
Acid dissociation constant
Encyclopedia
An acid dissociation constant, Ka, (also known as acidity constant, or acid-ionization constant) is a quantitative
measure of the strength of an acid
in solution. It is the equilibrium constant for a chemical reaction known as dissociation
in the context of acid-base reactions. The equilibrium can be written symbolically as:
where HA is a generic acid
that dissociates by splitting into A−, known as the conjugate base of the acid, and the hydrogen ion
or proton
, H+, which, in the case of aqueous solutions, exists as a solvated hydronium
ion. In the example shown in the figure, HA represents acetic acid
, and A− the acetate ion. The chemical species HA, A− and H+ are said to be in equilibrium when their concentrations do not change with the passing of time. The dissociation constant is usually written as a quotient of the equilibrium concentrations (in mol/L), denoted by [HA], [A−] and [H+]:
Due to the many orders of magnitude spanned by Ka values, a logarithmic
measure of the acid dissociation constant is more commonly used in practice. The logarithmic constant, pKa, which is equal to −log10 Ka, is sometimes also (but incorrectly) referred to as an acid dissociation constant:

The larger the value of pKa, the smaller the extent of dissociation. A weak acid
has a pKa value in the approximate range −2 to 12 in water. Acids with a pKa value of less than about −2 are said to be strong acids; a strong acid is almost completely dissociated in aqueous solution, to the extent that the concentration of the undissociated acid becomes undetectable. pKa values for strong acids can, however, be estimated by theoretical means or by extrapolating from measurements in non-aqueous solvent
s in which the dissociation constant is smaller, such as acetonitrile
and dimethylsulfoxide.
of the dissociation reaction; the pKa value is directly proportional to the standard Gibbs energy change
for the reaction. The value of the pKa changes with temperature and can be understood qualitatively based on Le Chatelier's principle
: when the reaction is endothermic
, the pKa decreases with increasing temperature; the opposite is true for exothermic
reactions. The underlying structural factors that influence the magnitude of the acid dissociation constant include Pauling's rules for acidity constants, inductive effect
s, mesomeric effect
s, and hydrogen bonding.
The quantitative behaviour of acids and bases in solution can be understood only if their pKa values are known. In particular, the pH
of a solution can be predicted when the analytical concentration and pKa values of all acids and bases are known; conversely, it is possible to calculate the equilibrium concentration of the acids and bases in solution when the pH is known. These calculations find application in many different areas of chemistry, biology, medicine, and geology. For example, many compounds used for medication are weak acids or bases, and a knowledge of the pKa values, together with the water–octanol partition coefficient
, can be used for estimating the extent to which the compound enters the blood stream. Acid dissociation constants are also essential in aquatic chemistry and chemical oceanography
, where the acidity of water plays a fundamental role. In living organisms, acid-base homeostasis
and enzyme kinetics
are dependent on the pKa values of the many acids and bases present in the cell and in the body. In chemistry, a knowledge of pKa values is necessary for the preparation of buffer solution
s and is also a prerequisite for a quantitative understanding of the interaction between acids or bases and metal ions to form complexes
. Experimentally, pKa values can be determined by potentiometric (pH) titration
, but for values of pKa less than about 2 or more than about 11, spectrophotometric
or NMR
measurements may be required due to practical difficulties with pH measurements.
in aqueous solution, releasing the hydrogen ion H+ (a proton):
The equilibrium constant for this dissociation reaction is known as a dissociation constant
. The liberated proton combines with a water molecule to give a hydronium (or oxonium) ion H3O+, and so Arrhenius later proposed that the dissociation should be written as an acid–base reaction:
Brønsted and Lowry generalised this further to a proton exchange reaction:
The acid loses a proton, leaving a conjugate base; the proton is transferred to the base, creating a conjugate acid. For aqueous solutions of an acid HA, the base is water; the conjugate base is A− and the conjugate acid is the hydronium ion. The Brønsted–Lowry definition applies to other solvents, such as dimethyl sulfoxide
: the solvent S acts as a base, accepting a proton and forming the conjugate acid SH+.
In solution chemistry, it is common to use H+ as an abbreviation for the solvated hydrogen ion, regardless of the solvent. In aqueous solution H+ denotes a solvated hydronium ion rather than a proton.
The designation of an acid or base as "conjugate" depends on the context. The conjugate acid BH+ of a base B dissociates according to
which is the reverse of the equilibrium
The hydroxide ion OH−, a well known base, is here acting as the conjugate base of the acid water. Acids and bases are thus regarded simply as donors and acceptors of protons respectively.
A broader definition of acid dissociation includes hydrolysis
, in which protons are produced by the splitting of water molecules. For example, boric acid
(B(OH)3) produces H3O+ as if it were a proton donor, but it has been confirmed by Raman spectroscopy
that this is due to the hydrolysis equilibrium:
Similarly, metal ion hydrolysis causes ions such as [Al(H2O)6]3+ to behave as weak acids:
the thermodynamic equilibrium constant, K can be defined by
can be defined by
where {A} is the activity
of the chemical species A etc. K is dimensionless since activity is dimensionless. Activities of the products of dissociation are placed in the numerator, activities of the reactants are placed in the denominator. See activity coefficient
is dimensionless since activity is dimensionless. Activities of the products of dissociation are placed in the numerator, activities of the reactants are placed in the denominator. See activity coefficient
for a derivation of this expression.
Since activity is the product of concentration
and activity coefficient
(γ) the definition could also be written as
where [HA] represents the concentration of HA and Γ is a quotient of activity coefficients.
To avoid the complications involved in using activities, dissociation constants are determined
, where possible, in a medium of high ionic strength
, that is, under conditions in which Γ can be assumed to be always constant. For example, the medium might be a solution of 0.1 M sodium nitrate
or 3 M potassium perchlorate
(1 M = 1 mol·dm−3, a unit of molar concentration). Furthermore, in all but the most concentrated solutions it can be assumed that the concentration of water, [H2O], is constant, approximately 55 mol·dm−3. On dividing K by the constant terms and writing [H+] for the concentration of the hydronium ion the expression
by the constant terms and writing [H+] for the concentration of the hydronium ion the expression
is obtained. This is the definition in common use. pKa is defined as −log10 Ka.
Note, however, that all published dissociation constant values refer to the specific ionic medium used in their determination and that different values are obtained with different conditions, as shown for acetic acid
in the illustration above. When published constants refer to an ionic strength other than the one required for a particular application, they may be adjusted by means of specific ion theory (SIT) and other theories.
Although Ka appears to have the dimension
of concentration it must in fact be dimensionless or it would not be possible to take its logarithm
. The illusion is the result of omitting the constant term [H2O] from the defining expression. Nevertheless it is not unusual, particularly in texts relating to biochemical equilibria, to see a value quoted with a dimension as, for example, "Ka = 300 M".
 After rearranging the expression defining Ka, and putting pH = −log10[H+], one obtains
After rearranging the expression defining Ka, and putting pH = −log10[H+], one obtains
This is a form of the Henderson–Hasselbalch equation, from which the following conclusions can be drawn.
In water, measurable pKa values range from about −2 for a strong acid to about 12 for a very weak acid (or strong base). All acids with a pKa value of less than −2 are more than 99% dissociated at pH 0 (1 M acid). This is known as solvent leveling since all such acids are brought to the same level of being strong acids, regardless of their pKa values. Likewise, all bases with a pKa value larger than the upper limit are more than 99% protonated at all attainable pH values and are classified as strong bases.
An example of a strong acid is hydrochloric acid
, HCl, which has a pKa value, estimated from thermodynamic quantities, of −9.3 in water. The concentration of undissociated acid in a 1 mol·dm−3 solution will be less than 0.01% of the concentrations of the products of dissociation. Hydrochloric acid is said to be "fully dissociated" in aqueous solution because the amount of undissociated acid is imperceptible. When the pKa and analytical concentration of the acid are known, the extent of dissociation and pH of a solution of a monoprotic acid can be easily calculated using an ICE table
.
A buffer solution
of a desired pH can be prepared as a mixture of a weak acid and its conjugate base. In practice the mixture can be created by dissolving the acid in water, and adding the requisite amount of strong acid or base. The pKa of the acid must be less than two units different from the target pH.
 Polyprotic acids are acids that can lose more than one proton. The constant for dissociation of the first proton may be denoted as Ka1 and the constants for dissociation of successive protons as Ka2, etc. Phosphoric acid
Polyprotic acids are acids that can lose more than one proton. The constant for dissociation of the first proton may be denoted as Ka1 and the constants for dissociation of successive protons as Ka2, etc. Phosphoric acid
, H3PO4, is an example of a polyprotic acid as it can lose three protons.
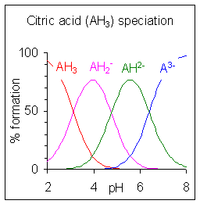
When the difference between successive pK values is about four or more, as in this example, each species may be considered as an acid in its own right; In fact salts of H2PO4− may be crystallised from solution by adjustment of pH to about 5.5 and salts of HPO42− may be crystallised from solution by adjustment of pH to about 10. The species distribution diagram shows that the concentrations of the two ions are maximum at pH 5.5 and 10.
When the difference between successive pK values is less than about four there is overlap between the pH range of existence of the species in equilibrium. The smaller the difference, the more the overlap. The case of citric acid is shown at the right; solutions of citric acid are buffered over the whole range of pH 2.5 to 7.5.
In general, it is true that successive pK values increase (Pauling's first rule). For example, for a diprotic acid, H2A, the two equilibria are
it can be seen that the second proton is removed from a negatively charged species. Since the proton carries a positive charge extra work is needed to remove it; that is the cause of the trend noted above. Phosphoric acid values (above) illustrate this rule, as do the values for vanadic acid, H3VO4. When an exception to the rule is found it indicates that a major change in structure is occurring. In the case of VO2+ (aq), the vanadium is octahedral
, 6-coordinate, whereas vanadic acid is tetrahedral
, 4-coordinate. This is the basis for an explanation of why pKa1 > pKa2 for vanadium(V) oxoacids.
, defined as AH. There are two dissociation equilibria to consider.
Substitute the expression for [AH] into the first equation
At the isoelectric point the concentration of the positively charged species, AH2+, is equal to the concentration of the negatively charged species, A-, so
Therefore, taking cologarithm
s, the pH is given by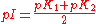
pI values for amino acids are listed at Proteinogenic amino acid#Chemical properties. When more than two charged species are in equilibrium with each other a full speciation calculation may be needed.
is given by
When, as is usually the case, the concentration of water can be assumed to be constant, this expression may be replaced by
The self-ionization
constant of water, Kw, is thus just a special case of an acid dissociation constant.
From these data it can be deduced that Kw = 10−14 at 24.87°C. At that temperature both hydrogen and hydroxide ions have a concentration of 10−7 mol dm−3.
HCO3− that is the conjugate base of the carbonic acid molecule
H2CO3 in the equilibrium
but also the conjugate acid of the carbonate ion
CO32− in (the reverse of) the equilibrium
Carbonic acid
equilibria are important for acid-base homeostasis
in the human body.
An Amino acid
is also amphoteric with the added complication that the neutral molecule is subject to an internal acid-base equilibrium in which the basic amino group attracts and binds the proton from the acidic carboxyl group, forming a zwitter ion
.
At pH less than about 5 both the carboxylate group and the amino group are protonated. As pH increases the acid dissociates according to
At high pH a second dissociation may take place.
Thus the zwitter ion, NH3+CHRCO2-, is amphoteric because it may either be protonated or deprotonated.
Using similar reasoning to that used before
Kb is related to Ka for the conjugate acid. In water, the concentration of the hydroxide
ion, [OH−], is related to the concentration of the hydrogen ion by Kw = [H+] [OH−], therefore
Substitution of the expression for [OH−] into the expression for Kb gives
When Ka, Kb and Kw are determined under the same conditions of temperature and ionic strength, it follows, taking cologarithm
s, that pKb = pKw − pKa. In aqueous solutions at 25 °C, pKw is 13.9965, so pKb ~ 14 − pKa.
In effect there is no need to define pKb separately from pKa, but it is done here because pKb values can be found in the older literature.
according to the van 't Hoff equation
R is the gas constant
and T is the absolute temperature
. Thus, for exothermic
reactions, (the standard enthalpy change, ΔH , is negative) K decreases with temperature, but for endothermic
, is negative) K decreases with temperature, but for endothermic
reactions (ΔH is positive) K increases with temperature.
is positive) K increases with temperature.
pKa values of organic compounds are often obtained using the aprotic solvents dimethyl sulfoxide
(DMSO) and acetonitrile
(ACN).
DMSO is widely used as an alternative to water because it has a lower dielectric constant than water, and is less polar and so dissolves non-polar, hydrophobic substances more easily. It has a measurable pKa range of about 1 to 30. Acetonitrile is less basic than DMSO, and, so, in general, acids are weaker and bases are stronger in this solvent. Some pKa values at 25oC for acetonitrile (ACN) and dimethyl sulfoxide (DMSO) are shown in the following tables. Values for water are included for comparison.
Ionization of acids is less in an acidic solvent than in water. For example, hydrogen chloride
is a weak acid when dissolved in acetic acid
. This is because acetic acid is a much weaker base than water.
