standard chemical potential of the species, R is the gas constant
is the standard chemical potential of the species, R is the gas constant
and T is the temperature. Setting the sum for the reactants j to be equal to the sum for the products, k, so that δGr (Eq) = 0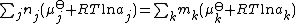
Rearranging the terms,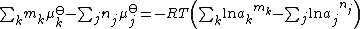
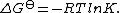
This relates the standard
Gibbs free energy change, ΔG to an equilibrium constant, K, the reaction quotient
to an equilibrium constant, K, the reaction quotient
of activity values at equilibrium.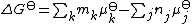
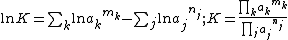
It follows that any equilibrium of this kind can be characterized either by the standard free energy change or by the equilibrium constant. In practice concentrations are more useful than activities. Activities can be calculated from concentrations if the activity coefficient
are known, but this is rarely the case. Sometimes activity coefficients can be calculated using, for example, Pitzer equations
or Specific ion interaction theory
. Otherwise conditions must be adjusted so that activity coefficients do not vary much. For ionic solutions this is achieved by using a background ionic medium at a high concentration relative to the concentrations of the species in equilibrium.
If activity coefficients are unknown they may be subsumed into the equilibrium constant, which becomes a concentration quotient. Each activity ai is assumed to be the product of a concentration, [Ai], and an activity coefficient, γi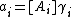
This expression for activity is placed in the expression defining the equilibrium constant.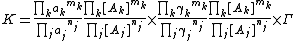
By setting the quotient of activity coefficients, Γ, equal to one This is equivalent to defining a new equilibrium constant as K / Γ the equilibrium constant is defined as a quotient of concentrations.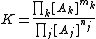
In more familiar notation, for a general equilibrium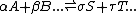
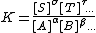
This definition is much more practical, but an equilibrium constant defined in terms of concentrations is dependent on conditions. In particular, equilibrium constants for species in aqueous solution are dependent on ionic strength
, as the quotient of activity coefficients varies with the ionic strength of the solution.
The values of the standard free energy change and of the equilibrium constant are temperature dependent. To a first approximation, the van 't Hoff equation may be used.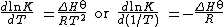
This shows that when the reaction is exothermic (ΔH , the standard enthalpy
, the standard enthalpy
change, is negative), then K decreases with increasing temperature, in accordance with Le Chatelier's principle
. The approximation involved is that the standard enthalpy change, ΔH , is independent of temperature, which is a good approximation only over a small temperature range. Thermodynamic arguments can be used to show that
, is independent of temperature, which is a good approximation only over a small temperature range. Thermodynamic arguments can be used to show that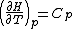
where Cp is the heat capacity at constant pressure.
, f, is used rather than activity. However, whereas activity is dimension
less, fugacity has the dimension of pressure
. A consequence is that chemical potential has to be defined in terms of a standard pressure, p
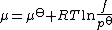
By convention p is usually taken to be 1 bar
is usually taken to be 1 bar
Fugacity can be expressed as the product of partial pressure
, p, and a fugacity coefficient, Φ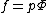
Fugacity coefficients are dimensionless and can be obtained experimentally at specific temperature and pressure, from measurements of deviations from ideal gas
behaviour. Equilibrium constants are defined in terms of fugacity. If the gases are at sufficiently low pressure that they behave as ideal gases, the equilibrium constant can be defined as a quotient of partial pressures.
An example of gas-phase equilibrium is provided by the Haber-Bosch process of ammonia
synthesis.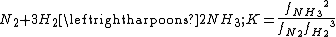
This reaction is strongly exothermic
, so the equilibrium constant decreases with temperature. However, a temperature of around 400°C is required in order to achieve a reasonable rate of reaction with currently available catalysts. Formation of ammonia is also favoured by high pressure, as the volume decreases when the reaction takes place. It is interesting to note that the same reaction, nitrogen fixation
, occurs at ambient temperatures in nature, when the catalyst is an enzyme
such as nitrogenase
. Much energy is needed initially to break the N-N triple bond even though the overall reaction is exothermic.
Gas-phase equilibria occur during combustion
and were studied as early as 1943 in connection with the development of the V2
rocket engine
.
The calculation of composition for a gaseous equilibrium at constant pressure is often carried out using ΔG values, rather than equilibrium constants.
acid
, H2A.The definitions given are association constants. A dissociation constant is the reciprocal of an association constant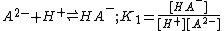
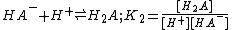
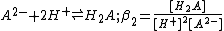
The three constants are not independent of each other and it is easy to see that β2= K1K2. The constants K1 and K2 are stepwise constants and β is an example of an overall constant.
 The concentrations of species in equilibrium are usually calculated under the assumption that activity coefficients are either known or can be ignored. In this case, each equilibrium constant for the formation of a complex in a set of multiple equilibria can be defined as follows
The concentrations of species in equilibrium are usually calculated under the assumption that activity coefficients are either known or can be ignored. In this case, each equilibrium constant for the formation of a complex in a set of multiple equilibria can be defined as follows
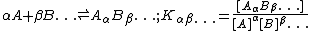
The concentrations of species containing reagent A are constrained by a condition of mass-balance
, that is, the total (or analytical) concentration, which is the sum of all species' concentrations, must be constant. There is one mass-balance equation for each reagent of the type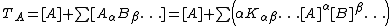
There are as many mass-balance equations as there are reagents, A, B .., so if the equilibrium constant values are known, there are n mass-balance equations in n unknowns, [A], [B].., the so-called free reagent concentrations. Solution of these equations gives all the information needed to calculate the concentrations of all the species.
Thus, the importance of an equilibrium constants lies in the fact that, once their values have been determined by experiment, they can be used to calculate the concentrations, known as the speciation
, of mixtures that contain the relevant species.
are the most widely used with aqueous solutions. The others are Spectrophotometric
, Fluorescence
(luminescence) measurements and NMR
chemical shift
measurements; simultaneous measurement of K and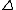 H for 1:1 adducts in biological systems is routinely carried out using Isothermal Titration Calorimetry
H for 1:1 adducts in biological systems is routinely carried out using Isothermal Titration Calorimetry
.
The experimental data will comprise a set of data points. At the i'th data point, the analytical concentrations of the reactants,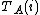 ,
, 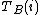 etc. will be experimentally known quantities and there will be one or more measured quantities, yi, that depend in some way on the analytical concentrations and equilibrium constants. A general computational procedure has three main components.
etc. will be experimentally known quantities and there will be one or more measured quantities, yi, that depend in some way on the analytical concentrations and equilibrium constants. A general computational procedure has three main components.
An acid is a proton donor; the proton is transferred to the base, a proton acceptor, creating a conjugate acid. For aqueous solutions of an acid HA, the base is water; the conjugate base is A− and the conjugate acid is the solvated hydrogen ion. In solution chemistry, it is usual to use H+ as an abbreviation for the solvated hydrogen ion, regardless of the solvent. In aqueous solution H+ denotes a solvated hydronium ion.The bare proton does not exist in aqueous solution. It is a very strong acid and combines the base, water, to form the hydronium ion
The hydronium ion forms various weak complexes by hydrogen bonding with more water molecules
The Brønsted–Lowry definition applies to other solvents, such as dimethyl sulfoxide
: the solvent S acts as a base, accepting a proton and forming the conjugate acid SH+.
A broader definition of acid dissociation includes hydrolysis
, in which protons are produced by the splitting of water molecules. For example, boric acid
, B(OH)3, acts as a weak acid, even though it is not a proton donor, because of the hydrolysis equilibrium
Similarly, metal ion hydrolysis causes ions such as [Al(H2O)6]3+ to behave as weak acids:
Acid-base equilibria are important in a very wide range of applications, such as acid-base homeostasis
, ocean acidification
, pharmacology
and analytical chemistry
.
, A. The host may be either a donor or an acceptor. In biochemistry
host-guest complexes are known as receptor
-ligand complexes; they are formed primarily by non-covalent bonding. Many host-guest complexes has 1:1 stoichiometry, but many others have more complex structures. The general equilibrium can be written as
The study of these complexes is important for supramolecular chemistry
and molecular recognition
. The objective of these studies is often to find systems with a high binding selectivity
of a host (receptor) for a particular target molecule or ion, the guest or ligand. An application is the development of chemical sensors. Finding a drug which either blocks a receptor, an antagonist
which forms a strong complex the receptor, or activate it, an agonist
, is an important pathway to drug discovery
.
s, metal ions will be present as aqua-ions
, so the reaction for the formation of the first complex could be written asElectrical charges are omitted from such expressions because the ligand, L, may or may not carry an electrical charge.
