Hemoglobin
Encyclopedia
Hemoglobin is the iron
-containing oxygen
-transport metalloprotein
in the red blood cell
s of all vertebrate
s, with the exception of the fish family Channichthyidae
, as well as the tissues of some invertebrate
s. Hemoglobin in the blood
carries oxygen from the respiratory organs (lung
s or gill
s) to the rest of the body (i.e., the tissues) where it releases the oxygen to burn nutrients to provide energy to power the functions of the organism, and collects the resultant carbon dioxide
to bring it back to the respiratory organs to be dispensed from the organism.
In mammal
s, the protein makes up about 97% of the red blood cells' dry content, and around 35% of the total content (including water).
Hemoglobin has an oxygen binding capacity of 1.34 ml O2 per gram of hemoglobin, which increases the total blood oxygen capacity seventy-fold compared to dissolved oxygen in blood. The mammalian hemoglobin molecule can bind (carry) up to four oxygen molecules.
Hemoglobin is involved in the transport of other gases: it carries some of the body's respiratory carbon dioxide
(about 10% of the total) as carbaminohemoglobin
, in which CO2 is bound to the globin protein. The molecule also carries the important regulatory molecule nitric oxide
bound to a globin protein thiol
group, releasing it at the same time as oxygen.
Hemoglobin is also found outside red blood cells and their progenitor lines. Other cells that contain hemoglobin include the A9 dopaminergic
neurons in the substantia nigra
, macrophage
s, alveolar cells, and mesangial cell
s in the kidney. In these tissues, hemoglobin has a non-oxygen-carrying function as an antioxidant
and a regulator of iron metabolism.
Hemoglobin and hemoglobin-like molecules are also found in many invertebrates, fungi, and plants. In these organisms, hemoglobins may carry oxygen, or they may act to transport and regulate other things such as carbon dioxide, nitric oxide, hydrogen sulfide and sulfide. A variant of the molecule, called leghemoglobin
, is used to scavenge oxygen, to keep it from poisoning anaerobic
systems, such as nitrogen-fixing nodules of leguminous plants.
published a series of articles in which he described growing hemoglobin crystals by successively diluting red blood cells with a solvent such as pure water, alcohol or ether, followed by slow evaporation of the solvent from the resulting protein solution. Hemoglobin's reversible oxygenation was described a few years later by Felix Hoppe-Seyler.
In 1959 Max Perutz
determined the molecular structure of hemoglobin by X-ray crystallography
. This work resulted in his sharing with John Kendrew
the 1962 Nobel Prize in Chemistry
.
The role of hemoglobin in the blood was elucidated by physiologist Claude Bernard
.
The name hemoglobin is derived from the words heme
and globin
, reflecting the fact that each subunit
of hemoglobin is a globular protein
with an embedded heme
group. Each heme group contains one iron atom, that can bind one oxygen molecule through ion
-induced dipole forces. The most common type of hemoglobin in mammals contains four such subunits.
There is more than one hemoglobin gene. The amino acid sequences of the globin proteins in hemoglobins usually differ between species. These differences grow with evolutionary distance between species. For example, the most common hemoglobin sequences in humans and chimpanzees are nearly identical, differing by only one amino acid in both the alpha and the beta globin protein chains. These differences grow larger between less closely related species.
Even within a species, different variants of hemoglobin always exist, although one sequence is usually a "most common" one in each species. Mutations in the genes
for the hemoglobin protein
in a species result in hemoglobin variants
. Many of these mutant forms of hemoglobin cause no disease. Some of these mutant forms of hemoglobin, however, cause a group of hereditary diseases termed the hemoglobinopathies
. The best known hemoglobinopathy is sickle-cell disease
, which was the first human disease whose mechanism
was understood at the molecular level. A (mostly) separate set of diseases called thalassemia
s involves underproduction of normal and sometimes abnormal hemoglobins, through problems and mutations in globin gene regulation. All these diseases produce anemia
.
Variations in hemoglobin amino acid sequences, as with other proteins, may be adaptive. For example, recent studies have suggested genetic variants in deer mice that help explain how deer mice that live in the mountains are able to survive in the thin air that accompanies high altitudes. A researcher from the University of Nebraska-Lincoln found mutations in four different genes that can account for differences between deer mice that live in lowland prairies versus the mountains. After examining wild mice captured from both highlands and lowlands, it was found that: the genes of the two breeds are “virtually identical–except for those that govern the oxygen-carrying capacity of their hemoglobin”. “The genetic difference enables highland mice to make more efficient use of their oxygen”, since less is available at higher altitudes, such as those in the mountains. Mammoth
hemoglobin featured mutations that allowed for oxygen delivery at lower temperatures, thus enabling mammoths to migrate to higher latitudes during the Pleistocene
.
of immature red blood cells, while the globin
protein parts are synthesized by ribosome
s in the cytosol. Production of Hb continues in the cell throughout its early development from the proerythroblast
to the reticulocyte
in the bone marrow
. At this point, the nucleus
is lost in mammalian red blood cells, but not in bird
s and many other species. Even after the loss of the nucleus in mammals, residual ribosomal RNA
allows further synthesis of Hb until the reticulocyte loses its RNA soon after entering the vasculature
(this hemoglobin-synthetic RNA in fact gives the reticulocyte its reticulated appearance and name).