Compare this reaction with what happens when acetic acid is dissolved in the more acidic solvent pure sulfuric acid
The unlikely geminal diol
species CH3C(OH)2+ is stable in these environments. For aqueous solutions the pH
scale is the most convenient acidity function
. Other acidity functions have been proposed for non-aqueous media, the most notable being the Hammett acidity function
, H0, for superacid
media and its modified version H− for superbasic
media.
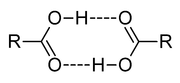 In aprotic solvents, oligomer
In aprotic solvents, oligomer
s, such as the well-known acetic acid dimer, may be formed by hydrogen bonding. An acid may also form hydrogen bonds to its conjugate base. This process, known as homoconjugation
, has the effect of enhancing the acidity of acids, lowering their effective pKa values, by stabilizing the conjugate base. Homoconjugation enhances the proton-donating power of toluenesulfonic acid in acetonitrile solution by a factor of nearly 800. In aqueous solutions, homoconjugation does not occur, because water forms stronger hydrogen bonds to the conjugate base than does the acid.
, in which the compound is more soluble. In the example shown at the right, the pKa value rises steeply with increasing percentage of dioxane as the dielectric constant of the mixture is decreasing.
A pKa value obtained in a mixed solvent cannot be used directly for aqueous solutions. The reason for this is that when the solvent is in its standard state its activity is defined as one. For example, the standard state of water:dioxane 9:1 is precisely that solvent mixture, with no added solutes. To obtain the pKa value for use with aqueous solutions it has to be extrapolated to zero co-solvent concentration from values obtained from various co-solvent mixtures.
These facts are obscured by the omission of the solvent from the expression that is normally used to define pKa, but pKa values obtained in a given mixed solvent can be compared to each other, giving relative acid strengths. The same is true of pKa values obtained in a particular non-aqueous solvent such a DMSO.
As of 2008, a universal, solvent-independent, scale for acid dissociation constants has not been developed, since there is no known way to compare the standard states of two different solvents.
 With organic acids inductive effects and mesomeric effect
With organic acids inductive effects and mesomeric effect
s affect the pKa values. A simple example is provided by the effect of replacing the hydrogen atoms in acetic acid by the more electronegative chlorine atom. The electron-withdrawing effect of the substituent makes ionisation easier, so successive pKa values decrease in the series 4.7, 2.8, 1.3 and 0.7 when 0,1, 2 or 3 chlorine atoms are present. The Hammett equation
, provides a general expression for the effect of substituents.
Ka is the dissociation constant of a substituted compound, Ka0 is the dissociation constant when the substituent is hydrogen, ρ is a property of the unsubstituted compound and σ has a particular value for each substituent. A plot of log Ka against σ is a straight line with intercept
log Ka0 and slope
ρ. This is an example of a linear free energy relationship as log Ka is proportional to the standard fee energy change. Hammett originally formulated the relationship with data from benzoic acid
with different substiuents in the ortho-
and para-
positions: some numerical values are in Hammett equation
. This and other studies allowed substituents to be ordered according to their electron-withdrawing
or electron-releasing
power, and to distinguish between inductive and mesomeric effects.
Alcohol
s do not normally behave as acids in water, but the presence of a double bond adjacent to the OH group can substantially decrease the pKa by the mechanism of keto-enol tautomerism
. Ascorbic acid
is an example of this effect. The diketone 2,4-pentanedione (acetylacetone
) is also a weak acid because of the keto-enol equilibrium. In aromatic compounds, such as phenol
, which have an OH substituent, conjugation
with the aromatic ring as a whole greatly increases the stability of the deprotonated form.
Structural effects can also be important. The difference between fumaric acid
and maleic acid
is a classic example. Fumaric acid is (E)-1,4-but-2-enedioic acid, a trans isomer
, whereas maleic acid is the corresponding cis isomer, i.e. (Z)-1,4-but-2-enedioic acid (see cis-trans isomerism). Fumaric acid has pKa values of approximately 3.0 and 4.5. By contrast, maleic acid has pKa values of approximately 1.5 and 6.5. The reason for this large difference is that when one proton is removed from the cis- isomer (maleic acid) a strong intramolecular
hydrogen bond
is formed with the nearby remaining carboxyl group. This favors the formation of the maleate H+, and it opposes the removal of the second proton from that species. In the trans isomer, the two carboxyl groups are always far apart, so hydrogen bonding is not observed.
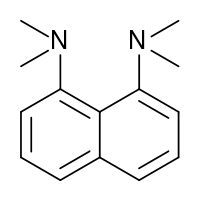
Proton sponge, 1,8-bis(dimethylamino)naphthalene, has a pKa value of 12.1. It is one of the strongest amine bases known. The high basicity is attributed to the relief of strain upon protonation and strong internal hydrogen bonding.
Effects of the solvent and solvation should be mentioned also in this section. It turns out, these influences are more subtle than that of a dielectric medium mentioned above. For example, the expected (by electronic effects of methyl substituents) and observed in gas phase order of basicity of methylamines, Me3N > Me2NH > MeNH2 > NH3, is changed by water to Me2NH > MeNH2 > Me3N > NH3. Neutral methylamine molecules are hydrogen-bonded to water molecules mailnly through one acceptor, N-HOH, interaction and only occasionally just one more donor bond, NH-OH2. Hence, methylamines are stabilized to about the same extent by hydration, regardless of the number of methyl groups. In stark contrast, corresponding methylammonium cations always utilize all the available protons for donor NH-OH2 bonding. Relative stabilization of methylammonium ions thus decreases with the number of methyl groups explaining the order of water basicity of methylamines.
change for the reaction, so for an acid dissociation constant
R is the gas constant
and T is the absolute temperature
. Note that pKa= −log Ka and 2.303 ≈ ln
10. At 25 °C ΔG in kJ·mol−1 = 5.708 pKa (1 kJ·mol−1 = 1000 Joule
in kJ·mol−1 = 5.708 pKa (1 kJ·mol−1 = 1000 Joule
s per mole
). Free energy is made up of an enthalpy
term and an entropy
term.
The standard enthalpy change can be determined by calorimetry
or by using the van 't Hoff equation, though the calorimetric method is preferable. When both the standard enthalpy change and acid dissociation constant have been determined, the standard entropy change is easily calculated from the equation above. In the following table, the entropy terms are calculated from the experimental values of pKa and ΔH . The data were critically selected and refer to 25 °C and zero ionic strength, in water.
. The data were critically selected and refer to 25 °C and zero ionic strength, in water.
The first point to note is that, when pKa is positive, the standard free energy change for the dissociation reaction is also positive. Second, some reactions are exothermic
and some are endothermic
, but, when ΔH is negative −TΔS
is negative −TΔS is the dominant factor, which determines that ΔG
is the dominant factor, which determines that ΔG is positive. Last, the entropy contribution is always unfavourable (ΔS
is positive. Last, the entropy contribution is always unfavourable (ΔS < 0) in these reactions. Ions in aqueous solution tend to orient the surrounding water molecules, which orders the solution and decreases the entropy. The contribution of an ion to the entropy is the partial molar entropy which is often negative, especially for small or highly charged ions. The ionization of a neutral acid involves formation of two ions so that the entropy decreases (ΔS
< 0) in these reactions. Ions in aqueous solution tend to orient the surrounding water molecules, which orders the solution and decreases the entropy. The contribution of an ion to the entropy is the partial molar entropy which is often negative, especially for small or highly charged ions. The ionization of a neutral acid involves formation of two ions so that the entropy decreases (ΔS < 0). On the second ionization of the same acid, there are now three ions and the anion has a charge, so the entropy again decreases.
< 0). On the second ionization of the same acid, there are now three ions and the anion has a charge, so the entropy again decreases.
Note that the standard free energy change for the reaction is for the changes from the reactants in their standard states to the products in their standard states. The free energy change at equilibrium is zero since the chemical potential
s of reactants and products are equal at equilibrium.
 The experimental determination of pKa values is commonly performed by means of titration
The experimental determination of pKa values is commonly performed by means of titration
s, in a medium of high ionic strength and at constant temperature. A typical procedure would be as follows. A solution of the compound in the medium is acidified with a strong acid to the point where the compound is fully protonated. The solution is then titrated with a strong base until all the protons have been removed. At each point in the titration pH is measured using a glass electrode
and a pH meter
. The equilibrium constants are found by fitting calculated pH values to the observed values, using the method of least squares
.
The total volume of added strong base should be small compared to the initial volume of titrand solution in order to keep the ionic strength nearly constant. This will ensure that pKa remains invariant during the titration.
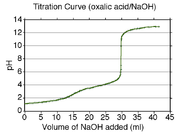
A calculated titration curve
for oxalic acid is shown at the right. Oxalic acid has pKa values of 1.27 and 4.27. Therefore the buffer regions will be centered at about pH 1.3 and pH 4.3. The buffer regions carry the information necessary to get the pKa values as the concentrations of acid and conjugate base change along a buffer region.
Between the two buffer regions there is an end-point, or equivalence point
, where the pH rises by about two units. This end-point is not sharp and is typical of a diprotic acid whose buffer regions overlap by a small amount: pKa2 − pKa1 is about three in this example. (If the difference in pK values were about two or less, the end-point would not be noticeable.) The second end-point begins at about pH 6.3 and is sharp. This indicates that all the protons have been removed. When this is so, the solution is not buffered and the pH rises steeply on addition of a small amount of strong base. However, the pH does not continue to rise indefinitely. A new buffer region begins at about pH 11 (pKw − 3), which is where self-ionization of water
becomes important.
It is very difficult to measure pH values of less than two in aqueous solution with a glass electrode, because the Nernst equation
breaks down at such low pH values. To determine pK values of less than about 2 or more than about 11 spectrophotometric or NMR measurements may be used instead of, or combined with, pH measurements.
When the glass electrode cannot be employed, as with non-aqueous solutions, spectrophotometric methods are frequently used. These may involve absorbance or fluorescence
measurements. In both cases the measured quantity is assumed to be proportional to the sum of contributions from each photo-active species; with absorbance measurements the Beer-Lambert law
is assumed to apply.
Aqueous solutions with normal water cannot be used for 1H NMR measurements but heavy water
, D2O, must be used instead. 13C NMR data, however, can be used with normal water and 1H NMR spectra can be used with non-aqueous media. The quantities measured with NMR are time-averaged chemical shift
s, as proton exchange is fast on the NMR time-scale. Other chemical shifts, such as those of 31P can be measured.
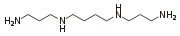 A base such as spermine
A base such as spermine
has a few different sites where protonation can occur. In this example the first proton can go on the terminal -NH2 group, or either of the internal -NH- groups. The pKa values for dissociation of spermine protonated at one or other of the sites are examples of micro-constants. They cannot be determined directly by means of pH, absorbance, fluorescence or NMR measurements. Nevertheless, the site of protonation is very important for biological function, so mathematical methods have been developed for the determination of micro-constants.
; for example, the pKa values of proteins and amino acid
side chains are of major importance for the activity of enzymes and the stability of proteins. Protein pKa values
cannot always be measured directly, but may be calculated using theoretical methods. Buffer solutions are used extensively to provide solutions at or near the physiological pH for the study of biochemical reactions; the design of these solutions depends on a knowledge of the pKa values of their components. Important buffer solutions include MOPS
, which provides a solution with pH 7.2, and tricine
, which is used in gel electrophoresis
. Buffering is an essential part of acid base physiology including acid-base homeostasis
, and is key to understanding disorders such as acid-base imbalance
. The isoelectric point
of a given molecule is a function of its pK values, so different molecules have different isoelectric points. This permits a technique called isoelectric focusing
, which is used for separation of proteins by 2-D gel polyacrylamide gel electrophoresis
.
Buffer solutions also play a key role in analytical chemistry
. They are used whenever there is a need to fix the pH of a solution at a particular value. Compared with an aqueous solution, the pH of a buffer solution is relatively insensitive to the addition of a small amount of strong acid or strong base. The buffer capacity of a simple buffer solution is largest when pH = pKa. In acid-base extraction
, the efficiency of extraction of a compound into an organic phase, such as an ether
, can be optimised by adjusting the pH of the aqueous phase using an appropriate buffer. At the optimum pH, the concentration of the electrically neutral species is maximised; such a species is more soluble in organic solvents having a low dielectric constant
than it is in water. This technique is used for the purification of weak acids and bases.
A pH indicator
is a weak acid or weak base that changes colour in the transition pH range, which is approximately pKa ± 1. The design of a universal indicator
requires a mixture of indicators whose adjacent pKa values differ by about two, so that their transition pH ranges just overlap.
In pharmacology
ionization of a compound alters its physical behaviour and macro properties such as solubility and lipophilicity
(log p). For example ionization of any compound will increase the solubility in water, but decrease the lipophilicity. This is exploited in drug development
to increase the concentration of a compound in the blood by adjusting the pKa of an ionizable group.