However, since water is in vast excess, the concentration of water is usually assumed to be constant and is omitted from equilibrium constant expressions. Often, the metal and the ligand are in competition for protons. For the equilibrium
a stability constant can be defined as follows.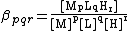
The definition can easily be extended to include any number of reagents. It includes hydroxide
complexes because the concentration of the hydroxide ions is related to the concentration of hydrogen ions by the self-ionization of water
Stability constants defined in this way, are association constants. This can lead to some confusion as pKa values
are dissociation constants. In general purpose computer programs it is customary to define all constants as association constants. The relationship between the two types of constant is given in association and dissociation constants.
In biochemistry
, an oxygen molecule can bind to an iron (II) atom in a heme
prosthetic group in hemoglobin
. The equilibrium is usually written, denoting hemoglobin by Hb, as
but this representation is incomplete as the Bohr effect
shows that the equilibrium concentrations are pH-dependent. A better representation would be
as this shows that when hydrogen ion concentration increases the equilibrium is shifted to the left in accordance with Le Chatelier's principle
. Hydrogen ion concentration can be increased by the presence of carbon dioxide, which behaves as a weak acid.
The iron atom can also bind to other molecules such as carbon monoxide
. Cigarette smoke contains some carbon monoxide so the equilibrium
is established in the blood of cigarette smokers.
Chelation therapy
is based on the principle of using chelating ligands with a high binding selectivity
for a particular metal to remove that metal from the human body.
Complexes with polyamino carboxylic acid
s find a wide range of applications. EDTA in particular is used extensively.
) equilibrium can be handled in exactly the same way as any other chemical equilibrium. For example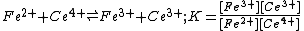
However, in the case of redox reactions it is convenient to split the overall reaction into two half-reactions. In this example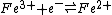
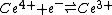
The standard free energy change, which is related to the equilibrium constant by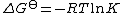
can be split into two components,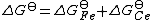
The concentration of free electrons is effectively zero as the electrons are transferred directly from the reductant to the oxidant. The standard electrode potential
, E0 for the each half-reaction is related to the standard free energy change by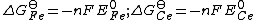
where n is the number of electrons transferred and F is the Faraday constant. Now, the free energy for an actual reaction is given by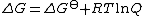
where R is the gas constant
and Q a reaction quotient
. Strictly speaking Q is a quotient of activities, but it is common practice to use concentrations instead of activities. Therefore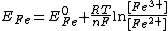
For any half-reaction, the redox potential of an actual mixture is given by the generalized expressionThe alternative expression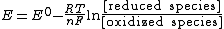
is sometimes used, as in Nernst equation
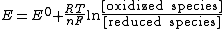
This is an example of the Nernst equation
. The potential is known as a reduction potential. Standard electrode potentials are available in a table of values
. Using these values, the actual electrode potential for a redox couple can be calculated as a function of the ratio of concentrations.
The equilibrium potential for a general redox half-reaction (See #Equilibrium constant above for an explanation of the symbols)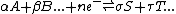
is given by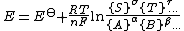
Use of this expression allows the effect of a species not involved in the redox reaction, such as the hydrogen ion in a half-reaction such as
to be taken into account.
The equilibrium constant for a full redox reaction can be obtained from the standard redox potentials of the constituent half-reactions. At equilibrium the potential for the two half-reactions must be equal to each other and, of course, the number of electrons exchanged must be the same in the two half reactions.
Redox equilibria play an important role in the electron transport chain
. The various cytochrome
s in the chain have different standard redox potentials, each one adapted for a specific redox reaction. This allows, for example, atmospheric oxygen
to be reduced in photosynthesis
. A distinct family of cytochromes, the cytochrome P450 oxidase
s, are involved in steroidogenesis and detoxification.
forms a saturated solution in a solvent
, the concentration of the solute, at a given temperature, is determined by the equilibrium constant at that temperature.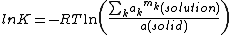
The activity of a pure substance in the solid state is one, by definition, so the expression simplifies to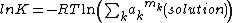
If the solute does not dissociate the summation is replaced by a single term, but if dissociation occurs, as with ionic substances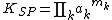
For example, with Na2SO4 m1=2 and m2=1 so the solubility product is written as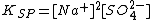
Concentrations, indicated by [..], are usually used in place of activities, but activity must be taken into account of the presence of another salt with no ions in common, the so-called salt effect. When another salt is present that has an ion in common, the common-ion effect
comes into play, reducing the solubility of the primary solute.
or distribution coefficient.The distinction between a partition coefficient and a distribution coefficient is of historical significance only. The partition coefficient is defined as the ratio of the analytical concentrations of the solute in the two phases. By convention the value is reported in logarithmic form.
The partition coefficient is defined at a specified temperature and, if applicable, pH of the aqueous phase. Partition coefficients are very important in pharmacology
because they determine the extent to which a substance can pass from the blood (an aqueous solution) through a cell wall which is like an organic solvent. They are usually measured using water and octanol
as the two solvents. Many pharmaceutical compounds are weak acid
s or weak base
s. Such a compound may exist with a different extent of protonation depending on pH
and the acid dissociation constant
. Because the organic phase has a low dielectric constant
the species with no electrical charge will be the most likely one to pass from the aqueous phase to the organic phase. Even at pH 7-7.2, the range of biological pH values, the aqueous phase may support an equilibrium between more than one protonated form. Log p is determined from the analytical concentration of the substance in the aqueous phase, that is, the sum of the concentration of the different species in equilibrium.
Solvent extraction is used extensively in separation and purification processes. In its simplest form a reaction is performed in an organic solvent and unwanted by-products are removed by extraction into water at a particular pH.
A metal ion may be extracted from an aqueous phase into an organic phase in which the salt is not soluble, by adding a ligand
. The ligand, La-, forms a complex with the metal ion, Mb+, [MLx])b-ax)+ which has a strongly hydrophobic outer surface. If the complex has no electrical charge it will be extracted relatively easily into the organic phase. If the complex is charged, it is extracted as an ion pair. The additional ligand is not always required. For example, uranyl nitrate
, UO2(NO3)2, is soluble in diethyl ether
because the solvent itself acts as a ligand. This property was used in the past for separating uranium from other metals whose salts are not soluble in ether. Currently extraction into kerosene
is preferred, using a ligand such as tri-n-butyl phosphate, TBP. In the PUREX
process, which is commonly used in nuclear reprocessing
, uranium(VI) is extracted from strong nitric acid as the electrically neutral complex [UO2(TBP)2(NO3)2]. The strong nitric acid provides a high concentration of nitrate ions which pushes the equilibrium in favour of the weak nitrato complex. Uranium is recovered by back-extraction (stripping) into weak nitric acid. Plutonium(IV) forms a similar complex, [PuO2(TBP)2(NO3)2] and the plutonium in this complex can be reduced to separate it from uranium.
Another important application of solvent extraction is in the separation of the lanthanoids. This process also uses TBP and the complexes are extracted into kerosene. Separation is achieved because the stability constant
for the formation of the TBP complex increases as the size of the lanthanoid ion decreases.
An instance of ion-pair extraction is in the use of a ligand to enable oxidation by potassium permanganate
, KMnO4, in an organic solvent. KMnO4 is not soluble in organic solvents. When a ligand, such as a crown ether
is added to an aqueous solution of KMnO4, it forms a hydrophobic complex with the potassium cation which allows the uncharged ion-pair, {[KL]+[MnO4]-} to be extracted into the organic solvent. See also: phase-transfer catalysis.
s for the stationary phase. A distribution constant, Kd can be defined as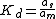
where as and am are the equilibrium activities in the stationary and mobile phases respectively. It can be shown that the rate of migration,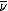 , is related to the distribution constant by
, is related to the distribution constant by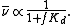
f is a factor which depends on the volumes of the two phases. Thus, the higher the affinity of the solute for the stationary phase, the slower the migration rate.