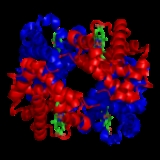
characteristic of many multi-subunit globular proteins. Most of the amino acids in hemoglobin form alpha helices, connected by short non-helical segments. Hydrogen bonds stabilize the helical sections inside this protein, causing attractions within the molecule, folding each polypeptide chain into a specific shape. Hemoglobin's quaternary structure comes from its four subunits in roughly a tetrahedral arrangement.
In most vertebrates, the hemoglobin molecule
is an assembly of four globular protein
subunits. Each subunit is composed of a protein
chain tightly associated with a non-protein heme
group. Each protein chain arranges into a set of alpha-helix structural segments connected together in a globin fold
arrangement, so called because this arrangement is the same folding motif used in other heme/globin proteins such as myoglobin
. This folding pattern contains a pocket that strongly binds the heme group.
A heme group consists of an iron (Fe) ion (charged atom) held in a heterocyclic
ring, known as a porphyrin
. This porphyrin ring consists of four pyrrole molecules cyclically linked together (by methene bridges) with the iron ion bound in the center. The iron ion, which is the site of oxygen binding, coordinates with the four nitrogen
s in the center of the ring, which all lie in one plane. The iron is bound strongly (covalently) to the globular protein via the imidazole
ring of the F8 histidine
residue (also known as the proximal histidine) below the porphyrin ring. A sixth position can reversibly bind oxygen by a coordinate covalent bond
, completing the octahedral group of six ligands. Oxygen binds in an "end-on bent" geometry where one oxygen atom binds Fe and the other protrudes at an angle. When oxygen is not bound, a very weakly bonded water molecule fills the site, forming a distorted octahedron
.
Even though carbon dioxide is carried by hemoglobin, it does not compete with oxygen for the iron-binding positions, but is actually bound to the protein chains of the structure.
The iron ion may be either in the Fe2+ or in the Fe3+ state, but ferrihemoglobin (methemoglobin) (Fe3+) cannot bind oxygen. In binding, oxygen temporarily and reversibly oxidizes (Fe2+) to (Fe3+) while oxygen temporally turns into superoxide
, thus iron must exist in the +2 oxidation state to bind oxygen. If superoxide ion associated to Fe3+ is protonated the hemoglobin iron will remain oxidized and incapable to bind oxygen. In such cases, the enzyme methemoglobin reductase
will be able to eventually reactivate methemoglobin by reducing the iron center.
In adult humans, the most common hemoglobin type is a tetramer (which contains 4 subunit proteins) called hemoglobin A, consisting of two α and two β subunits non-covalently bound, each made of 141 and 146 amino acid residues, respectively. This is denoted as α2β2. The subunits are structurally similar and about the same size. Each subunit has a molecular weight of about 17,000 daltons, for a total molecular weight of the tetramer of about 64,000 daltons (64,458 g/mol). Thus, 1 g/dL = 0.1551 mmol/L. Hemoglobin A is the most intensively studied of the hemoglobin molecules.
In human infants, the hemoglobin molecule is made up of 2 α chains and 2 gamma chains. The gamma chains are gradually replaced by β chains as the infant grows.
The four polypeptide chains are bound to each other by salt bridge
s, hydrogen bond
s, and the hydrophobic effect
. There are two kinds of contacts between the α and β chains: α1β1 and α1β2.
when oxygen binds to the heme component of the protein hemoglobin in red blood cells. This process occurs in the pulmonary capillaries adjacent to the alveoli of the lungs. The oxygen then travels through the blood stream to be dropped off at cells where it is utilized in glycolysis
and in the production of ATP
by the process of oxidative phosphorylation
. It does not, however, help to counteract a decrease in blood pH. Ventilation
, or breathing, may reverse this condition by removal of carbon dioxide
, thus causing a shift up in pH.
Hemoglobin exists in two forms, a taut form (T) and a relaxed form (R). Various factors such as low pH, high CO2 and high 2,3 BPG at the level of the tissues favor the taut form, which has low oxygen affinity and releases oxygen in the tissues. Conversely, a high pH, low CO2, or low 2,3 BPG favors the relaxed form which can better bind oxygen.
than deoxyhemoglobin, while at 940 nm its absorption is slightly higher. This difference is used for measurement of the amount of oxygen in patient's blood by an instrument called pulse oximeter
. This difference also accounts for the presentation of cyanosis
, the blue to purplish color that tissues develop during hypoxia
.
(suggesting at least one unpaired electron in the complex). The lowest-energy form of oxygen, and the lowest energy forms of the relevant oxidation states of iron, are these:
All of these structures are paramagnetic (have unpaired electrons), not diamagnetic. Thus, a non-intuitive (e.g., a higher-energy for at least one species) distribution of electrons in the combination of iron and oxygen must exist, in order to explain the observed diamagnetism and no unpaired electrons.