Knowledge of pKa values is important for the understanding of coordination complexes
, which are formed by the interaction of a metal ion, Mm+, acting as a Lewis acid
, with a ligand
, L, acting as a Lewis base. However, the ligand may also undergo protonation reactions, so the formation of a complex in aqueous solution could be represented symbolically by the reaction
To determine the equilibrium constant for this reaction, in which the ligand loses a proton, the pKa of the protonated ligand must be known. In practice, the ligand may be polyprotic; for example EDTA4−
can accept four protons; in that case, all pKa values must be known. In addition, the metal ion is subject to hydrolysis, that is, it behaves as a weak acid, so the pK values for the hydrolysis reactions must also be known.
Assessing the hazard
associated with an acid or base may require a knowledge of pKa values. For example, hydrogen cyanide is a very toxic gas, because the cyanide ion inhibits the iron-containing enzyme cytochrome c oxidase
. Hydrogen cyanide is a weak acid in aqueous solution with a pKa of about 9. In strongly alkaline solutions, above pH 11, say, it follows that sodium cyanide is "fully dissociated" so the hazard due to the hydrogen cyanide gas is much reduced. An acidic solution, on the other hand, is very hazardous because all the cyanide is in its acid form. Ingestion of cyanide by mouth is potentially fatal, independently of pH, because of the reaction with cytochrome c oxidase.
In environmental science
acid–base equilibria are important for lakes and rivers; for example, humic acid
s are important components of natural waters. Another example occurs in chemical oceanography
:
in order to quantify the solubility of iron(III) in seawater at various salinities
, the pKa values for the formation of the iron(III) hydrolysis products Fe(OH)2+, Fe(OH)2+ and Fe(OH)3 were determined, along with the solubility product of iron hydroxide.
More values can be found in thermodynamics, above.
Quantitative property
A quantitative property is one that exists in a range of magnitudes, and can therefore be measured with a number. Measurements of any particular quantitative property are expressed as a specific quantity, referred to as a unit, multiplied by a number. Examples of physical quantities are distance,...
measure of the strength of an acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
in solution. It is the equilibrium constant for a chemical reaction known as dissociation
Dissociation (chemistry)
Dissociation in chemistry and biochemistry is a general process in which ionic compounds separate or split into smaller particles, ions, or radicals, usually in a reversible manner...
in the context of acid-base reactions. The equilibrium can be written symbolically as:
- HA A− + H+,
where HA is a generic acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
that dissociates by splitting into A−, known as the conjugate base of the acid, and the hydrogen ion
Hydrogen ion
Hydrogen ion is recommended by IUPAC as a general term for all ions of hydrogen and its isotopes.Depending on the charge of the ion, two different classes can be distinguished: positively charged ions and negatively charged ions....
or proton
Proton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
, H+, which, in the case of aqueous solutions, exists as a solvated hydronium
Hydronium
In chemistry, a hydronium ion is the cation , a type of oxonium ion produced by protonation of water. This cation is often used to represent the nature of the proton in aqueous solution, where the proton is highly solvated...
ion. In the example shown in the figure, HA represents acetic acid
Acetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
, and A− the acetate ion. The chemical species HA, A− and H+ are said to be in equilibrium when their concentrations do not change with the passing of time. The dissociation constant is usually written as a quotient of the equilibrium concentrations (in mol/L), denoted by [HA], [A−] and [H+]:
Due to the many orders of magnitude spanned by Ka values, a logarithmic
Logarithmic scale
A logarithmic scale is a scale of measurement using the logarithm of a physical quantity instead of the quantity itself.A simple example is a chart whose vertical axis increments are labeled 1, 10, 100, 1000, instead of 1, 2, 3, 4...
measure of the acid dissociation constant is more commonly used in practice. The logarithmic constant, pKa, which is equal to −log10 Ka, is sometimes also (but incorrectly) referred to as an acid dissociation constant:

The larger the value of pKa, the smaller the extent of dissociation. A weak acid
Weak acid
A weak acid is an acid that dissociates incompletely. It does not release all of its hydrogens in a solution, donating only a partial amount of its protons to the solution...
has a pKa value in the approximate range −2 to 12 in water. Acids with a pKa value of less than about −2 are said to be strong acids; a strong acid is almost completely dissociated in aqueous solution, to the extent that the concentration of the undissociated acid becomes undetectable. pKa values for strong acids can, however, be estimated by theoretical means or by extrapolating from measurements in non-aqueous solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
s in which the dissociation constant is smaller, such as acetonitrile
Acetonitrile
Acetonitrile is the chemical compound with formula . This colourless liquid is the simplest organic nitrile. It is produced mainly as a byproduct of acrylonitrile manufacture...
and dimethylsulfoxide.
Theoretical background
The acid dissociation constant for an acid is a direct consequence of the underlying thermodynamicsChemical thermodynamics
Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics...
of the dissociation reaction; the pKa value is directly proportional to the standard Gibbs energy change
Gibbs free energy
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
for the reaction. The value of the pKa changes with temperature and can be understood qualitatively based on Le Chatelier's principle
Le Châtelier's principle
In chemistry, Le Chatelier's principle, also called the Chatelier's principle, can be used to predict the effect of a change in conditions on a chemical equilibrium. The principle is named after Henry Louis Le Chatelier and sometimes Karl Ferdinand Braun who discovered it independently...
: when the reaction is endothermic
Endothermic
In thermodynamics, the word endothermic describes a process or reaction in which the system absorbs energy from the surroundings in the form of heat. Its etymology stems from the prefix endo- and the Greek word thermasi,...
, the pKa decreases with increasing temperature; the opposite is true for exothermic
Exothermic
In thermodynamics, the term exothermic describes a process or reaction that releases energy from the system, usually in the form of heat, but also in the form of light , electricity , or sound...
reactions. The underlying structural factors that influence the magnitude of the acid dissociation constant include Pauling's rules for acidity constants, inductive effect
Inductive effect
In chemistry and physics, the inductive effect is an experimentally observable effect of the transmission of charge through a chain of atoms in a molecule by electrostatic induction...
s, mesomeric effect
Mesomeric effect
The mesomeric effect or resonance effect in chemistry is a property of substituents or functional groups in a chemical compound. The effect is used in a qualitative way and describes the electron withdrawing or releasing properties of substituents based on relevant resonance structures and is...
s, and hydrogen bonding.
The quantitative behaviour of acids and bases in solution can be understood only if their pKa values are known. In particular, the pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
of a solution can be predicted when the analytical concentration and pKa values of all acids and bases are known; conversely, it is possible to calculate the equilibrium concentration of the acids and bases in solution when the pH is known. These calculations find application in many different areas of chemistry, biology, medicine, and geology. For example, many compounds used for medication are weak acids or bases, and a knowledge of the pKa values, together with the water–octanol partition coefficient
Partition coefficient
In chemistry and the pharmaceutical sciences, a partition- or distribution coefficient is the ratio of concentrations of a compound in the two phases of a mixture of two immiscible solvents at equilibrium. The terms "gas/liquid partition coefficient" and "air/water partition coefficient" are...
, can be used for estimating the extent to which the compound enters the blood stream. Acid dissociation constants are also essential in aquatic chemistry and chemical oceanography
Chemical oceanography
Chemical oceanography is the study of ocean chemistry: the behavior of the chemical elements within the Earth's oceans. The ocean is unique in that it contains - in greater or lesser quantities - nearly every element in the periodic table....
, where the acidity of water plays a fundamental role. In living organisms, acid-base homeostasis
Acid-base homeostasis
Acid–base homeostasis is the part of human homeostasis concerning the proper balance between acids and bases, in other words, the pH. The body is very sensitive to its pH level, so strong mechanisms exist to maintain it...
and enzyme kinetics
Enzyme kinetics
Enzyme kinetics is the study of the chemical reactions that are catalysed by enzymes. In enzyme kinetics, the reaction rate is measured and the effects of varying the conditions of the reaction investigated...
are dependent on the pKa values of the many acids and bases present in the cell and in the body. In chemistry, a knowledge of pKa values is necessary for the preparation of buffer solution
Buffer solution
A buffer solution is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid. It has the property that the pH of the solution changes very little when a small amount of strong acid or base is added to it. Buffer solutions are used as a...
s and is also a prerequisite for a quantitative understanding of the interaction between acids or bases and metal ions to form complexes
Stability constants of complexes
A stability constant is an equilibrium constant for the formation of a complex in solution. It is a measure of the strength of the interaction between the reagents that come together to form the complex...
. Experimentally, pKa values can be determined by potentiometric (pH) titration
Titration
Titration, also known as titrimetry, is a common laboratory method of quantitative chemical analysis that is used to determine the unknown concentration of an identified analyte. Because volume measurements play a key role in titration, it is also known as volumetric analysis. A reagent, called the...
, but for values of pKa less than about 2 or more than about 11, spectrophotometric
Spectrophotometry
In chemistry, spectrophotometry is the quantitative measurement of the reflection or transmission properties of a material as a function of wavelength...
or NMR
Nuclear magnetic resonance
Nuclear magnetic resonance is a physical phenomenon in which magnetic nuclei in a magnetic field absorb and re-emit electromagnetic radiation...
measurements may be required due to practical difficulties with pH measurements.
Definitions
According to Arrhenius's original definition, an acid is a substance that dissociatesDissociation (chemistry)
Dissociation in chemistry and biochemistry is a general process in which ionic compounds separate or split into smaller particles, ions, or radicals, usually in a reversible manner...
in aqueous solution, releasing the hydrogen ion H+ (a proton):
- HA A− + H+.
The equilibrium constant for this dissociation reaction is known as a dissociation constant
Dissociation constant
In chemistry, biochemistry, and pharmacology, a dissociation constant is a specific type of equilibrium constant that measures the propensity of a larger object to separate reversibly into smaller components, as when a complex falls apart into its component molecules, or when a salt splits up into...
. The liberated proton combines with a water molecule to give a hydronium (or oxonium) ion H3O+, and so Arrhenius later proposed that the dissociation should be written as an acid–base reaction:
- HA + H2O A− + H3O+.
Brønsted and Lowry generalised this further to a proton exchange reaction:
- acid + base conjugate base + conjugate acid.
The acid loses a proton, leaving a conjugate base; the proton is transferred to the base, creating a conjugate acid. For aqueous solutions of an acid HA, the base is water; the conjugate base is A− and the conjugate acid is the hydronium ion. The Brønsted–Lowry definition applies to other solvents, such as dimethyl sulfoxide
Dimethyl sulfoxide
Dimethyl sulfoxide is an organosulfur compound with the formula 2SO. This colorless liquid is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water...
: the solvent S acts as a base, accepting a proton and forming the conjugate acid SH+.
In solution chemistry, it is common to use H+ as an abbreviation for the solvated hydrogen ion, regardless of the solvent. In aqueous solution H+ denotes a solvated hydronium ion rather than a proton.
The designation of an acid or base as "conjugate" depends on the context. The conjugate acid BH+ of a base B dissociates according to
- BH+ + OH− B + H2O
which is the reverse of the equilibrium
- H2O (acid) + B (base) OH− (conjugate base) + BH+ (conjugate acid).
The hydroxide ion OH−, a well known base, is here acting as the conjugate base of the acid water. Acids and bases are thus regarded simply as donors and acceptors of protons respectively.
A broader definition of acid dissociation includes hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
, in which protons are produced by the splitting of water molecules. For example, boric acid
Boric acid
Boric acid, also called hydrogen borate or boracic acid or orthoboric acid or acidum boricum, is a weak acid of boron often used as an antiseptic, insecticide, flame retardant, as a neutron absorber, and as a precursor of other chemical compounds. It exists in the form of colorless crystals or a...
(B(OH)3) produces H3O+ as if it were a proton donor, but it has been confirmed by Raman spectroscopy
Raman spectroscopy
Raman spectroscopy is a spectroscopic technique used to study vibrational, rotational, and other low-frequency modes in a system.It relies on inelastic scattering, or Raman scattering, of monochromatic light, usually from a laser in the visible, near infrared, or near ultraviolet range...
that this is due to the hydrolysis equilibrium:
- B(OH)3 + 2 H2O B(OH)4− + H3O+.
Similarly, metal ion hydrolysis causes ions such as [Al(H2O)6]3+ to behave as weak acids:
- [Al(H2O)6]3+ +H2O [Al(H2O)5(OH)]2+ + H3O+.
Equilibrium constant
An acid dissociation constant is a particular example of an equilibrium constant. For the specific equilibrium between a monoprotic acid, HA and its conjugate base A−, in water,- HA + H2O A− + H3O+
the thermodynamic equilibrium constant, K
 can be defined by
can be defined by
where {A} is the activity
Activity (chemistry)
In chemical thermodynamics, activity is a measure of the “effective concentration” of a species in a mixture, meaning that the species' chemical potential depends on the activity of a real solution in the same way that it would depend on concentration for an ideal solution.By convention, activity...
of the chemical species A etc. K
 is dimensionless since activity is dimensionless. Activities of the products of dissociation are placed in the numerator, activities of the reactants are placed in the denominator. See activity coefficient
is dimensionless since activity is dimensionless. Activities of the products of dissociation are placed in the numerator, activities of the reactants are placed in the denominator. See activity coefficientActivity coefficient
An activity coefficient is a factor used in thermodynamics to account for deviations from ideal behaviour in a mixture of chemical substances. In an ideal mixture, the interactions between each pair of chemical species are the same and, as a result, properties of the mixtures can be expressed...
for a derivation of this expression.