There is a wide variety of chromatographic techniques, depending on the nature of the stationary and mobile phases. When the stationary phase is solid, the analyte may form a complex with it. A water softener functions by selective complexation with a sulfonate
ion exchange resin
. Sodium ions form relatively weak complexes with the resin. When hard water
is passed through the resin, the divalent ions of magnesium and calcium displace the sodium ions and are retained on the resin, R.
The water coming out of the column is relatively rich in sodium ionsFeeding babies formula made up with sodium rich water can lead to hypernatremia
. and poor in calcium and magnesium which are retained on the column. The column is regenerated by passing a strong solution of sodium chloride through it, so that the resin- sodium complex is again formed on the column. Ion-exchange chromatography utilizes a resin such as chelex 100
in which iminodiacetate
residues, attached to a polymer backbone, form chelate complexes of differing strengths with different metal ions, allowing the ions such as Cu2+ and Ni2+ to be separated chromatographically.
Another example of complex formation is in chiral chromatography in which is used to separate enantiomer
s from each other. The stationary phase is itself chiral and forms complexes selectively with the enantiomers. In other types of chromatography with a solid stationary phase, such as thin layer chromatography
the analyte is selectively adsorbed onto the solid.
In gas-liquid chromatography
(GLC) the stationary phase is a liquid such as polydimethylsiloxane
, coated on a glass tube. Separation is achieved because the various components in the gas have different solubility in the stationary phase. GLC can be used to separate literally hundreds of components in a gas mixture such as cigarette smoke or essential oil
s, such as lavender oil.
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
. The unifying principle is that the free energy
Thermodynamic free energy
The thermodynamic free energy is the amount of work that a thermodynamic system can perform. The concept is useful in the thermodynamics of chemical or thermal processes in engineering and science. The free energy is the internal energy of a system less the amount of energy that cannot be used to...
of a system at equilibrium is the minimum possible, so that the slope of the free energy with respect to the reaction coordinate
Reaction coordinate
In chemistry, a reaction coordinate is an abstract one-dimensional coordinate which represents progress along a reaction pathway. It is usually a geometric parameter that changes during the conversion of one or more molecular entities....
is zero. This principle, applied to mixtures at equilibrium provides a definition of an equilibrium constant. Applications include acid-base
Acid dissociation constant
An acid dissociation constant, Ka, is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction known as dissociation in the context of acid-base reactions...
, host-guest
Host-guest chemistry
In supramolecular chemistry, host-guest chemistry describes complexes that are composed of two or more molecules or ions that are held together in unique structural relationships by forces other than those of full covalent bonds. Host-guest chemistry encompasses the idea of molecular recognition...
, metal-complex
Stability constants of complexes
A stability constant is an equilibrium constant for the formation of a complex in solution. It is a measure of the strength of the interaction between the reagents that come together to form the complex...
, solubility
Solubility
Solubility is the property of a solid, liquid, or gaseous chemical substance called solute to dissolve in a solid, liquid, or gaseous solvent to form a homogeneous solution of the solute in the solvent. The solubility of a substance fundamentally depends on the used solvent as well as on...
, partition
Partition coefficient
In chemistry and the pharmaceutical sciences, a partition- or distribution coefficient is the ratio of concentrations of a compound in the two phases of a mixture of two immiscible solvents at equilibrium. The terms "gas/liquid partition coefficient" and "air/water partition coefficient" are...
, chromatography
Chromatography
Chromatography is the collective term for a set of laboratory techniques for the separation of mixtures....
and redox
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
equilibria.
Thermodynamic equilibrium
A chemical system is said to be in equilibrium when the quantities of the chemical entities involved do not and cannot change in time without the application of an external influence. In this sense a system in chemical equilibrium is in a stableStable
A stable is a building in which livestock, especially horses, are kept. It most commonly means a building that is divided into separate stalls for individual animals...
state. The system at chemical equilibrium will be at a constant temperature, pressure (or volume) and composition. It will be insulated from exchange of heat with the surroundings, that is, it is a closed system
Closed system
-In physics:In thermodynamics, a closed system can exchange energy , but not matter, with its surroundings.In contrast, an isolated system cannot exchange any of heat, work, or matter with the surroundings, while an open system can exchange all of heat, work and matter.For a simple system, with...
. A change of temperature, pressure (or volume) constitutes an external influence and the equilibrium quantities will change as a result of such a change. If there is a possibility that the composition might change, but the rate of change is negligibly slow, the system is said to be in a metastable state. The equation of chemical equilibrium can be expressed symbolically as
- reactant(s) product(s)
The sign means "are in equilibrium with". This definition refers to macroscopic
Macroscopic
The macroscopic scale is the length scale on which objects or processes are of a size which is measurable and observable by the naked eye.When applied to phenomena and abstract objects, the macroscopic scale describes existence in the world as we perceive it, often in contrast to experiences or...
properties. Changes do occur at the microscopic level of atoms and molecules, but to such a minute extent that they are not measurable and in a balanced way so that the macroscopic quantities do not change. Chemical equilibrium is a dynamic state in which forward and backward reactions proceed at such rates that the macroscopic composition of the mixture is constant. Thus, equilibrium sign symbolizes the fact that reactions occur in both forward
 and backward
and backward  directions.
directions.
Steady state (chemistry)
In chemistry, a steady state is a situation in which all state variables are constant in spite of ongoing processes that strive to change them. For an entire system to be at steady state, i.e. for all state variables of a system to be constant, there must be a flow through the system...
, on the other hand, is not necessarily an equilibrium state in the chemical sense. For example, in a radioactive decay chain
Decay chain
In nuclear science, the decay chain refers to the radioactive decay of different discrete radioactive decay products as a chained series of transformations...
the concentrations of intermediate isotopes are constant because the rate of production is equal to the rate of decay. It is not a chemical equilibrium because the decay process occurs in one direction only.
Thermodynamic equilibrium is characterized by the free energy for the whole (closed) system being a minimum. For systems at constant volume the Helmholtz free energy
Helmholtz free energy
In thermodynamics, the Helmholtz free energy is a thermodynamic potential that measures the “useful” work obtainable from a closed thermodynamic system at a constant temperature and volume...
is minimum and for systems at constant pressure the Gibbs free energy
Gibbs free energy
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
is minimum. Thus a metastable state is one for which the free energy change between reactants and products is not minimal even though the composition does not change in time.
The existence of this minimum is due to the free energy of mixing of reactants and products being always negative. For ideal solution
Ideal solution
In chemistry, an ideal solution or ideal mixture is a solution with thermodynamic properties analogous to those of a mixture of ideal gases. The enthalpy of solution is zero as is the volume change on mixing; the closer to zero the enthalpy of solution is, the more "ideal" the behavior of the...
s the enthalpy
Enthalpy
Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
of mixing is zero, so the minimum exists because the entropy
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
of mixing is always positive. The slope of the reaction free energy, δGr with respect to the reaction coordinate
Reaction coordinate
In chemistry, a reaction coordinate is an abstract one-dimensional coordinate which represents progress along a reaction pathway. It is usually a geometric parameter that changes during the conversion of one or more molecular entities....
, ξ, is zero when the free energy is at its minimum value.
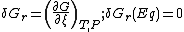
Equilibrium constant
Chemical potentialChemical potential
Chemical potential, symbolized by μ, is a measure first described by the American engineer, chemist and mathematical physicist Josiah Willard Gibbs. It is the potential that a substance has to produce in order to alter a system...
is the partial molar free energy. The potential, μi, of the ith species in a chemical reaction is the partial derivative of the free energy with respect to the number of moles of that species, Ni
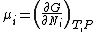
A general chemical equilibrium can be written asThe general expression is not used much in chemistry. To help understand the notation consider the equilibrium
- H2SO4 + 2 OH- SO42- + 2 H2O
for this reaction n1=1, n2=2,m1=1 and m2=2, Reactant1=H2SO4, Reactant2=OH-, Product1=SO42- and Product2=H2O.
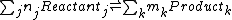
nj are the stoichiometric coefficients of the reactants in the equilibrium equation, and mj are the coefficients of the products. The value of δGr for these reactions is a function of the chemical potentials of all the species.
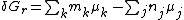
The chemical potential, μi, of the ith species can be calculated in terms of its activity
Activity (chemistry)
In chemical thermodynamics, activity is a measure of the “effective concentration” of a species in a mixture, meaning that the species' chemical potential depends on the activity of a real solution in the same way that it would depend on concentration for an ideal solution.By convention, activity...
, ai.
