
Specific ion interaction theory
Encyclopedia
Specific ion Interaction Theory (SIT theory) is a theory used to estimate single-ion
activity coefficient
s in electrolyte
solutions at relatively high concentrations. It does so by taking into consideration interaction coefficients between the various ions present in solution. Interaction coefficients are determined from equilibrium constant values obtained with solutions at various ionic strength
s. The determination of SIT interaction coefficients also yields the value of the equilibrium constant at infinite dilution.
theory. These activity coefficients are needed because an equilibrium constant is defined in thermodynamics
as a quotient of activities
but is usually measured using concentration
s. The protonation of a monobasic acid
will be used to simplify the exposition. The equilibrium for protonation of the conjugate base, A- of the acid, may be written as
for which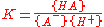
where {HA} signifies an activity of the chemical species HA etc.. The role of water in the equilibrium has been ignored as in all but the most concentrated solutions the activity of water is a constant. Note that K is defined here as an association constant, the reciprocal of an acid dissociation constant
.
Each activity term can be expressed as the product of a concentration and an activity coefficient
. For example,
where the square brackets signify a concentration and γ is an activity coefficient. Thus the equlilbrim constant can be expressed as a product of a concentration quotient and an activity coefficient quotient.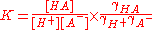
Taking logarithms.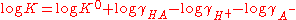
K0 is the hypothetical value that the equilibrium constant would have if the solution of the acid were so dilute that the activity coefficients were all equal to one.
It is common practise to determine
equilibrium constants in solutions containing an electrolyte at high ionic strength such that the activity coefficients are effectively constant. However, when the ionic strength is changed the measured equilibrium constant will also change, so there is a need to estimate individual (single ion) activity coefficients. Debye-Huckel theory provides a means to do this, but it is accurate only at very low concentrations. Hence the need for an extension to Debye-Hückel theory. Two main approaches have been used. SIT theory, discussed here and Pitzer equations
.
was invented. Subsequently Ciavatta developed the theory further.
The activity coefficient of the jth ion in solution is written as γj when concentrations are on the molal concentration
scale and as yj when concentrations are on the molar concentration scale. (The molal concentration scale is preferred in thermodynamics because molal concentrations are independent of temperature). The basic idea of SIT theory is that the activity coefficient can be expressed as (molal concentrations)
(molal concentrations)
or (molar concentrations)
(molar concentrations)
where z is the electrical charge on the ion, I is the ionic strength, ε and b are interaction coefficients and m and c are concentrations. The summation extends over the other ions present in solution, which includes the ions produced by the background electrolyte. The first term in these expressions comes from Debye-Hückel theory. The second term shows how the contributions from "interaction" are dependent on concentration. Thus, the interaction coefficients are used as corrections to Debye-Hückel theory when concentrations are higher than the region of validity of that theory.
The activity coefficient of a neutral species can be assumed to depend linearly on ionic strength, as in
where km is a Setschenow coefficient.
In the example of a monobasic acid HA, assuming that the background electrolyte is the salt NaNO3, the interaction coefficients will be for interaction between H+ and NO3-, and between A- and Na+.
has also been compared to both SIT and Pitzer equations
.
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
activity coefficient
Activity coefficient
An activity coefficient is a factor used in thermodynamics to account for deviations from ideal behaviour in a mixture of chemical substances. In an ideal mixture, the interactions between each pair of chemical species are the same and, as a result, properties of the mixtures can be expressed...
s in electrolyte
Electrolyte
In chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
solutions at relatively high concentrations. It does so by taking into consideration interaction coefficients between the various ions present in solution. Interaction coefficients are determined from equilibrium constant values obtained with solutions at various ionic strength
Ionic strength
The ionic strength of a solution is a measure of the concentration of ions in that solution. Ionic compounds, when dissolved in water, dissociate into ions. The total electrolyte concentration in solution will affect important properties such as the dissociation or the solubility of different salts...
s. The determination of SIT interaction coefficients also yields the value of the equilibrium constant at infinite dilution.
Background
The need for this theory arises from the need to derive activity coefficients of solutes when their concentrations are too high to be predicted accurately by Debye-HückelDebye–Hückel theory
The Debye–Hückel theory was proposed by Peter Debye and Erich Hückel as a theoretical explanation for departures from ideality in solutions of electrolytes. It was based on an extremely simplified model of the electrolyte solution but nevertheless gave accurate predictions of mean activity...
theory. These activity coefficients are needed because an equilibrium constant is defined in thermodynamics
Thermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
as a quotient of activities
Activity (chemistry)
In chemical thermodynamics, activity is a measure of the “effective concentration” of a species in a mixture, meaning that the species' chemical potential depends on the activity of a real solution in the same way that it would depend on concentration for an ideal solution.By convention, activity...
but is usually measured using concentration
Concentration
In chemistry, concentration is defined as the abundance of a constituent divided by the total volume of a mixture. Four types can be distinguished: mass concentration, molar concentration, number concentration, and volume concentration...
s. The protonation of a monobasic acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
will be used to simplify the exposition. The equilibrium for protonation of the conjugate base, A- of the acid, may be written as
- A- + H+ AH
for which
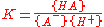
where {HA} signifies an activity of the chemical species HA etc.. The role of water in the equilibrium has been ignored as in all but the most concentrated solutions the activity of water is a constant. Note that K is defined here as an association constant, the reciprocal of an acid dissociation constant
Acid dissociation constant
An acid dissociation constant, Ka, is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction known as dissociation in the context of acid-base reactions...