Since activity is the product of concentration
Concentration
In chemistry, concentration is defined as the abundance of a constituent divided by the total volume of a mixture. Four types can be distinguished: mass concentration, molar concentration, number concentration, and volume concentration...
and activity coefficient
Activity coefficient
An activity coefficient is a factor used in thermodynamics to account for deviations from ideal behaviour in a mixture of chemical substances. In an ideal mixture, the interactions between each pair of chemical species are the same and, as a result, properties of the mixtures can be expressed...
(γ) the definition could also be written as
where [HA] represents the concentration of HA and Γ is a quotient of activity coefficients.
To avoid the complications involved in using activities, dissociation constants are determined
Determination of equilibrium constants
Equilibrium constants are determined in order to quantify chemical equilibria. When an equilibrium constant is expressed as a concentration quotient,K=\frac...
, where possible, in a medium of high ionic strength
Ionic strength
The ionic strength of a solution is a measure of the concentration of ions in that solution. Ionic compounds, when dissolved in water, dissociate into ions. The total electrolyte concentration in solution will affect important properties such as the dissociation or the solubility of different salts...
, that is, under conditions in which Γ can be assumed to be always constant. For example, the medium might be a solution of 0.1 M sodium nitrate
Sodium nitrate
Sodium nitrate is the chemical compound with the formula NaNO3. This salt, also known as Chile saltpeter or Peru saltpeter to distinguish it from ordinary saltpeter, potassium nitrate, is a white solid which is very soluble in water...
or 3 M potassium perchlorate
Potassium perchlorate
Potassium perchlorate is the inorganic salt with the chemical formula KClO4. Like other perchlorates, this salt is a strong oxidizer and potentially reacts with many organic substances...
(1 M = 1 mol·dm−3, a unit of molar concentration). Furthermore, in all but the most concentrated solutions it can be assumed that the concentration of water, [H2O], is constant, approximately 55 mol·dm−3. On dividing K
 by the constant terms and writing [H+] for the concentration of the hydronium ion the expression
by the constant terms and writing [H+] for the concentration of the hydronium ion the expression
is obtained. This is the definition in common use. pKa is defined as −log10 Ka.
Note, however, that all published dissociation constant values refer to the specific ionic medium used in their determination and that different values are obtained with different conditions, as shown for acetic acid
Acetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
in the illustration above. When published constants refer to an ionic strength other than the one required for a particular application, they may be adjusted by means of specific ion theory (SIT) and other theories.
Although Ka appears to have the dimension
Dimensional analysis
In physics and all science, dimensional analysis is a tool to find or check relations among physical quantities by using their dimensions. The dimension of a physical quantity is the combination of the basic physical dimensions which describe it; for example, speed has the dimension length per...
of concentration it must in fact be dimensionless or it would not be possible to take its logarithm
Logarithm
The logarithm of a number is the exponent by which another fixed value, the base, has to be raised to produce that number. For example, the logarithm of 1000 to base 10 is 3, because 1000 is 10 to the power 3: More generally, if x = by, then y is the logarithm of x to base b, and is written...
. The illusion is the result of omitting the constant term [H2O] from the defining expression. Nevertheless it is not unusual, particularly in texts relating to biochemical equilibria, to see a value quoted with a dimension as, for example, "Ka = 300 M".
Monoprotic acids

This is a form of the Henderson–Hasselbalch equation, from which the following conclusions can be drawn.
- At half-neutralization [A−]/[HA] = 1; since log(1) =0, the pH at half-neutralization is numerically equal to pKa. Conversely, when pH = pKa, the concentration of HA is equal to the concentration of A−.
- The buffer regionBuffer solutionA buffer solution is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid. It has the property that the pH of the solution changes very little when a small amount of strong acid or base is added to it. Buffer solutions are used as a...
extends over the approximate range pKa ± 2, though buffering is weak outside the range pKa ± 1. At pKa ± 1, [A−]/[HA] = 10 or 1/10. - If the pH is known, the ratio may be calculated. This ratio is independent of the analytical concentration of the acid.
In water, measurable pKa values range from about −2 for a strong acid to about 12 for a very weak acid (or strong base). All acids with a pKa value of less than −2 are more than 99% dissociated at pH 0 (1 M acid). This is known as solvent leveling since all such acids are brought to the same level of being strong acids, regardless of their pKa values. Likewise, all bases with a pKa value larger than the upper limit are more than 99% protonated at all attainable pH values and are classified as strong bases.
An example of a strong acid is hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
, HCl, which has a pKa value, estimated from thermodynamic quantities, of −9.3 in water. The concentration of undissociated acid in a 1 mol·dm−3 solution will be less than 0.01% of the concentrations of the products of dissociation. Hydrochloric acid is said to be "fully dissociated" in aqueous solution because the amount of undissociated acid is imperceptible. When the pKa and analytical concentration of the acid are known, the extent of dissociation and pH of a solution of a monoprotic acid can be easily calculated using an ICE table
ICE table
An ICE table, ICE chart, or ICE box is a tabular system of keeping track of changing concentrations in an equilibrium reaction. ICE stands for "Initial, Change, Equilibrium". It is used in chemistry to keep track of the changes in amount of substance of the reactants and also organize a set of...
.
A buffer solution
Buffer solution
A buffer solution is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid. It has the property that the pH of the solution changes very little when a small amount of strong acid or base is added to it. Buffer solutions are used as a...
of a desired pH can be prepared as a mixture of a weak acid and its conjugate base. In practice the mixture can be created by dissolving the acid in water, and adding the requisite amount of strong acid or base. The pKa of the acid must be less than two units different from the target pH.
Polyprotic acids

Phosphoric acid
Phosphoric acid, also known as orthophosphoric acid or phosphoric acid, is a mineral acid having the chemical formula H3PO4. Orthophosphoric acid molecules can combine with themselves to form a variety of compounds which are also referred to as phosphoric acids, but in a more general way...
, H3PO4, is an example of a polyprotic acid as it can lose three protons.
| equilibrium | pKa value |
|---|---|
| H3PO4 H2PO4− + H+ | pKa1 = 2.15 |
| H2PO4− HPO42− + H+ | pKa2 = 7.20 |
| HPO42− PO43− + H+ | pKa3 = 12.37 |
When the difference between successive pK values is about four or more, as in this example, each species may be considered as an acid in its own right; In fact salts of H2PO4− may be crystallised from solution by adjustment of pH to about 5.5 and salts of HPO42− may be crystallised from solution by adjustment of pH to about 10. The species distribution diagram shows that the concentrations of the two ions are maximum at pH 5.5 and 10.
When the difference between successive pK values is less than about four there is overlap between the pH range of existence of the species in equilibrium. The smaller the difference, the more the overlap. The case of citric acid is shown at the right; solutions of citric acid are buffered over the whole range of pH 2.5 to 7.5.
In general, it is true that successive pK values increase (Pauling's first rule). For example, for a diprotic acid, H2A, the two equilibria are
- H2A HA− + H+
- HA− A2− + H+
it can be seen that the second proton is removed from a negatively charged species. Since the proton carries a positive charge extra work is needed to remove it; that is the cause of the trend noted above. Phosphoric acid values (above) illustrate this rule, as do the values for vanadic acid, H3VO4. When an exception to the rule is found it indicates that a major change in structure is occurring. In the case of VO2+ (aq), the vanadium is octahedral
Octahedral molecular geometry
In chemistry, octahedral molecular geometry describes the shape of compounds where in six atoms or groups of atoms or ligands are symmetrically arranged around a central atom, defining the vertices of an octahedron...
, 6-coordinate, whereas vanadic acid is tetrahedral
Tetrahedral molecular geometry
In a tetrahedral molecular geometry a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. The bond angles are cos−1 ≈ 109.5° when all four substituents are the same, as in CH4. This molecular geometry is common throughout the first...
, 4-coordinate. This is the basis for an explanation of why pKa1 > pKa2 for vanadium(V) oxoacids.
| equilibrium | pKa value |
|---|---|
| [VO2(H2O)4]+ H3VO4 + H+ + 2H2O | pKa1 = 4.2 |
| H3VO4 H2VO4− + H+ | pKa2 = 2.60 |
| H2VO4− HVO42− + H+ | pKa3 = 7.92 |
| HVO42− VO43− + H+ | pKa4 = 13.27 |
Isoelectric point
For substances in solution the isoelectric point (pI) is defined as the pH at which the sum, weighted by charge value, of concentrations of positively charged species is equal to the weighted sum of concentrations of negatively charged species. In the case that there is one species of each type, the isoelectric point can be obtained directly from the pK values. Take the example of glycineGlycine
Glycine is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its 'side chain', glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG cf. the genetic code.Glycine is a colourless, sweet-tasting crystalline solid...
, defined as AH. There are two dissociation equilibria to consider.
- AH2+ AH + H+; [AH][H+] = K1[AH2+]
- AH A- + H+; [A-][H+] = K2[AH]
Substitute the expression for [AH] into the first equation
- [A-][H+]2 = K1K2[AH2+]
At the isoelectric point the concentration of the positively charged species, AH2+, is equal to the concentration of the negatively charged species, A-, so
- [H+]2 = K1K2
Therefore, taking cologarithm
Cologarithm
In mathematics, the base-b cologarithm, sometimes shortened to colog, of a number is the base-b logarithm of the reciprocal of the number...
s, the pH is given by
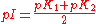
pI values for amino acids are listed at Proteinogenic amino acid#Chemical properties. When more than two charged species are in equilibrium with each other a full speciation calculation may be needed.
Water self-ionization
Water has both acidic and basic properties. The equilibrium constant for the equilibrium- 2 H2O OH− + H3O+
is given by
When, as is usually the case, the concentration of water can be assumed to be constant, this expression may be replaced by
The self-ionization
Self-ionization of water
The self-ionization of water is the chemical reaction in which a proton is transferred from one water molecule to another, in pure water or an aqueous solution, to create the two ions, hydronium, H3O+ and hydroxide, OH−...
constant of water, Kw, is thus just a special case of an acid dissociation constant.
| T/°C | 0 | 5 | 10 | 15 | 20 | 25 | 30 | 35 | 40 | 45 | 50 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| pKw | 14.943 | 14.734 | 14.535 | 14.346 | 14.167 | 13.997 | 13.830 | 13.680 | 13.535 | 13.396 | 13.262 |
From these data it can be deduced that Kw = 10−14 at 24.87°C. At that temperature both hydrogen and hydroxide ions have a concentration of 10−7 mol dm−3.
Amphoteric substances
An amphoteric substance is one that can act as an acid or as a base, depending on pH. Water (above) is amphoteric. Another example of an amphoteric molecule is the bicarbonate ionBicarbonate
In inorganic chemistry, bicarbonate is an intermediate form in the deprotonation of carbonic acid...
HCO3− that is the conjugate base of the carbonic acid molecule
Carbonic acid
Carbonic acid is the inorganic compound with the formula H2CO3 . It is also a name sometimes given to solutions of carbon dioxide in water, because such solutions contain small amounts of H2CO3. Carbonic acid forms two kinds of salts, the carbonates and the bicarbonates...
H2CO3 in the equilibrium
- H2CO3 + H2O HCO3− + H3O+
but also the conjugate acid of the carbonate ion
Carbonate
In chemistry, a carbonate is a salt of carbonic acid, characterized by the presence of the carbonate ion, . The name may also mean an ester of carbonic acid, an organic compound containing the carbonate group C2....
CO32− in (the reverse of) the equilibrium
- HCO3− + OH− CO32− + H2O.
Carbonic acid
Carbonic acid
Carbonic acid is the inorganic compound with the formula H2CO3 . It is also a name sometimes given to solutions of carbon dioxide in water, because such solutions contain small amounts of H2CO3. Carbonic acid forms two kinds of salts, the carbonates and the bicarbonates...
equilibria are important for acid-base homeostasis
Acid-base homeostasis
Acid–base homeostasis is the part of human homeostasis concerning the proper balance between acids and bases, in other words, the pH. The body is very sensitive to its pH level, so strong mechanisms exist to maintain it...
in the human body.
An Amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
is also amphoteric with the added complication that the neutral molecule is subject to an internal acid-base equilibrium in which the basic amino group attracts and binds the proton from the acidic carboxyl group, forming a zwitter ion
Zwitterion
In chemistry, a zwitterion is a neutral molecule with a positive and a negative electrical charge at different locations within that molecule. Zwitterions are sometimes also called inner salts.-Examples:...
.
- NH2CHRCO2H NH3+CHRCO2-
At pH less than about 5 both the carboxylate group and the amino group are protonated. As pH increases the acid dissociates according to
- NH3+CHRCO2H NH3+CHRCO2- + H+
At high pH a second dissociation may take place.
- NH3+CHRCO2- NH2CHRCO2- + H+
Thus the zwitter ion, NH3+CHRCO2-, is amphoteric because it may either be protonated or deprotonated.