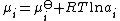
μi
 is the standard chemical potential of the species, R is the gas constant
is the standard chemical potential of the species, R is the gas constantGas constant
The gas constant is a physical constant which is featured in many fundamental equations in the physical sciences, such as the ideal gas law and the Nernst equation. It is equivalent to the Boltzmann constant, but expressed in units of energy The gas constant (also known as the molar, universal,...
and T is the temperature. Setting the sum for the reactants j to be equal to the sum for the products, k, so that δGr (Eq) = 0
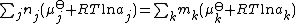
Rearranging the terms,
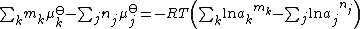
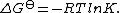
This relates the standard
Standard state
In chemistry, the standard state of a material is a reference point used to calculate its properties under different conditions. In principle, the choice of standard state is arbitrary, although the International Union of Pure and Applied Chemistry recommends a conventional set of standard states...
Gibbs free energy change, ΔG
 to an equilibrium constant, K, the reaction quotient
to an equilibrium constant, K, the reaction quotientReaction quotient
In chemistry, a reaction quotient: Qr is a function of the activities or concentrations of the chemical species involved in a chemical reaction. In the special case that the reaction is at equilibrium the reaction quotient is equal to the equilibrium constant....
of activity values at equilibrium.
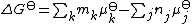
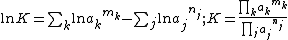
It follows that any equilibrium of this kind can be characterized either by the standard free energy change or by the equilibrium constant. In practice concentrations are more useful than activities. Activities can be calculated from concentrations if the activity coefficient
Activity coefficient
An activity coefficient is a factor used in thermodynamics to account for deviations from ideal behaviour in a mixture of chemical substances. In an ideal mixture, the interactions between each pair of chemical species are the same and, as a result, properties of the mixtures can be expressed...
are known, but this is rarely the case. Sometimes activity coefficients can be calculated using, for example, Pitzer equations
Pitzer equations
Pitzer equations are important for the understanding of the behaviour of ions dissolved in natural waters such as rivers, lakes and sea-water. The parameters of the Pitzer equations are linear combinations of parameters, of a virial expansion of the excess Gibbs free energy, which characterise...
or Specific ion interaction theory
Specific ion interaction theory
Specific ion Interaction Theory is a theory used to estimate single-ion activity coefficients in electrolyte solutions at relatively high concentrations. It does so by taking into consideration interaction coefficients between the various ions present in solution...
. Otherwise conditions must be adjusted so that activity coefficients do not vary much. For ionic solutions this is achieved by using a background ionic medium at a high concentration relative to the concentrations of the species in equilibrium.
If activity coefficients are unknown they may be subsumed into the equilibrium constant, which becomes a concentration quotient. Each activity ai is assumed to be the product of a concentration, [Ai], and an activity coefficient, γi
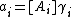
This expression for activity is placed in the expression defining the equilibrium constant.
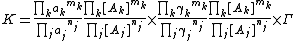
By setting the quotient of activity coefficients, Γ, equal to one This is equivalent to defining a new equilibrium constant as K / Γ the equilibrium constant is defined as a quotient of concentrations.
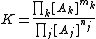
In more familiar notation, for a general equilibrium
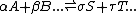
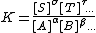
This definition is much more practical, but an equilibrium constant defined in terms of concentrations is dependent on conditions. In particular, equilibrium constants for species in aqueous solution are dependent on ionic strength
Ionic strength
The ionic strength of a solution is a measure of the concentration of ions in that solution. Ionic compounds, when dissolved in water, dissociate into ions. The total electrolyte concentration in solution will affect important properties such as the dissociation or the solubility of different salts...
, as the quotient of activity coefficients varies with the ionic strength of the solution.
The values of the standard free energy change and of the equilibrium constant are temperature dependent. To a first approximation, the van 't Hoff equation may be used.
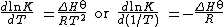
This shows that when the reaction is exothermic (ΔH
 , the standard enthalpy
, the standard enthalpyEnthalpy
Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
change, is negative), then K decreases with increasing temperature, in accordance with Le Chatelier's principle
Le Châtelier's principle
In chemistry, Le Chatelier's principle, also called the Chatelier's principle, can be used to predict the effect of a change in conditions on a chemical equilibrium. The principle is named after Henry Louis Le Chatelier and sometimes Karl Ferdinand Braun who discovered it independently...
. The approximation involved is that the standard enthalpy change, ΔH
 , is independent of temperature, which is a good approximation only over a small temperature range. Thermodynamic arguments can be used to show that
, is independent of temperature, which is a good approximation only over a small temperature range. Thermodynamic arguments can be used to show that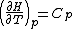
where Cp is the heat capacity at constant pressure.
Equilibria involving gases
When dealing with gases, fugacityFugacity
In chemical thermodynamics, the fugacity of a real gas is an effective pressure which replaces the true mechanical pressure in accurate chemical equilibrium calculations. It is equal to the pressure of an ideal gas which has the same chemical potential as the real gas. For example, nitrogen gas ...
, f, is used rather than activity. However, whereas activity is dimension
Dimension
In physics and mathematics, the dimension of a space or object is informally defined as the minimum number of coordinates needed to specify any point within it. Thus a line has a dimension of one because only one coordinate is needed to specify a point on it...
less, fugacity has the dimension of pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
. A consequence is that chemical potential has to be defined in terms of a standard pressure, p

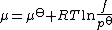
By convention p
 is usually taken to be 1 bar
is usually taken to be 1 barBar (unit)
The bar is a unit of pressure equal to 100 kilopascals, and roughly equal to the atmospheric pressure on Earth at sea level. Other units derived from the bar are the megabar , kilobar , decibar , centibar , and millibar...
Fugacity can be expressed as the product of partial pressure
Partial pressure
In a mixture of ideal gases, each gas has a partial pressure which is the pressure which the gas would have if it alone occupied the volume. The total pressure of a gas mixture is the sum of the partial pressures of each individual gas in the mixture....
, p, and a fugacity coefficient, Φ
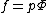
Fugacity coefficients are dimensionless and can be obtained experimentally at specific temperature and pressure, from measurements of deviations from ideal gas
Ideal gas
An ideal gas is a theoretical gas composed of a set of randomly-moving, non-interacting point particles. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics.At normal conditions such as...
behaviour. Equilibrium constants are defined in terms of fugacity. If the gases are at sufficiently low pressure that they behave as ideal gases, the equilibrium constant can be defined as a quotient of partial pressures.
An example of gas-phase equilibrium is provided by the Haber-Bosch process of ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
synthesis.
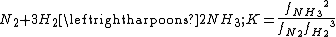
This reaction is strongly exothermic
Exothermic
In thermodynamics, the term exothermic describes a process or reaction that releases energy from the system, usually in the form of heat, but also in the form of light , electricity , or sound...
, so the equilibrium constant decreases with temperature. However, a temperature of around 400°C is required in order to achieve a reasonable rate of reaction with currently available catalysts. Formation of ammonia is also favoured by high pressure, as the volume decreases when the reaction takes place. It is interesting to note that the same reaction, nitrogen fixation
Nitrogen fixation
Nitrogen fixation is the natural process, either biological or abiotic, by which nitrogen in the atmosphere is converted into ammonia . This process is essential for life because fixed nitrogen is required to biosynthesize the basic building blocks of life, e.g., nucleotides for DNA and RNA and...
, occurs at ambient temperatures in nature, when the catalyst is an enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
such as nitrogenase
Nitrogenase
Nitrogenases are enzymes used by some organisms to fix atmospheric nitrogen gas . It is the only known family of enzymes that accomplish this process. Dinitrogen is quite inert because of the strength of its N-N triple bond...
. Much energy is needed initially to break the N-N triple bond even though the overall reaction is exothermic.
Gas-phase equilibria occur during combustion
Combustion
Combustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
and were studied as early as 1943 in connection with the development of the V2
V-2 rocket
The V-2 rocket , technical name Aggregat-4 , was a ballistic missile that was developed at the beginning of the Second World War in Germany, specifically targeted at London and later Antwerp. The liquid-propellant rocket was the world's first long-range combat-ballistic missile and first known...
rocket engine
Rocket engine
A rocket engine, or simply "rocket", is a jet engineRocket Propulsion Elements; 7th edition- chapter 1 that uses only propellant mass for forming its high speed propulsive jet. Rocket engines are reaction engines and obtain thrust in accordance with Newton's third law...