.
Each activity term can be expressed as the product of a concentration and an activity coefficient
Activity coefficient
An activity coefficient is a factor used in thermodynamics to account for deviations from ideal behaviour in a mixture of chemical substances. In an ideal mixture, the interactions between each pair of chemical species are the same and, as a result, properties of the mixtures can be expressed...
. For example,
- {HA} = [HA] × γHA
where the square brackets signify a concentration and γ is an activity coefficient. Thus the equlilbrim constant can be expressed as a product of a concentration quotient and an activity coefficient quotient.
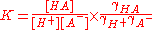
Taking logarithms.
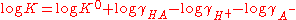
K0 is the hypothetical value that the equilibrium constant would have if the solution of the acid were so dilute that the activity coefficients were all equal to one.
It is common practise to determine
Determination of equilibrium constants
Equilibrium constants are determined in order to quantify chemical equilibria. When an equilibrium constant is expressed as a concentration quotient,K=\frac...
equilibrium constants in solutions containing an electrolyte at high ionic strength such that the activity coefficients are effectively constant. However, when the ionic strength is changed the measured equilibrium constant will also change, so there is a need to estimate individual (single ion) activity coefficients. Debye-Huckel theory provides a means to do this, but it is accurate only at very low concentrations. Hence the need for an extension to Debye-Hückel theory. Two main approaches have been used. SIT theory, discussed here and Pitzer equations
Pitzer equations
Pitzer equations are important for the understanding of the behaviour of ions dissolved in natural waters such as rivers, lakes and sea-water. The parameters of the Pitzer equations are linear combinations of parameters, of a virial expansion of the excess Gibbs free energy, which characterise...
.
Development
SIT theory was first proposed by Brønsted and was further developed by Guggenheim. Scatchard. extended the theory to allow the interaction coefficients to vary with ionic strength. The theory was mainly of theoretical interest until 1945 because of the difficulty of determining equilibrium constants before the glass electrodeGlass electrode
A glass electrode is a type of ion-selective electrode made of a doped glass membrane that is sensitive to a specific ion. It is an important part of the instrumentation for chemical analysis and physico-chemical studies. In modern practice, widely used membranous ion-selective electrodes are part...
was invented. Subsequently Ciavatta developed the theory further.
The activity coefficient of the jth ion in solution is written as γj when concentrations are on the molal concentration
Concentration
In chemistry, concentration is defined as the abundance of a constituent divided by the total volume of a mixture. Four types can be distinguished: mass concentration, molar concentration, number concentration, and volume concentration...
scale and as yj when concentrations are on the molar concentration scale. (The molal concentration scale is preferred in thermodynamics because molal concentrations are independent of temperature). The basic idea of SIT theory is that the activity coefficient can be expressed as
 (molal concentrations)
(molal concentrations)or
 (molar concentrations)
(molar concentrations)where z is the electrical charge on the ion, I is the ionic strength, ε and b are interaction coefficients and m and c are concentrations. The summation extends over the other ions present in solution, which includes the ions produced by the background electrolyte. The first term in these expressions comes from Debye-Hückel theory. The second term shows how the contributions from "interaction" are dependent on concentration. Thus, the interaction coefficients are used as corrections to Debye-Hückel theory when concentrations are higher than the region of validity of that theory.
The activity coefficient of a neutral species can be assumed to depend linearly on ionic strength, as in

where km is a Setschenow coefficient.
In the example of a monobasic acid HA, assuming that the background electrolyte is the salt NaNO3, the interaction coefficients will be for interaction between H+ and NO3-, and between A- and Na+.
Determination and application
Firstly, equilibrium constants are determined at a number of different ionic strengths, at a chosen temperature and particular background electrolyte. The interaction coefficients are then determined by fitting to the observed equilibrium constant values. The procedure also provides the value of K at infinite dilution. It is not limited to monobasic acids. and can also be applied to metal complexes. The SIT and Pitzer approaches have been compared recently.. The Bromley equationBromley equation
The Bromley equation was developed in 1973 with the objective of calculating activity coefficients for aqueous electrolyte solutions whose concentrations are above the range of validity of the Debye–Hückel equation This equation, together with Specific ion interaction theory and Pitzer equations...
has also been compared to both SIT and Pitzer equations
Pitzer equations
Pitzer equations are important for the understanding of the behaviour of ions dissolved in natural waters such as rivers, lakes and sea-water. The parameters of the Pitzer equations are linear combinations of parameters, of a virial expansion of the excess Gibbs free energy, which characterise...
.
External links
- SIT program A PC program to correct stability constants for changes in ionic strength using SIT theory and to estimate SIT parameters with full statistics. Contains an editable database of published SIT parameters. It also provides routines to inter-convert MolaRities (c) and MolaLities (m), and lg K(c) and lg K(m).