Bases
Historically, the equilibrium constant Kb for a base has been defined as the association constant for protonation of the base, B, to form the conjugate acid, HB+.- B + H2O HB+ + OH−
Using similar reasoning to that used before
Kb is related to Ka for the conjugate acid. In water, the concentration of the hydroxide
Hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and a hydrogen atom held together by a covalent bond, and carrying a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, as a ligand, a nucleophile, and a...
ion, [OH−], is related to the concentration of the hydrogen ion by Kw = [H+] [OH−], therefore
Substitution of the expression for [OH−] into the expression for Kb gives
When Ka, Kb and Kw are determined under the same conditions of temperature and ionic strength, it follows, taking cologarithm
Cologarithm
In mathematics, the base-b cologarithm, sometimes shortened to colog, of a number is the base-b logarithm of the reciprocal of the number...
s, that pKb = pKw − pKa. In aqueous solutions at 25 °C, pKw is 13.9965, so pKb ~ 14 − pKa.
In effect there is no need to define pKb separately from pKa, but it is done here because pKb values can be found in the older literature.
Temperature dependence
All equilibrium constants vary with temperatureTemperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
according to the van 't Hoff equation
R is the gas constant
Gas constant
The gas constant is a physical constant which is featured in many fundamental equations in the physical sciences, such as the ideal gas law and the Nernst equation. It is equivalent to the Boltzmann constant, but expressed in units of energy The gas constant (also known as the molar, universal,...
and T is the absolute temperature
Kelvin
The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all...
. Thus, for exothermic
Exothermic
In thermodynamics, the term exothermic describes a process or reaction that releases energy from the system, usually in the form of heat, but also in the form of light , electricity , or sound...
reactions, (the standard enthalpy change, ΔH
 , is negative) K decreases with temperature, but for endothermic
, is negative) K decreases with temperature, but for endothermicEndothermic
In thermodynamics, the word endothermic describes a process or reaction in which the system absorbs energy from the surroundings in the form of heat. Its etymology stems from the prefix endo- and the Greek word thermasi,...
reactions (ΔH
 is positive) K increases with temperature.
is positive) K increases with temperature.Acidity in nonaqueous solutions
A solvent will be more likely to promote ionization of a dissolved acidic molecule in the following circumstances.- It is a protic solventProtic solventIn chemistry a protic solvent is a solvent that has a hydrogen atom bound to an oxygen or a nitrogen . In general terms, any molecular solvent that contains dissociable H+ is called a protic solvent. The molecules of such solvents can donate an H+...
, capable of forming hydrogen bonds. - It has a high donor numberDonor numberIn chemistry a donor number or DN is a qualitative measure of Lewis basicity. A donor number is defined as the negative enthalpy value for the 1:1 adduct formation between a Lewis base and the standard Lewis acid SbCl5 , in dilute solution in the noncoordinating solvent 1,2-dichloroethane with a...
, making it a strong Lewis base. - it has a high dielectric constantDielectric constantThe relative permittivity of a material under given conditions reflects the extent to which it concentrates electrostatic lines of flux. In technical terms, it is the ratio of the amount of electrical energy stored in a material by an applied voltage, relative to that stored in a vacuum...
(relative permittivity), making it a good solvent for ionic species.
pKa values of organic compounds are often obtained using the aprotic solvents dimethyl sulfoxide
Dimethyl sulfoxide
Dimethyl sulfoxide is an organosulfur compound with the formula 2SO. This colorless liquid is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water...
(DMSO) and acetonitrile
Acetonitrile
Acetonitrile is the chemical compound with formula . This colourless liquid is the simplest organic nitrile. It is produced mainly as a byproduct of acrylonitrile manufacture...
(ACN).
| Solvent | Donor number | Dielectric constant |
|---|---|---|
| Acetonitrile | 14 | 37 |
| Dimethylsulfoxide | 30 | 47 |
| Water | 18 | 78 |
DMSO is widely used as an alternative to water because it has a lower dielectric constant than water, and is less polar and so dissolves non-polar, hydrophobic substances more easily. It has a measurable pKa range of about 1 to 30. Acetonitrile is less basic than DMSO, and, so, in general, acids are weaker and bases are stronger in this solvent. Some pKa values at 25oC for acetonitrile (ACN) and dimethyl sulfoxide (DMSO) are shown in the following tables. Values for water are included for comparison.
| HA A− + H+ | ACN | DMSO | water |
|---|---|---|---|
| p-Toluenesulfonic acid P-Toluenesulfonic acid p-Toluenesulfonic acid or tosylic acid is an organic compound with the formula CH3C6H4SO3H. It is a white solid that is soluble in water, alcohols, and other polar organic solvents. The 4-CH3C6H4SO2- group is known as the Tosyl group and is often abbreviated as Ts or Tos... |
8.5 | 0.9 | strong |
| 2,4-Dinitrophenol 2,4-Dinitrophenol 2,4-Dinitrophenol , C6H4N2O5, is a cellular metabolic poison. It uncouples oxidative phosphorylation by carrying protons across the mitochondrial membrane, leading to a rapid consumption of energy without generation of ATP.... |
16.66 | 5.1 | 3.9 |
| Benzoic acid Benzoic acid Benzoic acid , C7H6O2 , is a colorless crystalline solid and the simplest aromatic carboxylic acid. The name derived from gum benzoin, which was for a long time the only source for benzoic acid. Its salts are used as a food preservative and benzoic acid is an important precursor for the synthesis... |
21.51 | 11.1 | 4.2 |
| Acetic acid Acetic acid Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell... |
23.51 | 12.6 | 4.756 |
| Phenol Phenol Phenol, also known as carbolic acid, phenic acid, is an organic compound with the chemical formula C6H5OH. It is a white crystalline solid. The molecule consists of a phenyl , bonded to a hydroxyl group. It is produced on a large scale as a precursor to many materials and useful compounds... |
29.14 | 18.0 | 9.99 |
| BH+ B + H+ | |||
| Pyrrolidine Pyrrolidine Pyrrolidine, also known as tetrahydropyrrole, is an organic compound with the molecular formula C4H9N. It is a cyclic secondary amine with a five-membered heterocycle containing four carbon atoms and one nitrogen atom... |
19.56 | 10.8 | 11.4 |
| Triethylamine Triethylamine Triethylamine is the chemical compound with the formula N3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine, for which TEA is also a common abbreviation.... |
18.82 | 9.0 | 10.72 |
| Proton sponge | 18.62 | 7.5 | 12.1 |
| Pyridine Pyridine Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom... |
12.53 | 3.4 | 5.2 |
| Aniline Aniline Aniline, phenylamine or aminobenzene is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the prototypical aromatic amine. Being a precursor to many industrial chemicals, its main use is in the manufacture of precursors to polyurethane... |
10.62 | 3.6 | 9.4 |
Ionization of acids is less in an acidic solvent than in water. For example, hydrogen chloride
Hydrogen chloride
The compound hydrogen chloride has the formula HCl. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric humidity. Hydrogen chloride gas and hydrochloric acid are important in technology and industry...
is a weak acid when dissolved in acetic acid
Acetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
. This is because acetic acid is a much weaker base than water.
- HCl + CH3CO2H Cl− + CH3C(OH)2+
- acid + base conjugate base + conjugate acid
Compare this reaction with what happens when acetic acid is dissolved in the more acidic solvent pure sulfuric acid
- H2SO4 + CH3CO2H HSO4− + CH3C(OH)2+
The unlikely geminal diol
Diol
A diol or glycol is a chemical compound containing two hydroxyl groups A geminal diol has two hydroxyl groups bonded to the same atom...
species CH3C(OH)2+ is stable in these environments. For aqueous solutions the pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
scale is the most convenient acidity function
Acidity function
An acidity function is a measure of the acidity of a medium or solvent system, usually expressed in terms of its ability to donate protons to a solute . The pH scale is by far the most commonly used acidity function, and is ideal for dilute aqueous solutions...
. Other acidity functions have been proposed for non-aqueous media, the most notable being the Hammett acidity function
Hammett acidity function
The Hammett acidity function is a measure of acidity that is used for very concentrated solutions of strong acids, including superacids. It was proposed by the physical organic chemist Louis Plack Hammett and is the best-known acidity function used to extend the measure of acidity beyond the...
, H0, for superacid
Superacid
According to the classical definition superacid is an acid with an acidity greater than that of 100% pure sulfuric acid, which has a Hammett acidity function of −12. According to the modern definition, superacid is a medium, in which the chemical potential of the proton is higher than in pure...
media and its modified version H− for superbasic
Superbase
In chemistry, a superbase is an extremely strong base, that is a compound that has a high affinity for protons. Hydroxide ion is the strongest base possible in aqueous solutions, but bases exist with pKb's well outside of the aqueous range. Such bases are valuable in organic synthesis and are...
media.

Oligomer
In chemistry, an oligomer is a molecule that consists of a few monomer units , in contrast to a polymer that, at least in principle, consists of an unlimited number of monomers. Dimers, trimers, and tetramers are oligomers. Many oils are oligomeric, such as liquid paraffin...
s, such as the well-known acetic acid dimer, may be formed by hydrogen bonding. An acid may also form hydrogen bonds to its conjugate base. This process, known as homoconjugation
Homoconjugation
In chemistry, homoconjugation has two meanings, one in acid-base chemistry and one in structural organic chemistry.-Meaning in acid-base chemistry:...
, has the effect of enhancing the acidity of acids, lowering their effective pKa values, by stabilizing the conjugate base. Homoconjugation enhances the proton-donating power of toluenesulfonic acid in acetonitrile solution by a factor of nearly 800. In aqueous solutions, homoconjugation does not occur, because water forms stronger hydrogen bonds to the conjugate base than does the acid.
Mixed solvents
When a compound has limited solubility in water it is common practice (in the pharmaceutical industry, for example) to determine pKa values in a solvent mixture such as water/dioxane or water/methanolMethanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
, in which the compound is more soluble. In the example shown at the right, the pKa value rises steeply with increasing percentage of dioxane as the dielectric constant of the mixture is decreasing.
A pKa value obtained in a mixed solvent cannot be used directly for aqueous solutions. The reason for this is that when the solvent is in its standard state its activity is defined as one. For example, the standard state of water:dioxane 9:1 is precisely that solvent mixture, with no added solutes. To obtain the pKa value for use with aqueous solutions it has to be extrapolated to zero co-solvent concentration from values obtained from various co-solvent mixtures.
These facts are obscured by the omission of the solvent from the expression that is normally used to define pKa, but pKa values obtained in a given mixed solvent can be compared to each other, giving relative acid strengths. The same is true of pKa values obtained in a particular non-aqueous solvent such a DMSO.
As of 2008, a universal, solvent-independent, scale for acid dissociation constants has not been developed, since there is no known way to compare the standard states of two different solvents.
Factors that affect pKa values
Pauling's second rule states that the value of the first pKa for acids of the formula XOm(OH) n is approximately independent of n and X and is approximately 8 for m = 0, 2 for m = 1, −3 for m = 2 and < −10 for m = 3. This correlates with the oxidation state of the central atom, X: the higher the oxidation state the stronger the oxyacid. For example, pKa for HClO is 7.2, for HClO2 is 2.0, for HClO3 is −1 and HClO4 is a strong acid.
Mesomeric effect
The mesomeric effect or resonance effect in chemistry is a property of substituents or functional groups in a chemical compound. The effect is used in a qualitative way and describes the electron withdrawing or releasing properties of substituents based on relevant resonance structures and is...
s affect the pKa values. A simple example is provided by the effect of replacing the hydrogen atoms in acetic acid by the more electronegative chlorine atom. The electron-withdrawing effect of the substituent makes ionisation easier, so successive pKa values decrease in the series 4.7, 2.8, 1.3 and 0.7 when 0,1, 2 or 3 chlorine atoms are present. The Hammett equation
Hammett equation
The Hammett equation in organic chemistry describes a linear free-energy relationship relating reaction rates and equilibrium constants for many reactions involving benzoic acid derivatives with meta- and para-substituents to each other with just two parameters: a substituent constant and a...
, provides a general expression for the effect of substituents.
- log Ka = log Ka0 + ρσ.
Ka is the dissociation constant of a substituted compound, Ka0 is the dissociation constant when the substituent is hydrogen, ρ is a property of the unsubstituted compound and σ has a particular value for each substituent. A plot of log Ka against σ is a straight line with intercept
Y-intercept
In coordinate geometry, using the common convention that the horizontal axis represents a variable x and the vertical axis represents a variable y, a y-intercept is a point where the graph of a function or relation intersects with the y-axis of the coordinate system...
log Ka0 and slope
Slope
In mathematics, the slope or gradient of a line describes its steepness, incline, or grade. A higher slope value indicates a steeper incline....
ρ. This is an example of a linear free energy relationship as log Ka is proportional to the standard fee energy change. Hammett originally formulated the relationship with data from benzoic acid
Benzoic acid
Benzoic acid , C7H6O2 , is a colorless crystalline solid and the simplest aromatic carboxylic acid. The name derived from gum benzoin, which was for a long time the only source for benzoic acid. Its salts are used as a food preservative and benzoic acid is an important precursor for the synthesis...
with different substiuents in the ortho-
Arene substitution patterns
Arene substitution patterns are part of organic chemistry IUPAC nomenclature and pinpoint the position of substituents other than hydrogen in relation to each other on an aromatic hydrocarbon.- Ortho, meta, and para substitution :...
and para-
Arene substitution patterns
Arene substitution patterns are part of organic chemistry IUPAC nomenclature and pinpoint the position of substituents other than hydrogen in relation to each other on an aromatic hydrocarbon.- Ortho, meta, and para substitution :...
positions: some numerical values are in Hammett equation
Hammett equation
The Hammett equation in organic chemistry describes a linear free-energy relationship relating reaction rates and equilibrium constants for many reactions involving benzoic acid derivatives with meta- and para-substituents to each other with just two parameters: a substituent constant and a...