.
The calculation of composition for a gaseous equilibrium at constant pressure is often carried out using ΔG values, rather than equilibrium constants.
Multiple equilibria
Two or more equilibria can exist at the same time. When this is so, equilibrium constants can be ascribed to individual equilibria, but they are not always unique. For example, three equilibrium constants can be defined for a dibasicDibasic
Dibasic is an adjective meaning:# containing two hydrogen atoms that can be replaced by metal ions, or# "of or relating to salts or acids forming salts with two atoms of a univalent metal."...
acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
, H2A.The definitions given are association constants. A dissociation constant is the reciprocal of an association constant
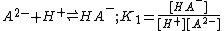
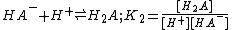
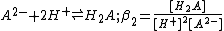
The three constants are not independent of each other and it is easy to see that β2= K1K2. The constants K1 and K2 are stepwise constants and β is an example of an overall constant.
Speciation

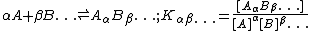
The concentrations of species containing reagent A are constrained by a condition of mass-balance
Conservation of mass
The law of conservation of mass, also known as the principle of mass/matter conservation, states that the mass of an isolated system will remain constant over time...
, that is, the total (or analytical) concentration, which is the sum of all species' concentrations, must be constant. There is one mass-balance equation for each reagent of the type
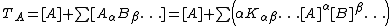
There are as many mass-balance equations as there are reagents, A, B .., so if the equilibrium constant values are known, there are n mass-balance equations in n unknowns, [A], [B].., the so-called free reagent concentrations. Solution of these equations gives all the information needed to calculate the concentrations of all the species.
Thus, the importance of an equilibrium constants lies in the fact that, once their values have been determined by experiment, they can be used to calculate the concentrations, known as the speciation
Speciation of ions
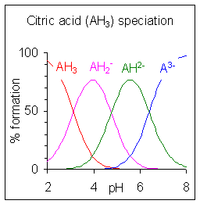
Speciation of ions refers to the changing concentration of varying forms of an ion as the pH of the solution changes.The pH of a solution of a monoprotic weak acid can be expressed in terms of the extent of dissociation. After rearranging the expression defining the acid dissociation constant, and...
, of mixtures that contain the relevant species.
Determination
There are five main types of experimental data that are used for the determination of solution equilibrium constants. Potentiometric data obtained with a glass electrodeGlass electrode
A glass electrode is a type of ion-selective electrode made of a doped glass membrane that is sensitive to a specific ion. It is an important part of the instrumentation for chemical analysis and physico-chemical studies. In modern practice, widely used membranous ion-selective electrodes are part...
are the most widely used with aqueous solutions. The others are Spectrophotometric
Spectrophotometry
In chemistry, spectrophotometry is the quantitative measurement of the reflection or transmission properties of a material as a function of wavelength...
, Fluorescence
Fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation of a different wavelength. It is a form of luminescence. In most cases, emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation...
(luminescence) measurements and NMR
NMR
NMR may refer to:Applications of Nuclear Magnetic Resonance:* Nuclear magnetic resonance* NMR spectroscopy* Solid-state nuclear magnetic resonance* Protein nuclear magnetic resonance spectroscopy* Proton NMR* Carbon-13 NMR...
chemical shift
Chemical shift
In nuclear magnetic resonance spectroscopy, the chemical shift is the resonant frequency of a nucleus relative to a standard. Often the position and number of chemical shifts are diagnostic of the structure of a molecule...
measurements; simultaneous measurement of K and
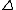 H for 1:1 adducts in biological systems is routinely carried out using Isothermal Titration Calorimetry
H for 1:1 adducts in biological systems is routinely carried out using Isothermal Titration CalorimetryIsothermal Titration Calorimetry
Isothermal titration calorimetry is a physical technique used to determine the thermodynamic parameters of interactions in solution. It is most often used to study the binding of small molecules to larger macromolecules .-Thermodynamic measurements:ITC is a quantitative technique that can...
.
The experimental data will comprise a set of data points. At the i'th data point, the analytical concentrations of the reactants,
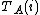 ,
, 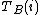 etc. will be experimentally known quantities and there will be one or more measured quantities, yi, that depend in some way on the analytical concentrations and equilibrium constants. A general computational procedure has three main components.
etc. will be experimentally known quantities and there will be one or more measured quantities, yi, that depend in some way on the analytical concentrations and equilibrium constants. A general computational procedure has three main components.
- Definition of a chemical model of the equilibria. The model consists of a list of reagents, A, B, etc. and the complexes formed from them, with stoichiometries ApBq... Known or estimated values of the equilibrium constants for the formation of all complexes must be supplied.
- Calculation of the concentrations of all the chemical species in each solution. The free concentrations are calculated by solving the equations of mass-balance, and the concentrations of the complexes are calculated using the equilibrium constant definitions. A quantity corresponding to the observed quantity can then be calculated using physical principles such as the Nernst potential or Beer-Lambert lawBeer-Lambert lawIn optics, the Beer–Lambert law, also known as Beer's law or the Lambert–Beer law or the Beer–Lambert–Bouguer law relates the absorption of light to the properties of the material through which the light is travelling.-Equations:The law states that there is a logarithmic dependence between the...
which relate the calculated quantity to the concentrations of the species. - Refinement of the equilibrium constants. Usually a Non-linear least squaresNon-linear least squaresNon-linear least squares is the form of least squares analysis which is used to fit a set of m observations with a model that is non-linear in n unknown parameters . It is used in some forms of non-linear regression. The basis of the method is to approximate the model by a linear one and to...
procedure is used. A weighted sum of squares, U, is minimized.
-
- The weights, wi and quantities y may be vectors. Values of the equilibrium constants are refined in an iterative procedure.
Acid-base equilibria
Brønsted and Lowry characterized an acid-base equilibrium as involving a proton exchange reaction:- acid + base conjugate base + conjugate acid.
An acid is a proton donor; the proton is transferred to the base, a proton acceptor, creating a conjugate acid. For aqueous solutions of an acid HA, the base is water; the conjugate base is A− and the conjugate acid is the solvated hydrogen ion. In solution chemistry, it is usual to use H+ as an abbreviation for the solvated hydrogen ion, regardless of the solvent. In aqueous solution H+ denotes a solvated hydronium ion.The bare proton does not exist in aqueous solution. It is a very strong acid and combines the base, water, to form the hydronium ion
- H+ + H2O → H3O+
The hydronium ion forms various weak complexes by hydrogen bonding with more water molecules
The Brønsted–Lowry definition applies to other solvents, such as dimethyl sulfoxide
Dimethyl sulfoxide
Dimethyl sulfoxide is an organosulfur compound with the formula 2SO. This colorless liquid is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water...
: the solvent S acts as a base, accepting a proton and forming the conjugate acid SH+.
A broader definition of acid dissociation includes hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
, in which protons are produced by the splitting of water molecules. For example, boric acid
Boric acid
Boric acid, also called hydrogen borate or boracic acid or orthoboric acid or acidum boricum, is a weak acid of boron often used as an antiseptic, insecticide, flame retardant, as a neutron absorber, and as a precursor of other chemical compounds. It exists in the form of colorless crystals or a...
, B(OH)3, acts as a weak acid, even though it is not a proton donor, because of the hydrolysis equilibrium
- B(OH)3 + H2O B(OH)4− + H+.
Similarly, metal ion hydrolysis causes ions such as [Al(H2O)6]3+ to behave as weak acids:
- [Al(H2O)6]3+ [Al(H2O)5(OH)]2+ + H+.
Acid-base equilibria are important in a very wide range of applications, such as acid-base homeostasis
Acid-base homeostasis
Acid–base homeostasis is the part of human homeostasis concerning the proper balance between acids and bases, in other words, the pH. The body is very sensitive to its pH level, so strong mechanisms exist to maintain it...
, ocean acidification
Ocean acidification
Ocean acidification is the name given to the ongoing decrease in the pH and increase in acidity of the Earth's oceans, caused by the uptake of anthropogenic carbon dioxide from the atmosphere....
, pharmacology
Pharmacology
Pharmacology is the branch of medicine and biology concerned with the study of drug action. More specifically, it is the study of the interactions that occur between a living organism and chemicals that affect normal or abnormal biochemical function...
and analytical chemistry
Analytical chemistry
Analytical chemistry is the study of the separation, identification, and quantification of the chemical components of natural and artificial materials. Qualitative analysis gives an indication of the identity of the chemical species in the sample and quantitative analysis determines the amount of...
.
Host-guest equilibria
A host-guest complex, also known as a donor-acceptor complex, may be formed from a Lewis base, B, and a Lewis acidLewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
, A. The host may be either a donor or an acceptor. In biochemistry
Biochemistry
Biochemistry, sometimes called biological chemistry, is the study of chemical processes in living organisms, including, but not limited to, living matter. Biochemistry governs all living organisms and living processes...
host-guest complexes are known as receptor
Receptor (biochemistry)
In biochemistry, a receptor is a molecule found on the surface of a cell, which receives specific chemical signals from neighbouring cells or the wider environment within an organism...
-ligand complexes; they are formed primarily by non-covalent bonding. Many host-guest complexes has 1:1 stoichiometry, but many others have more complex structures. The general equilibrium can be written as
- pA +qB ApBq
The study of these complexes is important for supramolecular chemistry
Supramolecular chemistry
Supramolecular chemistry refers to the area of chemistry beyond the molecules and focuses on the chemical systems made up of a discrete number of assembled molecular subunits or components...
and molecular recognition
Molecular recognition
The term molecular recognition refers to the specific interaction between two or more molecules through noncovalent bonding such as hydrogen bonding, metal coordination, hydrophobic forces, van der Waals forces, π-π interactions, electrostatic and/or electromagnetic effects...
. The objective of these studies is often to find systems with a high binding selectivity
Binding selectivity
Binding selectivity refers to the differing affinities with which different ligands bind to a substrate forming a complex. A selectivity coefficient is the equilibrium constant for the reaction of displacement by one ligand of another ligand in a complex with the substrate...
of a host (receptor) for a particular target molecule or ion, the guest or ligand. An application is the development of chemical sensors. Finding a drug which either blocks a receptor, an antagonist
Antagonist
An antagonist is a character, group of characters, or institution, that represents the opposition against which the protagonist must contend...
which forms a strong complex the receptor, or activate it, an agonist
Agonist
An agonist is a chemical that binds to a receptor of a cell and triggers a response by that cell. Agonists often mimic the action of a naturally occurring substance...
, is an important pathway to drug discovery
Drug discovery
In the fields of medicine, biotechnology and pharmacology, drug discovery is the process by which drugs are discovered or designed.In the past most drugs have been discovered either by identifying the active ingredient from traditional remedies or by serendipitous discovery...
.
Complexes of metals
The formation of a complex between a metal ion, M, and a ligand, L, is in fact usually a substitution reaction. For example, In aqueous solutionAqueous solution
An aqueous solution is a solution in which the solvent is water. It is usually shown in chemical equations by appending aq to the relevant formula, such as NaCl. The word aqueous means pertaining to, related to, similar to, or dissolved in water...
s, metal ions will be present as aqua-ions
Metal ions in aqueous solution
A metal ion in aqueous solution is a cation, dissolved in water, of chemical formula [Mn]z+. The solvation number, n, determined by a variety of experimental methods is 4 for Li+ and Be2+ and 6 for elements in rows 3 and 4 of the periodic table. Lanthanide and actinide aqua ions have solvation...
, so the reaction for the formation of the first complex could be written asElectrical charges are omitted from such expressions because the ligand, L, may or may not carry an electrical charge.
- [M(H2O)n] + L [M(H2O)n-1L] +H2O
However, since water is in vast excess, the concentration of water is usually assumed to be constant and is omitted from equilibrium constant expressions. Often, the metal and the ligand are in competition for protons. For the equilibrium
- pM + qL +rH MpLqHr
a stability constant can be defined as follows.
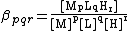
The definition can easily be extended to include any number of reagents. It includes hydroxide
Hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and a hydrogen atom held together by a covalent bond, and carrying a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, as a ligand, a nucleophile, and a...
complexes because the concentration of the hydroxide ions is related to the concentration of hydrogen ions by the self-ionization of water
Self-ionization of water
The self-ionization of water is the chemical reaction in which a proton is transferred from one water molecule to another, in pure water or an aqueous solution, to create the two ions, hydronium, H3O+ and hydroxide, OH−...
- [OH-] = KW [H+]-1
Stability constants defined in this way, are association constants. This can lead to some confusion as pKa values
Acid dissociation constant
An acid dissociation constant, Ka, is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction known as dissociation in the context of acid-base reactions...
are dissociation constants. In general purpose computer programs it is customary to define all constants as association constants. The relationship between the two types of constant is given in association and dissociation constants.
In biochemistry
Biochemistry
Biochemistry, sometimes called biological chemistry, is the study of chemical processes in living organisms, including, but not limited to, living matter. Biochemistry governs all living organisms and living processes...
, an oxygen molecule can bind to an iron (II) atom in a heme
Heme
A heme or haem is a prosthetic group that consists of an iron atom contained in the center of a large heterocyclic organic ring called a porphyrin. Not all porphyrins contain iron, but a substantial fraction of porphyrin-containing metalloproteins have heme as their prosthetic group; these are...
prosthetic group in hemoglobin
Hemoglobin
Hemoglobin is the iron-containing oxygen-transport metalloprotein in the red blood cells of all vertebrates, with the exception of the fish family Channichthyidae, as well as the tissues of some invertebrates...
. The equilibrium is usually written, denoting hemoglobin by Hb, as
- Hb + O2 HbO2
but this representation is incomplete as the Bohr effect
Bohr effect
Bohr effect is a property of hemoglobin first described in 1904 by the Danish physiologist Christian Bohr , which states that an increasing concentration of protons and/or carbon dioxide will reduce the oxygen affinity of hemoglobin...
shows that the equilibrium concentrations are pH-dependent. A better representation would be
- [HbH]+ + O2 HbO2 + H+
as this shows that when hydrogen ion concentration increases the equilibrium is shifted to the left in accordance with Le Chatelier's principle
Le Châtelier's principle
In chemistry, Le Chatelier's principle, also called the Chatelier's principle, can be used to predict the effect of a change in conditions on a chemical equilibrium. The principle is named after Henry Louis Le Chatelier and sometimes Karl Ferdinand Braun who discovered it independently...
. Hydrogen ion concentration can be increased by the presence of carbon dioxide, which behaves as a weak acid.
- H2O + CO2 HCO3- + H+
The iron atom can also bind to other molecules such as carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
. Cigarette smoke contains some carbon monoxide so the equilibrium
- HbO2 + CO Hb(CO) + O2
is established in the blood of cigarette smokers.
Chelation therapy
Chelation therapy
Chelation therapy is the administration of chelating agents to remove heavy metals from the body. For the most common forms of heavy metal intoxication—those involving lead, arsenic or mercury—the standard of care in the United States dictates the use of dimercaptosuccinic acid...
is based on the principle of using chelating ligands with a high binding selectivity
Binding selectivity
Binding selectivity refers to the differing affinities with which different ligands bind to a substrate forming a complex. A selectivity coefficient is the equilibrium constant for the reaction of displacement by one ligand of another ligand in a complex with the substrate...
for a particular metal to remove that metal from the human body.
Complexes with polyamino carboxylic acid
Polyamino carboxylic acid
thumb|left|120px|a metal complex with the [[EDTA]] anionthumb|120px|the [[glycine|glycinate]] ion can form a chelate complex with a metal ionthumb|120px| β [[alanine]]...
s find a wide range of applications. EDTA in particular is used extensively.
Redox equilibria
A reduction-oxidation (redoxRedox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
) equilibrium can be handled in exactly the same way as any other chemical equilibrium. For example
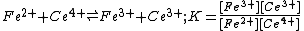
However, in the case of redox reactions it is convenient to split the overall reaction into two half-reactions. In this example
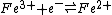
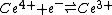
The standard free energy change, which is related to the equilibrium constant by
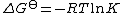
can be split into two components,
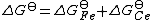
The concentration of free electrons is effectively zero as the electrons are transferred directly from the reductant to the oxidant. The standard electrode potential
Standard electrode potential
In electrochemistry, the standard electrode potential, abbreviated E° or E , is the measure of individual potential of a reversible electrode at standard state, which is with solutes at an effective concentration of 1 mol dm−3, and gases at a pressure of 1 atm...