. This and other studies allowed substituents to be ordered according to their electron-withdrawing
Inductive effect
In chemistry and physics, the inductive effect is an experimentally observable effect of the transmission of charge through a chain of atoms in a molecule by electrostatic induction...
or electron-releasing
Inductive effect
In chemistry and physics, the inductive effect is an experimentally observable effect of the transmission of charge through a chain of atoms in a molecule by electrostatic induction...
power, and to distinguish between inductive and mesomeric effects.
Alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s do not normally behave as acids in water, but the presence of a double bond adjacent to the OH group can substantially decrease the pKa by the mechanism of keto-enol tautomerism
Keto-enol tautomerism
In organic chemistry, keto-enol tautomerism refers to a chemical equilibrium between a keto form and an enol . The enol and keto forms are said to be tautomers of each other...
. Ascorbic acid
Ascorbic acid
Ascorbic acid is a naturally occurring organic compound with antioxidant properties. It is a white solid, but impure samples can appear yellowish. It dissolves well in water to give mildly acidic solutions. Ascorbic acid is one form of vitamin C. The name is derived from a- and scorbutus , the...
is an example of this effect. The diketone 2,4-pentanedione (acetylacetone
Acetylacetone
Acetylacetone is an organic compound that famously exists in two tautomeric forms that rapidly interconvert. The less stable tautomer is a diketone formally named pentane-2,4-dione. The more common tautomer is the enol form. The pair of tautomers rapidly interconvert and are treated as a single...
) is also a weak acid because of the keto-enol equilibrium. In aromatic compounds, such as phenol
Phenol
Phenol, also known as carbolic acid, phenic acid, is an organic compound with the chemical formula C6H5OH. It is a white crystalline solid. The molecule consists of a phenyl , bonded to a hydroxyl group. It is produced on a large scale as a precursor to many materials and useful compounds...
, which have an OH substituent, conjugation
Conjugated system
In chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds, which in general may lower the overall energy of the molecule and increase stability. Lone pairs, radicals or carbenium ions may be part of the...
with the aromatic ring as a whole greatly increases the stability of the deprotonated form.
Structural effects can also be important. The difference between fumaric acid
Fumaric acid
Fumaric acid or trans-butenedioic acid is the chemical compound with the formula HO2CCH=CHCO2H. This white crystalline compound is one of two isomeric unsaturated dicarboxylic acids, the other being maleic acid. In fumaric acid the carboxylic acid groups are trans and in maleic acid they are cis...
and maleic acid
Maleic acid
Maleic acid is an organic compound that is a dicarboxylic acid, a molecule with two carboxyl groups. Maleic acid is the cis-isomer of butenedioic acid, whereas fumaric acid is the trans-isomer...
is a classic example. Fumaric acid is (E)-1,4-but-2-enedioic acid, a trans isomer
Isomer
In chemistry, isomers are compounds with the same molecular formula but different structural formulas. Isomers do not necessarily share similar properties, unless they also have the same functional groups. There are many different classes of isomers, like stereoisomers, enantiomers, geometrical...
, whereas maleic acid is the corresponding cis isomer, i.e. (Z)-1,4-but-2-enedioic acid (see cis-trans isomerism). Fumaric acid has pKa values of approximately 3.0 and 4.5. By contrast, maleic acid has pKa values of approximately 1.5 and 6.5. The reason for this large difference is that when one proton is removed from the cis- isomer (maleic acid) a strong intramolecular
Intramolecular
Intramolecular in chemistry describes a process or characteristic limited within the structure of a single molecule, a property or phenomenon limited to the extent of a single molecule.- Examples :...
hydrogen bond
Hydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
is formed with the nearby remaining carboxyl group. This favors the formation of the maleate H+, and it opposes the removal of the second proton from that species. In the trans isomer, the two carboxyl groups are always far apart, so hydrogen bonding is not observed.
Proton sponge, 1,8-bis(dimethylamino)naphthalene, has a pKa value of 12.1. It is one of the strongest amine bases known. The high basicity is attributed to the relief of strain upon protonation and strong internal hydrogen bonding.
Effects of the solvent and solvation should be mentioned also in this section. It turns out, these influences are more subtle than that of a dielectric medium mentioned above. For example, the expected (by electronic effects of methyl substituents) and observed in gas phase order of basicity of methylamines, Me3N > Me2NH > MeNH2 > NH3, is changed by water to Me2NH > MeNH2 > Me3N > NH3. Neutral methylamine molecules are hydrogen-bonded to water molecules mailnly through one acceptor, N-HOH, interaction and only occasionally just one more donor bond, NH-OH2. Hence, methylamines are stabilized to about the same extent by hydration, regardless of the number of methyl groups. In stark contrast, corresponding methylammonium cations always utilize all the available protons for donor NH-OH2 bonding. Relative stabilization of methylammonium ions thus decreases with the number of methyl groups explaining the order of water basicity of methylamines.
Thermodynamics
An equilibrium constant is related to the standard Gibbs energyGibbs free energy
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
change for the reaction, so for an acid dissociation constant
- ΔG
 = −RT ln Ka.
= −RT ln Ka.
R is the gas constant
Gas constant
The gas constant is a physical constant which is featured in many fundamental equations in the physical sciences, such as the ideal gas law and the Nernst equation. It is equivalent to the Boltzmann constant, but expressed in units of energy The gas constant (also known as the molar, universal,...
and T is the absolute temperature
Kelvin
The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all...
. Note that pKa= −log Ka and 2.303 ≈ ln
Natural logarithm
The natural logarithm is the logarithm to the base e, where e is an irrational and transcendental constant approximately equal to 2.718281828...
10. At 25 °C ΔG
 in kJ·mol−1 = 5.708 pKa (1 kJ·mol−1 = 1000 Joule
in kJ·mol−1 = 5.708 pKa (1 kJ·mol−1 = 1000 JouleJoule
The joule ; symbol J) is a derived unit of energy or work in the International System of Units. It is equal to the energy expended in applying a force of one newton through a distance of one metre , or in passing an electric current of one ampere through a resistance of one ohm for one second...
s per mole
Mole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
). Free energy is made up of an enthalpy
Enthalpy
Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
term and an entropy
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
term.
- ΔG
 = ΔH
= ΔH − TΔS
− TΔS
The standard enthalpy change can be determined by calorimetry
Calorimetry
Calorimetry is the science of measuring the heat of chemical reactions or physical changes. Calorimetry is performed with a calorimeter. The word calorimetry is derived from the Latin word calor, meaning heat...
or by using the van 't Hoff equation, though the calorimetric method is preferable. When both the standard enthalpy change and acid dissociation constant have been determined, the standard entropy change is easily calculated from the equation above. In the following table, the entropy terms are calculated from the experimental values of pKa and ΔH
 . The data were critically selected and refer to 25 °C and zero ionic strength, in water.
. The data were critically selected and refer to 25 °C and zero ionic strength, in water.| Compound | Equilibrium | pKa | ΔG /kJ·mol−1 /kJ·mol−1 | ΔH /kJ·mol−1 /kJ·mol−1 | —TΔS /kJ·mol−1 /kJ·mol−1 |
|---|---|---|---|---|---|
| HA = Acetic acid Acetic acid Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell... |
HA H+ + A− | 4.756 | 22.147† | −0.41 | 22.56‡ |
| H2A+ = GlycineH+ Glycine Glycine is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its 'side chain', glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG cf. the genetic code.Glycine is a colourless, sweet-tasting crystalline solid... |
H2A+ HA + H+ | 2.351 | 13.420 | 4.00 | 9.419 |
| HA H+ + A− | 9.78 | 55.825 | 44.20 | 11.6 | |
| H2A = Maleic acid Maleic acid Maleic acid is an organic compound that is a dicarboxylic acid, a molecule with two carboxyl groups. Maleic acid is the cis-isomer of butenedioic acid, whereas fumaric acid is the trans-isomer... |
H2A HA− + H+ | 1.92 | 10.76 | 1.10 | 9.85 |
| HA− H+ + A2− | 6.27 | 35.79 | −3.60 | 39.4 | |
| H3A = Citric acid Citric acid Citric acid is a weak organic acid. It is a natural preservative/conservative and is also used to add an acidic, or sour, taste to foods and soft drinks... |
H3A H2A− + H+ | 3.128 | 17.855 | 4.07 | 13.78 |
| H2A− HA2− + H+ | 4.76 | 27.176 | 2.23 | 24.9 | |
| HA2− A3− + H+ | 6.40 | 36.509 | −3.38 | 39.9 | |
| H3A = Boric acid Boric acid Boric acid, also called hydrogen borate or boracic acid or orthoboric acid or acidum boricum, is a weak acid of boron often used as an antiseptic, insecticide, flame retardant, as a neutron absorber, and as a precursor of other chemical compounds. It exists in the form of colorless crystals or a... |
H3A H2A− + H+ | 9.237 | 52.725 | 13.80 | 38.92 |
| H3A = Phosphoric acid Phosphoric acid Phosphoric acid, also known as orthophosphoric acid or phosphoric acid, is a mineral acid having the chemical formula H3PO4. Orthophosphoric acid molecules can combine with themselves to form a variety of compounds which are also referred to as phosphoric acids, but in a more general way... |
H3A H2A− + H+ | 2.148 | 12.261 | −8.00 | 20.26 |
| H2A− HA2− + H+ | 7.20 | 41.087 | 3.60 | 37.5 | |
| HA2− A3− + H+ | 12.35 | 80.49 | 16.00 | 54.49 | |
| HA− = Hydrogen sulfate | HA− A2− + H+ | 1.99 | 11.36 | −22.40 | 33.74 |
| H2A = Oxalic acid Oxalic acid Oxalic acid is an organic compound with the formula H2C2O4. This colourless solid is a dicarboxylic acid. In terms of acid strength, it is about 3,000 times stronger than acetic acid. Oxalic acid is a reducing agent and its conjugate base, known as oxalate , is a chelating agent for metal cations... |
H2A HA− + H+ | 1.27 | 7.27 | −3.90 | 11.15 |
| HA− A2− + H+ | 4.266 | 24.351 | 7.00 | 31.35 |
- † ΔG
 = 2.303RT pKa
= 2.303RT pKa - ‡ Computed here, from ΔH and ΔG values supplied in the citation, using —TΔS
 = ΔG
= ΔG — ΔH
— ΔH
| Compound | Equilibrium | pKa | ΔH /kJ·mol−1 /kJ·mol−1 | —TΔS /kJ·mol−1 /kJ·mol−1 |
|---|---|---|---|---|
| B = Ammonia Ammonia Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or... |
HB+ B + H+ | 9.245 | 51.95 | 0.8205 |
| B = Methylamine Methylamine Methylamine is the organic compound with a formula of CH3NH2. This colourless gas is a derivative of ammonia, but with one H atom replaced by a methyl group. It is the simplest primary amine. It is sold as a solution in methanol, ethanol, THF, and water, or as the anhydrous gas in pressurized... |
HB+ B + H+ | 10.645 | 55.34 | 5.422 |
| B = Triethylamine Triethylamine Triethylamine is the chemical compound with the formula N3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine, for which TEA is also a common abbreviation.... |
HB+ B + H+ | 10.72 | 43.13 | 18.06 |
The first point to note is that, when pKa is positive, the standard free energy change for the dissociation reaction is also positive. Second, some reactions are exothermic
Exothermic
In thermodynamics, the term exothermic describes a process or reaction that releases energy from the system, usually in the form of heat, but also in the form of light , electricity , or sound...
and some are endothermic
Endothermic
In thermodynamics, the word endothermic describes a process or reaction in which the system absorbs energy from the surroundings in the form of heat. Its etymology stems from the prefix endo- and the Greek word thermasi,...
, but, when ΔH
 is negative −TΔS
is negative −TΔS is the dominant factor, which determines that ΔG
is the dominant factor, which determines that ΔG is positive. Last, the entropy contribution is always unfavourable (ΔS
is positive. Last, the entropy contribution is always unfavourable (ΔS < 0) in these reactions. Ions in aqueous solution tend to orient the surrounding water molecules, which orders the solution and decreases the entropy. The contribution of an ion to the entropy is the partial molar entropy which is often negative, especially for small or highly charged ions. The ionization of a neutral acid involves formation of two ions so that the entropy decreases (ΔS
< 0) in these reactions. Ions in aqueous solution tend to orient the surrounding water molecules, which orders the solution and decreases the entropy. The contribution of an ion to the entropy is the partial molar entropy which is often negative, especially for small or highly charged ions. The ionization of a neutral acid involves formation of two ions so that the entropy decreases (ΔS < 0). On the second ionization of the same acid, there are now three ions and the anion has a charge, so the entropy again decreases.