, E0 for the each half-reaction is related to the standard free energy change by
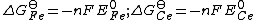
where n is the number of electrons transferred and F is the Faraday constant. Now, the free energy for an actual reaction is given by
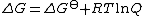
where R is the gas constant
Gas constant
The gas constant is a physical constant which is featured in many fundamental equations in the physical sciences, such as the ideal gas law and the Nernst equation. It is equivalent to the Boltzmann constant, but expressed in units of energy The gas constant (also known as the molar, universal,...
and Q a reaction quotient
Reaction quotient
In chemistry, a reaction quotient: Qr is a function of the activities or concentrations of the chemical species involved in a chemical reaction. In the special case that the reaction is at equilibrium the reaction quotient is equal to the equilibrium constant....
. Strictly speaking Q is a quotient of activities, but it is common practice to use concentrations instead of activities. Therefore
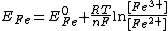
For any half-reaction, the redox potential of an actual mixture is given by the generalized expressionThe alternative expression
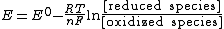
is sometimes used, as in Nernst equation
Nernst equation
In electrochemistry, the Nernst equation is an equation that can be used to determine the equilibrium reduction potential of a half-cell in an electrochemical cell. It can also be used to determine the total voltage for a full electrochemical cell...
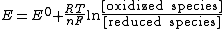
This is an example of the Nernst equation
Nernst equation
In electrochemistry, the Nernst equation is an equation that can be used to determine the equilibrium reduction potential of a half-cell in an electrochemical cell. It can also be used to determine the total voltage for a full electrochemical cell...
. The potential is known as a reduction potential. Standard electrode potentials are available in a table of values
Standard electrode potential (data page)
The values of standard electrode potentials are given in the table below in volts relative to the standard hydrogen electrode and are assembled from referencesThe values are for the following conditions:* the temperature of 298.15 K ;...
. Using these values, the actual electrode potential for a redox couple can be calculated as a function of the ratio of concentrations.
The equilibrium potential for a general redox half-reaction (See #Equilibrium constant above for an explanation of the symbols)
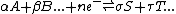
is given by
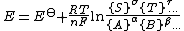
Use of this expression allows the effect of a species not involved in the redox reaction, such as the hydrogen ion in a half-reaction such as
- MnO4- + 8H+ +5e- Mn2+ + 4H2O
to be taken into account.
The equilibrium constant for a full redox reaction can be obtained from the standard redox potentials of the constituent half-reactions. At equilibrium the potential for the two half-reactions must be equal to each other and, of course, the number of electrons exchanged must be the same in the two half reactions.
Redox equilibria play an important role in the electron transport chain
Electron transport chain
An electron transport chain couples electron transfer between an electron donor and an electron acceptor with the transfer of H+ ions across a membrane. The resulting electrochemical proton gradient is used to generate chemical energy in the form of adenosine triphosphate...
. The various cytochrome
Cytochrome
Cytochromes are, in general, membrane-bound hemoproteins that contain heme groups and carry out electron transport.They are found either as monomeric proteins or as subunits of bigger enzymatic complexes that catalyze redox reactions....
s in the chain have different standard redox potentials, each one adapted for a specific redox reaction. This allows, for example, atmospheric oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
to be reduced in photosynthesis
Photosynthesis
Photosynthesis is a chemical process that converts carbon dioxide into organic compounds, especially sugars, using the energy from sunlight. Photosynthesis occurs in plants, algae, and many species of bacteria, but not in archaea. Photosynthetic organisms are called photoautotrophs, since they can...
. A distinct family of cytochromes, the cytochrome P450 oxidase
Cytochrome P450 oxidase
The cytochrome P450 superfamily is a large and diverse group of enzymes. The function of most CYP enzymes is to catalyze the oxidation of organic substances. The substrates of CYP enzymes include metabolic intermediates such as lipids and steroidal hormones, as well as xenobiotic substances...
s, are involved in steroidogenesis and detoxification.
Solubility
When a soluteSolution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
forms a saturated solution in a solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
, the concentration of the solute, at a given temperature, is determined by the equilibrium constant at that temperature.
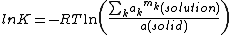
The activity of a pure substance in the solid state is one, by definition, so the expression simplifies to
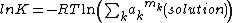
If the solute does not dissociate the summation is replaced by a single term, but if dissociation occurs, as with ionic substances
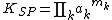
For example, with Na2SO4 m1=2 and m2=1 so the solubility product is written as
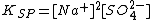
Concentrations, indicated by [..], are usually used in place of activities, but activity must be taken into account of the presence of another salt with no ions in common, the so-called salt effect. When another salt is present that has an ion in common, the common-ion effect
Common-ion effect
The common ion effect is an effect which results when two substances, which both ionize to give the same ion, are involved in a chemical equilibrium.-Solubility effects:...
comes into play, reducing the solubility of the primary solute.
Partition
When a solution of a substance in one solvent is brought into equilibrium with a second solvent that is immiscible with the first solvent, the dissolved substance may be partitioned between the two solvents. The ratio of concentrations in the two solvents is known as a partition coefficientPartition coefficient
In chemistry and the pharmaceutical sciences, a partition- or distribution coefficient is the ratio of concentrations of a compound in the two phases of a mixture of two immiscible solvents at equilibrium. The terms "gas/liquid partition coefficient" and "air/water partition coefficient" are...
or distribution coefficient.The distinction between a partition coefficient and a distribution coefficient is of historical significance only. The partition coefficient is defined as the ratio of the analytical concentrations of the solute in the two phases. By convention the value is reported in logarithmic form.
The partition coefficient is defined at a specified temperature and, if applicable, pH of the aqueous phase. Partition coefficients are very important in pharmacology
Pharmacology
Pharmacology is the branch of medicine and biology concerned with the study of drug action. More specifically, it is the study of the interactions that occur between a living organism and chemicals that affect normal or abnormal biochemical function...
because they determine the extent to which a substance can pass from the blood (an aqueous solution) through a cell wall which is like an organic solvent. They are usually measured using water and octanol
Octanol
Octanol is a straight chain fatty alcohol with eight carbon atoms and the molecular formula CH37OH. Although the term octanol usually refers exclusively to the primary alcohol 1-octanol, there are other less common isomers of octanol such as the secondary alcohols 2-octanol, 3-octanol and...
as the two solvents. Many pharmaceutical compounds are weak acid
Weak acid
A weak acid is an acid that dissociates incompletely. It does not release all of its hydrogens in a solution, donating only a partial amount of its protons to the solution...
s or weak base
Weak base
In chemistry, a weak base is a chemical base that does not ionize fully in an aqueous solution. As Brønsted–Lowry bases are proton acceptors, a weak base may also be defined as a chemical base in which protonation is incomplete. This results in a relatively low pH compared to strong bases...
s. Such a compound may exist with a different extent of protonation depending on pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
and the acid dissociation constant
Acid dissociation constant
An acid dissociation constant, Ka, is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction known as dissociation in the context of acid-base reactions...
. Because the organic phase has a low dielectric constant
Dielectric constant
The relative permittivity of a material under given conditions reflects the extent to which it concentrates electrostatic lines of flux. In technical terms, it is the ratio of the amount of electrical energy stored in a material by an applied voltage, relative to that stored in a vacuum...
the species with no electrical charge will be the most likely one to pass from the aqueous phase to the organic phase. Even at pH 7-7.2, the range of biological pH values, the aqueous phase may support an equilibrium between more than one protonated form. Log p is determined from the analytical concentration of the substance in the aqueous phase, that is, the sum of the concentration of the different species in equilibrium.
Solvent extraction is used extensively in separation and purification processes. In its simplest form a reaction is performed in an organic solvent and unwanted by-products are removed by extraction into water at a particular pH.
A metal ion may be extracted from an aqueous phase into an organic phase in which the salt is not soluble, by adding a ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
. The ligand, La-, forms a complex with the metal ion, Mb+, [MLx])b-ax)+ which has a strongly hydrophobic outer surface. If the complex has no electrical charge it will be extracted relatively easily into the organic phase. If the complex is charged, it is extracted as an ion pair. The additional ligand is not always required. For example, uranyl nitrate
Uranyl nitrate
Uranyl nitrate is a water soluble yellow uranium salt. The yellow-green crystals of uranium nitrate hexahydrate are triboluminescent.Uranyl nitrate can be prepared by reaction of uranium salts with nitric acid...
, UO2(NO3)2, is soluble in diethyl ether
Diethyl ether
Diethyl ether, also known as ethyl ether, simply ether, or ethoxyethane, is an organic compound in the ether class with the formula . It is a colorless, highly volatile flammable liquid with a characteristic odor...
because the solvent itself acts as a ligand. This property was used in the past for separating uranium from other metals whose salts are not soluble in ether. Currently extraction into kerosene
Kerosene
Kerosene, sometimes spelled kerosine in scientific and industrial usage, also known as paraffin or paraffin oil in the United Kingdom, Hong Kong, Ireland and South Africa, is a combustible hydrocarbon liquid. The name is derived from Greek keros...
is preferred, using a ligand such as tri-n-butyl phosphate, TBP. In the PUREX
PUREX
PUREX is an acronym standing for Plutonium - URanium EXtraction — de facto standard aqueous nuclear reprocessing method for the recovery of uranium and plutonium from used nuclear fuel. It is based on liquid-liquid extraction ion-exchange.The PUREX process was invented by Herbert H. Anderson and...
process, which is commonly used in nuclear reprocessing
Nuclear reprocessing
Nuclear reprocessing technology was developed to chemically separate and recover fissionable plutonium from irradiated nuclear fuel. Reprocessing serves multiple purposes, whose relative importance has changed over time. Originally reprocessing was used solely to extract plutonium for producing...
, uranium(VI) is extracted from strong nitric acid as the electrically neutral complex [UO2(TBP)2(NO3)2]. The strong nitric acid provides a high concentration of nitrate ions which pushes the equilibrium in favour of the weak nitrato complex. Uranium is recovered by back-extraction (stripping) into weak nitric acid. Plutonium(IV) forms a similar complex, [PuO2(TBP)2(NO3)2] and the plutonium in this complex can be reduced to separate it from uranium.
Another important application of solvent extraction is in the separation of the lanthanoids. This process also uses TBP and the complexes are extracted into kerosene. Separation is achieved because the stability constant
Stability constants of complexes
A stability constant is an equilibrium constant for the formation of a complex in solution. It is a measure of the strength of the interaction between the reagents that come together to form the complex...
for the formation of the TBP complex increases as the size of the lanthanoid ion decreases.
An instance of ion-pair extraction is in the use of a ligand to enable oxidation by potassium permanganate
Potassium permanganate
Potassium permanganate is an inorganic chemical compound with the formula KMnO4. It is a salt consisting of K+ and MnO4− ions. Formerly known as permanganate of potash or Condy's crystals, it is a strong oxidizing agent. It dissolves in water to give intensely purple solutions, the...
, KMnO4, in an organic solvent. KMnO4 is not soluble in organic solvents. When a ligand, such as a crown ether
Crown ether
Crown ethers are cyclic chemical compounds that consist of a ring containing several ether groups. The most common crown ethers are oligomers of ethylene oxide, the repeating unit being ethyleneoxy, i.e., -CH2CH2O-. Important members of this series are the tetramer , the pentamer , and the hexamer...
is added to an aqueous solution of KMnO4, it forms a hydrophobic complex with the potassium cation which allows the uncharged ion-pair, {[KL]+[MnO4]-} to be extracted into the organic solvent. See also: phase-transfer catalysis.
Chromatography
In chromatography substances are separated by partition between a stationary phase and a mobile phase. The analyte is dissolved in the mobile phase, and passes over the stationary phase. Separation occurs because of differing affinities of the analyteAnalyte
An analyte, or component , is a substance or chemical constituent that is of interest in an analytical procedure. Grammatically, it is important to note that experiments always seek to measure properties of analytes—and that analytes themselves can never be measured. For instance, one cannot...
s for the stationary phase. A distribution constant, Kd can be defined as
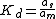
where as and am are the equilibrium activities in the stationary and mobile phases respectively. It can be shown that the rate of migration,
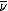 , is related to the distribution constant by
, is related to the distribution constant by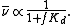
f is a factor which depends on the volumes of the two phases. Thus, the higher the affinity of the solute for the stationary phase, the slower the migration rate.
There is a wide variety of chromatographic techniques, depending on the nature of the stationary and mobile phases. When the stationary phase is solid, the analyte may form a complex with it. A water softener functions by selective complexation with a sulfonate
Sulfonate
A sulfonate is a salt or ester of a sulfonic acid. It contains the functional group R-SO2O-.- Sulfonate salts:Anions with the general formula RSO2O− are called sulfonates. They are the conjugate bases of sulfonic acids with formula RSO2OH. As sulfonic acids tend to be strong acids, the...
ion exchange resin
Ion exchange resin
An ion-exchange resin or ion-exchange polymer is an insoluble matrix normally in the form of small beads, usually white or yellowish, fabricated from an organic polymer substrate. The material has highly developed structure of pores on the surface of which are sites with easily trapped and...
. Sodium ions form relatively weak complexes with the resin. When hard water
Hard water
Hard water is water that has high mineral content . Hard water has high concentrations of Ca2+ and Mg2+ ions. Hard water is generally not harmful to one's health but can pose serious problems in industrial settings, where water hardness is monitored to avoid costly breakdowns in boilers, cooling...
is passed through the resin, the divalent ions of magnesium and calcium displace the sodium ions and are retained on the resin, R.
- RNa + M2+ RM+ + Na+
The water coming out of the column is relatively rich in sodium ionsFeeding babies formula made up with sodium rich water can lead to hypernatremia
Hypernatremia
Hypernatremia or hypernatraemia is an electrolyte disturbance that is defined by an elevated sodium level in the blood. Hypernatremia is generally not caused by an excess of sodium, but rather by a relative deficit of free water in the body...
. and poor in calcium and magnesium which are retained on the column. The column is regenerated by passing a strong solution of sodium chloride through it, so that the resin- sodium complex is again formed on the column. Ion-exchange chromatography utilizes a resin such as chelex 100
Chelex 100
Chelex 100 is a chelating material from Bio-Rad used to purify other compounds via ion exchange. It is noteworthy for its ability to bind transition metal ions.It is a styrene-divinylbenzene co-polymer containing iminodiacetic acid groups....
in which iminodiacetate
Iminodiacetic acid
Iminodiacetic acid, HN2, often abbreviated to IDA, is an dicarboxylic acid amine. The iminodiacetate anion can act as a tridentate ligand to form a metal complex with two, fused, five membered chelate rings...
residues, attached to a polymer backbone, form chelate complexes of differing strengths with different metal ions, allowing the ions such as Cu2+ and Ni2+ to be separated chromatographically.
Another example of complex formation is in chiral chromatography in which is used to separate enantiomer
Enantiomer
In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable , much as one's left and right hands are the same except for opposite orientation. It can be clearly understood if you try to place your hands one over the other without...
s from each other. The stationary phase is itself chiral and forms complexes selectively with the enantiomers. In other types of chromatography with a solid stationary phase, such as thin layer chromatography
Thin layer chromatography
Thin layer chromatography is a chromatography technique used to separate mixtures. Thin layer chromatography is performed on a sheet of glass, plastic, or aluminum foil, which is coated with a thin layer of adsorbent material, usually silica gel, aluminium oxide, or cellulose...
the analyte is selectively adsorbed onto the solid.
In gas-liquid chromatography
Gas-liquid chromatography
Gas chromatography , is a common type of chromatography used in analytical chemistry for separating and analysing compounds that can be vaporized without decomposition. Typical uses of GC include testing the purity of a particular substance, or separating the different components of a mixture...
(GLC) the stationary phase is a liquid such as polydimethylsiloxane
Polydimethylsiloxane
Polydimethylsiloxane belongs to a group of polymeric organosilicon compounds that are commonly referred to as silicones. PDMS is the most widely used silicon-based organic polymer, and is particularly known for its unusual rheological properties. PDMS is optically clear, and, in general, is...
, coated on a glass tube. Separation is achieved because the various components in the gas have different solubility in the stationary phase. GLC can be used to separate literally hundreds of components in a gas mixture such as cigarette smoke or essential oil
Essential oil
An essential oil is a concentrated hydrophobic liquid containing volatile aroma compounds from plants. Essential oils are also known as volatile oils, ethereal oils or aetherolea, or simply as the "oil of" the plant from which they were extracted, such as oil of clove...
s, such as lavender oil.
External links
- Chemical Equilibrium Downloadable book