< 0). On the second ionization of the same acid, there are now three ions and the anion has a charge, so the entropy again decreases.Note that the standard free energy change for the reaction is for the changes from the reactants in their standard states to the products in their standard states. The free energy change at equilibrium is zero since the chemical potential
Chemical potential
Chemical potential, symbolized by μ, is a measure first described by the American engineer, chemist and mathematical physicist Josiah Willard Gibbs. It is the potential that a substance has to produce in order to alter a system...
s of reactants and products are equal at equilibrium.
Experimental determination

Titration
Titration, also known as titrimetry, is a common laboratory method of quantitative chemical analysis that is used to determine the unknown concentration of an identified analyte. Because volume measurements play a key role in titration, it is also known as volumetric analysis. A reagent, called the...
s, in a medium of high ionic strength and at constant temperature. A typical procedure would be as follows. A solution of the compound in the medium is acidified with a strong acid to the point where the compound is fully protonated. The solution is then titrated with a strong base until all the protons have been removed. At each point in the titration pH is measured using a glass electrode
Glass electrode
A glass electrode is a type of ion-selective electrode made of a doped glass membrane that is sensitive to a specific ion. It is an important part of the instrumentation for chemical analysis and physico-chemical studies. In modern practice, widely used membranous ion-selective electrodes are part...
and a pH meter
PH meter
A pH meter is an electronic instrument used for measuring the pH of a liquid...
. The equilibrium constants are found by fitting calculated pH values to the observed values, using the method of least squares
Least squares
The method of least squares is a standard approach to the approximate solution of overdetermined systems, i.e., sets of equations in which there are more equations than unknowns. "Least squares" means that the overall solution minimizes the sum of the squares of the errors made in solving every...
.
The total volume of added strong base should be small compared to the initial volume of titrand solution in order to keep the ionic strength nearly constant. This will ensure that pKa remains invariant during the titration.
A calculated titration curve
Titration curve
Titrations are often recorded on titration curves, whose compositions are generally identical: the independent variable is the volume of the titrant, while the dependent variable is the pH of the solution...
for oxalic acid is shown at the right. Oxalic acid has pKa values of 1.27 and 4.27. Therefore the buffer regions will be centered at about pH 1.3 and pH 4.3. The buffer regions carry the information necessary to get the pKa values as the concentrations of acid and conjugate base change along a buffer region.
Between the two buffer regions there is an end-point, or equivalence point
Equivalence point
The equivalence point, or stoichiometric point, of a chemical reaction when a titrant is added and is stoichiometrically equal to the amount of moles of substance present in the sample: the smallest amount of titrant that is sufficient to fully neutralize or react with the analyte...
, where the pH rises by about two units. This end-point is not sharp and is typical of a diprotic acid whose buffer regions overlap by a small amount: pKa2 − pKa1 is about three in this example. (If the difference in pK values were about two or less, the end-point would not be noticeable.) The second end-point begins at about pH 6.3 and is sharp. This indicates that all the protons have been removed. When this is so, the solution is not buffered and the pH rises steeply on addition of a small amount of strong base. However, the pH does not continue to rise indefinitely. A new buffer region begins at about pH 11 (pKw − 3), which is where self-ionization of water
Self-ionization of water
The self-ionization of water is the chemical reaction in which a proton is transferred from one water molecule to another, in pure water or an aqueous solution, to create the two ions, hydronium, H3O+ and hydroxide, OH−...
becomes important.
It is very difficult to measure pH values of less than two in aqueous solution with a glass electrode, because the Nernst equation
Nernst equation
In electrochemistry, the Nernst equation is an equation that can be used to determine the equilibrium reduction potential of a half-cell in an electrochemical cell. It can also be used to determine the total voltage for a full electrochemical cell...
breaks down at such low pH values. To determine pK values of less than about 2 or more than about 11 spectrophotometric or NMR measurements may be used instead of, or combined with, pH measurements.
When the glass electrode cannot be employed, as with non-aqueous solutions, spectrophotometric methods are frequently used. These may involve absorbance or fluorescence
Fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation of a different wavelength. It is a form of luminescence. In most cases, emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation...
measurements. In both cases the measured quantity is assumed to be proportional to the sum of contributions from each photo-active species; with absorbance measurements the Beer-Lambert law
Beer-Lambert law
In optics, the Beer–Lambert law, also known as Beer's law or the Lambert–Beer law or the Beer–Lambert–Bouguer law relates the absorption of light to the properties of the material through which the light is travelling.-Equations:The law states that there is a logarithmic dependence between the...
is assumed to apply.
Aqueous solutions with normal water cannot be used for 1H NMR measurements but heavy water
Heavy water
Heavy water is water highly enriched in the hydrogen isotope deuterium; e.g., heavy water used in CANDU reactors is 99.75% enriched by hydrogen atom-fraction...
, D2O, must be used instead. 13C NMR data, however, can be used with normal water and 1H NMR spectra can be used with non-aqueous media. The quantities measured with NMR are time-averaged chemical shift
Chemical shift
In nuclear magnetic resonance spectroscopy, the chemical shift is the resonant frequency of a nucleus relative to a standard. Often the position and number of chemical shifts are diagnostic of the structure of a molecule...
s, as proton exchange is fast on the NMR time-scale. Other chemical shifts, such as those of 31P can be measured.
Micro-constants
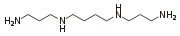
Spermine
Spermine is a polyamine involved in cellular metabolism found in all eukaryotic cells. Formed from spermidine, it is found in a wide variety of organisms and tissues and is an essential growth factor in some bacteria. It is found as a polycation at physiological pH...
has a few different sites where protonation can occur. In this example the first proton can go on the terminal -NH2 group, or either of the internal -NH- groups. The pKa values for dissociation of spermine protonated at one or other of the sites are examples of micro-constants. They cannot be determined directly by means of pH, absorbance, fluorescence or NMR measurements. Nevertheless, the site of protonation is very important for biological function, so mathematical methods have been developed for the determination of micro-constants.
Applications and significance
A knowledge of pKa values is important for the quantitative treatment of systems involving acid–base equilibria in solution. Many applications exist in biochemistryBiochemistry
Biochemistry, sometimes called biological chemistry, is the study of chemical processes in living organisms, including, but not limited to, living matter. Biochemistry governs all living organisms and living processes...
; for example, the pKa values of proteins and amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
side chains are of major importance for the activity of enzymes and the stability of proteins. Protein pKa values
Protein pKa calculations
In computational biology, protein pKa calculations are used to estimate the pKa values of amino acids as they exist within proteins. These calculations complement the pKa values reported for amino acids in their free state, and are used frequently within the fields of molecular modeling, structural...
cannot always be measured directly, but may be calculated using theoretical methods. Buffer solutions are used extensively to provide solutions at or near the physiological pH for the study of biochemical reactions; the design of these solutions depends on a knowledge of the pKa values of their components. Important buffer solutions include MOPS
MOPS
MOPS is the common name for the compound 3-propanesulfonic acid, a buffer introduced by Good et al. in the 1960s. It is a structural analog to MES. Its chemical structure contains a morpholine ring. HEPES is a similar pH buffering compound that contains a piperazine ring...
, which provides a solution with pH 7.2, and tricine
Tricine
Tricine is an organic compound that is used in buffer solutions. The name tricine comes from tris and glycine from which it was derived. It is a zwitterionic amino acid with a useful buffering range of pH 7.4-8.8. It is a white crystalline powder that is moderately soluble in water. It has a pH...
, which is used in gel electrophoresis
Gel electrophoresis
Gel electrophoresis is a method used in clinical chemistry to separate proteins by charge and or size and in biochemistry and molecular biology to separate a mixed population of DNA and RNA fragments by length, to estimate the size of DNA and RNA fragments or to separate proteins by charge...
. Buffering is an essential part of acid base physiology including acid-base homeostasis
Acid-base homeostasis
Acid–base homeostasis is the part of human homeostasis concerning the proper balance between acids and bases, in other words, the pH. The body is very sensitive to its pH level, so strong mechanisms exist to maintain it...
, and is key to understanding disorders such as acid-base imbalance
Acid-base imbalance
Acid–base imbalance is an abnormality of the human body's normal balance of acids and bases that causes the plasma pH to deviate out of the normal range . In the fetus, the normal range differs based on which umbilical vessel is sampled...
. The isoelectric point
Isoelectric point
The isoelectric point , sometimes abbreviated to IEP, is the pH at which a particular molecule or surface carries no net electrical charge....
of a given molecule is a function of its pK values, so different molecules have different isoelectric points. This permits a technique called isoelectric focusing
Isoelectric focusing
Isoelectric focusing , also known as electrofocusing, is a technique for separating different molecules by their electric charge differences...
, which is used for separation of proteins by 2-D gel polyacrylamide gel electrophoresis
Two-dimensional gel electrophoresis
Two-dimensional gel electrophoresis, abbreviated as 2-DE or 2-D electrophoresis, is a form of gel electrophoresis commonly used to analyze proteins...
.
Buffer solutions also play a key role in analytical chemistry
Analytical chemistry
Analytical chemistry is the study of the separation, identification, and quantification of the chemical components of natural and artificial materials. Qualitative analysis gives an indication of the identity of the chemical species in the sample and quantitative analysis determines the amount of...
. They are used whenever there is a need to fix the pH of a solution at a particular value. Compared with an aqueous solution, the pH of a buffer solution is relatively insensitive to the addition of a small amount of strong acid or strong base. The buffer capacity of a simple buffer solution is largest when pH = pKa. In acid-base extraction
Acid-base extraction
Acid-base extraction is a procedure using sequential liquid–liquid extractions to purify acids and bases from mixtures based on their chemical properties....
, the efficiency of extraction of a compound into an organic phase, such as an ether
Ether
Ethers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"...
, can be optimised by adjusting the pH of the aqueous phase using an appropriate buffer. At the optimum pH, the concentration of the electrically neutral species is maximised; such a species is more soluble in organic solvents having a low dielectric constant
Dielectric constant
The relative permittivity of a material under given conditions reflects the extent to which it concentrates electrostatic lines of flux. In technical terms, it is the ratio of the amount of electrical energy stored in a material by an applied voltage, relative to that stored in a vacuum...
than it is in water. This technique is used for the purification of weak acids and bases.
A pH indicator
PH indicator
A pH indicator is a halochromic chemical compound that is added in small amounts to a solution so that the pH of the solution can be determined visually. Hence a pH indicator is a chemical detector for hydronium ions or hydrogen ions in the Arrhenius model. Normally, the indicator causes the...
is a weak acid or weak base that changes colour in the transition pH range, which is approximately pKa ± 1. The design of a universal indicator
Universal indicator
A Universal indicator is a pH indicator composed of a blend of several compounds that exhibits several smooth colour changes over a pH value range from 1-14 to indicate the acidity or basicity of solutions. Although there are a number of commercially available universal pH indicators, most are a...
requires a mixture of indicators whose adjacent pKa values differ by about two, so that their transition pH ranges just overlap.
In pharmacology
Pharmacology
Pharmacology is the branch of medicine and biology concerned with the study of drug action. More specifically, it is the study of the interactions that occur between a living organism and chemicals that affect normal or abnormal biochemical function...
ionization of a compound alters its physical behaviour and macro properties such as solubility and lipophilicity
Partition coefficient
In chemistry and the pharmaceutical sciences, a partition- or distribution coefficient is the ratio of concentrations of a compound in the two phases of a mixture of two immiscible solvents at equilibrium. The terms "gas/liquid partition coefficient" and "air/water partition coefficient" are...
(log p). For example ionization of any compound will increase the solubility in water, but decrease the lipophilicity. This is exploited in drug development
Drug development
Drug development is a blanket term used to define the process of bringing a new drug to the market once a lead compound has been identified through the process of drug discovery...
to increase the concentration of a compound in the blood by adjusting the pKa of an ionizable group.
Knowledge of pKa values is important for the understanding of coordination complexes
Complex (chemistry)
In chemistry, a coordination complex or metal complex, is an atom or ion , bonded to a surrounding array of molecules or anions, that are in turn known as ligands or complexing agents...
, which are formed by the interaction of a metal ion, Mm+, acting as a Lewis acid
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
, with a ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
, L, acting as a Lewis base. However, the ligand may also undergo protonation reactions, so the formation of a complex in aqueous solution could be represented symbolically by the reaction
-
- [M(H2O)n]m+ +LH [M(H2O)n−1L](m−1)+ + H3O+
To determine the equilibrium constant for this reaction, in which the ligand loses a proton, the pKa of the protonated ligand must be known. In practice, the ligand may be polyprotic; for example EDTA4−
EDTA
Ethylenediaminetetraacetic acid, widely abbreviated as EDTA , is a polyamino carboxylic acid and a colourless, water-soluble solid. Its conjugate base is named ethylenediaminetetraacetate. It is widely used to dissolve limescale. Its usefulness arises because of its role as a hexadentate ligand...
can accept four protons; in that case, all pKa values must be known. In addition, the metal ion is subject to hydrolysis, that is, it behaves as a weak acid, so the pK values for the hydrolysis reactions must also be known.
Assessing the hazard
Risk assessment
Risk assessment is a step in a risk management procedure. Risk assessment is the determination of quantitative or qualitative value of risk related to a concrete situation and a recognized threat...
associated with an acid or base may require a knowledge of pKa values. For example, hydrogen cyanide is a very toxic gas, because the cyanide ion inhibits the iron-containing enzyme cytochrome c oxidase
Cytochrome c oxidase
The enzyme cytochrome c oxidase or Complex IV is a large transmembrane protein complex found in bacteria and the mitochondrion.It is the last enzyme in the respiratory electron transport chain of mitochondria located in the mitochondrial membrane...
. Hydrogen cyanide is a weak acid in aqueous solution with a pKa of about 9. In strongly alkaline solutions, above pH 11, say, it follows that sodium cyanide is "fully dissociated" so the hazard due to the hydrogen cyanide gas is much reduced. An acidic solution, on the other hand, is very hazardous because all the cyanide is in its acid form. Ingestion of cyanide by mouth is potentially fatal, independently of pH, because of the reaction with cytochrome c oxidase.
In environmental science
Environmental science
Environmental science is an interdisciplinary academic field that integrates physical and biological sciences, to the study of the environment, and the solution of environmental problems...
acid–base equilibria are important for lakes and rivers; for example, humic acid
Humic acid
Humic acid is a principal component of humic substances, which are the major organic constituents of soil , peat, coal, many upland streams, dystrophic lakes, and ocean water. It is produced by biodegradation of dead organic matter...
s are important components of natural waters. Another example occurs in chemical oceanography
Chemical oceanography
Chemical oceanography is the study of ocean chemistry: the behavior of the chemical elements within the Earth's oceans. The ocean is unique in that it contains - in greater or lesser quantities - nearly every element in the periodic table....
:
in order to quantify the solubility of iron(III) in seawater at various salinities
Salinity
Salinity is the saltiness or dissolved salt content of a body of water. It is a general term used to describe the levels of different salts such as sodium chloride, magnesium and calcium sulfates, and bicarbonates...
, the pKa values for the formation of the iron(III) hydrolysis products Fe(OH)2+, Fe(OH)2+ and Fe(OH)3 were determined, along with the solubility product of iron hydroxide.
Values for common substances
There are multiple techniques to determine the pKa of a chemical, leading to some discrepancies between different sources. Well measured values are typically within 0.1 units of each other. Data presented here were taken at 25 °C in water.More values can be found in thermodynamics, above.
| Chemical Name | Equilibrium | pKa |
|---|---|---|
| B = Adenine Adenine Adenine is a nucleobase with a variety of roles in biochemistry including cellular respiration, in the form of both the energy-rich adenosine triphosphate and the cofactors nicotinamide adenine dinucleotide and flavin adenine dinucleotide , and protein synthesis, as a chemical component of DNA... |
BH22+ BH+ + H+ | 4.17 |
| BH+ B + H+ | 9.65 | |
| H3A = Arsenic acid Arsenic acid Arsenic acid is the chemical compound with the formula H3AsO4. More descriptively written as AsO3, this colorless acid is the arsenic analogue of phosphoric acid. Arsenate and phosphate salts behave very similarly. Arsenic acid as such has not been isolated, but only found in solution where it... |
H3A H2A− + H+ | 2.22 |
| H2A− HA2− + H+ | 6.98 | |
| HA2− A3− + H+ | 11.53 | |
| HA = Benzoic acid Benzoic acid Benzoic acid , C7H6O2 , is a colorless crystalline solid and the simplest aromatic carboxylic acid. The name derived from gum benzoin, which was for a long time the only source for benzoic acid. Its salts are used as a food preservative and benzoic acid is an important precursor for the synthesis... |
HA H+ + A− | 4.204 |
| HA = Butanoic acid | HA H+ + A− | 4.82 |
| H2A = Chromic acid Chromic acid The term chromic acid is usually used for a mixture made by adding concentrated sulfuric acid to a dichromate, which may contain a variety of compounds, including solid chromium trioxide. This kind of chromic acid may be used as a cleaning mixture for glass. Chromic acid may also refer to the... |
H2A HA− + H+ | 0.98 |
| HA− A2− + H+ | 6.5 | |
| B = Codeine Codeine Codeine or 3-methylmorphine is an opiate used for its analgesic, antitussive, and antidiarrheal properties... |
BH+ B + H+ | 8.17 |
| HA = Cresol Cresol Cresols are organic compounds which are methylphenols. They are a widely occurring natural and manufactured group of aromatic organic compounds which are categorized as phenols . Depending on the temperature, cresols can be solid or liquid because they have melting points not far from room... |
HA H+ + A− | 10.29 |
| HA = Formic acid Formic acid Formic acid is the simplest carboxylic acid. Its chemical formula is HCOOH or HCO2H. It is an important intermediate in chemical synthesis and occurs naturally, most notably in the venom of bee and ant stings. In fact, its name comes from the Latin word for ant, formica, referring to its early... |
HA H+ + A− | 3.751 |
| HA = Hydrofluoric acid Hydrofluoric acid Hydrofluoric acid is a solution of hydrogen fluoride in water. It is a valued source of fluorine and is the precursor to numerous pharmaceuticals such as fluoxetine and diverse materials such as PTFE .... |
HA H+ + A− | 3.17 |
| HA = Hydrocyanic acid | HA H+ + A− | 9.21 |
| HA = Hydrogen selenide Hydrogen selenide Hydrogen selenide is the inorganic compound with the formula H2Se. It is the simplest and virtually the only hydride of selenium. H2Se is a colorless, flammable gas under standard conditions. It is the most toxic selenium compound with an exposure limit: 0.05 ppm over an 8 hour period... |
HA H+ + A− | 3.89 |
| HA = Hydrogen peroxide Hydrogen peroxide Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent... (90%) |
HA H+ + A− | 11.7 |
| HA = Lactic acid Lactic acid Lactic acid, also known as milk acid, is a chemical compound that plays a role in various biochemical processes and was first isolated in 1780 by the Swedish chemist Carl Wilhelm Scheele. Lactic acid is a carboxylic acid with the chemical formula C3H6O3... |
HA H+ + A− | 3.86 |
| HA = Propionic acid Propionic acid Propanoic acid is a naturally occurring carboxylic acid with chemical formula CH3CH2COOH. It is a clear liquid with a pungent odor... |
HA H+ + A− | 4.87 |
| HA = Phenol Phenol Phenol, also known as carbolic acid, phenic acid, is an organic compound with the chemical formula C6H5OH. It is a white crystalline solid. The molecule consists of a phenyl , bonded to a hydroxyl group. It is produced on a large scale as a precursor to many materials and useful compounds... |
HA H+ + A− | 9.99 |
| H2A = L-(+)-Ascorbic Acid Vitamin C Vitamin C or L-ascorbic acid or L-ascorbate is an essential nutrient for humans and certain other animal species. In living organisms ascorbate acts as an antioxidant by protecting the body against oxidative stress... |
H2A HA− + H+ | 4.17 |
| HA− A2− + H+ | 11.57 | |
See also
- Acids in wineAcids in wineThe acids in wine are an important component in both winemaking and the finished product of wine. They are present in both grapes and wine, having direct influences on the color, balance and taste of the wine as well as the growth and vitality of yeasts during fermentation and protecting the wine...
: tartaric, malic and citric are the principal acids in wine. - Ocean acidificationOcean acidificationOcean acidification is the name given to the ongoing decrease in the pH and increase in acidity of the Earth's oceans, caused by the uptake of anthropogenic carbon dioxide from the atmosphere....
: dissolution of atmospheric carbon dioxide affects seawater pH. The reaction depends on total inorganic carbonTotal inorganic carbonThe total inorganic carbon or dissolved inorganic carbon is the sum of inorganic carbon species in a solution. The inorganic carbon species include carbon dioxide, carbonic acid, bicarbonate anion, and carbonate. It is customary to express carbon dioxide and carbonic acid simultaneously as CO2*...
and on solubility equilibria with solid carbonates such as limestoneLimestoneLimestone is a sedimentary rock composed largely of the minerals calcite and aragonite, which are different crystal forms of calcium carbonate . Many limestones are composed from skeletal fragments of marine organisms such as coral or foraminifera....
and dolomiteDolomiteDolomite is a carbonate mineral composed of calcium magnesium carbonate CaMg2. The term is also used to describe the sedimentary carbonate rock dolostone....
. - Grotthuss mechanismGrotthuss mechanismThe Grotthuss mechanism is the mechanism by which an 'excess' proton or protonic defect diffuses through the hydrogen bond network of water molecules or other hydrogen-bonded liquids through the formation or cleavage of covalent bonds....
: how protons are transferred between hydronium ions and water molecules, accounting for the exceptionally high ionic mobility of the proton (animation). - Predominance diagramPredominance diagramthumb|200px|Predominance diagram for chromateA predominance diagram purports to show the conditions of concentration and pH where a chemical species has the highest concentration in solutions in which there are multiple acid-base equilibria. The lines on a predominance diagram indicate where...
: relates to equilibria involving polyoxyanionsOxyanionAn oxyanion or oxoanion is a chemical compound with the generic formula AxOyz− . Oxoanions are formed by a large majority of the chemical elements. The formulae of simple oxoanions are determined by the octet rule...
. pKa values are needed to construct these diagrams. - Proton affinityProton affinityThe proton affinity, Epa, of an anion or of a neutral atom or molecule is a measure of its gas-phase basicity. It is the energy released in the following reactions:-Acid/base chemistry:...
: a measure of basicity in the gas phase. - Stability constants of complexesStability constants of complexesA stability constant is an equilibrium constant for the formation of a complex in solution. It is a measure of the strength of the interaction between the reagents that come together to form the complex...
: formation of a complex can often be seen as a competition between proton and metal ion for a ligand, which is the product of dissociation of an acid. - Hammett acidity functionHammett acidity functionThe Hammett acidity function is a measure of acidity that is used for very concentrated solutions of strong acids, including superacids. It was proposed by the physical organic chemist Louis Plack Hammett and is the best-known acidity function used to extend the measure of acidity beyond the...
: a measure of acidity that is used for very concentrated solutions of strong acids, including superacids.
Further reading
(Previous edition published as ) (Non-aqueous solvents) (translation editor: Mary R. Masson) Chapter 4: Solvent Effects on the Position of Homogeneous Chemical Equilibria.External links
- Extensive bibliography of pKa values in DMSODimethyl sulfoxideDimethyl sulfoxide is an organosulfur compound with the formula 2SO. This colorless liquid is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water...
, acetonitrileAcetonitrileAcetonitrile is the chemical compound with formula . This colourless liquid is the simplest organic nitrile. It is produced mainly as a byproduct of acrylonitrile manufacture...
, THFThFFollicular B helper T cells , are antigen-experienced CD4+ T cells found in the B cell follicles of secondary lymphoid organs such as lymph nodes, spleens and Peyer's patches, and are identified by their constitutive expression of the B cell follicle homing receptor CXCR5...
, heptaneHeptanen-Heptane is the straight-chain alkane with the chemical formula H3C5CH3 or C7H16. When used as a test fuel component in anti-knock test engines, a 100% heptane fuel is the zero point of the octane rating scale...
, 1,2-dichloroethane1,2-DichloroethaneThe chemical compound 1,2-dichloroethane, commonly known by its old name of ethylene dichloride , is a chlorinated hydrocarbon, mainly used to produce vinyl chloride monomer , the major precursor for PVC production. It is a colourless liquid with a chloroform-like odour...
, and in the gas phase. - All-in-one freeware for pH and acid-base equilibrium calculations and for simulation and analysis of potentiometric titrationPotentiometric titrationPotentiometric titration is a technique similar to direct titration of a redox reaction. No indicator is used, instead the voltage across the analyte, typically an electrolyte solution is measured. To do this, two electrodes are used, a neutral electrode and a standard reference electrode. The...
curves with spreadsheets. - Includes a database with aqueous, non-aqueous, and gaseous phase pKa values than can be searched using SMILESSimplified molecular input line entry specificationThe simplified molecular-input line-entry specification or SMILES is a specification in form of a line notation for describing the structure of chemical molecules using short ASCII strings...
or CAS registry numberCAS registry numberCAS Registry Numbersare unique numerical identifiers assigned by the "Chemical Abstracts Service" toevery chemical described in the...
s. - pKa values for various acid and bases. Includes a table of some solubility products.
- Explanations of the relevance of these properties to pharmacologyPharmacologyPharmacology is the branch of medicine and biology concerned with the study of drug action. More specifically, it is the study of the interactions that occur between a living organism and chemicals that affect normal or abnormal biochemical function...
.- Free online prediction tool (Marvin) pKa, logP, logD etc. From ChemAxonChemaxonChemAxon is a software company specializing in application programming interfaces and end user applications for cheminformatics and life science research...
- Chemicalize.orgChemicalize.orgchemicalize.org is a free chemical structure miner and web search engine developed and owned by ChemAxon. The main purpose of chemicalize.org is to identify chemical names on websites and convert them to chemical structures...
:List of predicted structure based properties
- Free online prediction tool (Marvin) pKa, logP, logD etc. From ChemAxon