The three logical possibilities to produce diamagnetic (no net spin) Hb-O2 are:
Direct experimental data:
Thus, the nearest formal oxidation state of iron in Hb-O2 is the +3 state, with oxygen in the -1 state (as superoxide .O2-). The diamagnetism in this configuration arises from the single unpaired electron on superoxide aligning antiferromagnetically from the single unpaired electron on iron, to give no net spin to the entire configuration, in accordance with diamagnetic oxyhemoglobin from experiment.
The second choice of the three logical possibilities above for diamagnetic oxyhemoglobin being found correct by experiment, is not surprising: singlet oxygen (possibility #1) and large separations of charge (possibility #3) are both unfavorably high-energy states. Iron's shift to a higher oxidation state in Hb-O2 decreases the atom's size, and allows it into the plane of the porphyrin ring, pulling on the coordinated histidine residue and initiating the allosteric changes seen in the globulins.
Early postulates by bio-inorganic chemists claimed that possibility #1 (above) was correct and that iron should exist in oxidation state II. This seemed particularly likely since the iron oxidation state III as methemoglobin, when not accompanied by superoxide .O2- to "hold" the oxidation electron, was known to render hemoglobin incapable of binding normal triplet O2 as it occurs in the air. It was thus assumed that iron remained as Fe(II) when oxygen gas was bound in the lungs. The iron chemistry in this previous classical model was elegant, but the required presence of the required diamagnetic high-energy singlet oxygen was never explained. It was classically argued that the binding of an oxygen molecule placed high-spin iron(II) in an octahedral field of strong-field ligands; this change in field would increase the crystal field splitting energy
, causing iron's electrons to pair into the low-spin configuration, which would be diamagnetic in Fe(II). This forced low-spin pairing is indeed thought to happen in iron when oxygen binds, but is not enough to explain iron's change in size. Extraction of an additional electron from iron by oxygen is required to explain both iron's smaller size and observed increased oxidation state, and oxygen's weaker bond.
It should be noted that the assignment of a whole-number oxidation state is a formalism, as the covalent bonds are not required to have perfect bond orders involving whole electron-transfer. Thus, all three models for paramagnetic Hb-O2 may contribute to some small degree (by resonance) to the actual electronic configuration of Hb-O2. However, the model of iron in Hb-O2 being Fe(III) is more correct than the classical idea that it remains Fe(II).
, which binds to hemoglobin in a cooperative
manner, hemoglobin ligands also include competitive inhibitors
such as carbon monoxide
(CO) and allosteric
ligands such as carbon dioxide
(CO2) and nitric oxide
(NO). The carbon dioxide is bound to amino groups of the globin proteins as carbaminohemoglobin
, and is thought to account for about 10% of carbon dioxide transport in mammals. Nitric oxide is bound to specific thiol groups in the globin protein to form an S-nitrosothiol which dissociates into free nitric oxide and thiol again, as the hemoglobin releases oxygen from its heme site. This nitric oxide transport to peripheral tissues is hypothesized to assist oxygen transport in tissues, by releasing vasodilatory nitric oxide to tissues in which oxygen levels are low.
 When oxygen binds to the iron complex, it causes the iron atom to move back toward the center of the plane of the porphyrin ring (see moving diagram). At the same time, the imidazole side-chain of the histidine residue interacting at the other pole of the iron is pulled toward the porphyrin ring. This interaction forces the plane of the ring sideways toward the outside of the tetramer, and also induces a strain in the protein helix containing the histidine as it moves nearer to the iron atom. This strain is transmitted to the remaining three monomers in the tetramer, where it induces a similar conformational change in the other heme sites such that binding of oxygen to these sites becomes easier.
When oxygen binds to the iron complex, it causes the iron atom to move back toward the center of the plane of the porphyrin ring (see moving diagram). At the same time, the imidazole side-chain of the histidine residue interacting at the other pole of the iron is pulled toward the porphyrin ring. This interaction forces the plane of the ring sideways toward the outside of the tetramer, and also induces a strain in the protein helix containing the histidine as it moves nearer to the iron atom. This strain is transmitted to the remaining three monomers in the tetramer, where it induces a similar conformational change in the other heme sites such that binding of oxygen to these sites becomes easier.
In the tetrameric form of normal adult hemoglobin, the binding of oxygen is, thus, a cooperative
process. The binding affinity of hemoglobin for oxygen is increased by the oxygen saturation of the molecule, with the first oxygens bound influencing the shape of the binding sites for the next oxygens, in a way favorable for binding. This positive cooperative binding is achieved through steric
conformational changes of the hemoglobin protein complex as discussed above; i.e., when one subunit protein in hemoglobin becomes oxygenated, a conformational or structural change in the whole complex is initiated, causing the other subunits to gain an increased affinity for oxygen. As a consequence, the oxygen binding curve of hemoglobin is sigmoidal
, or S-shaped, as opposed to the normal hyperbolic
curve associated with noncooperative binding.
The dynamic mechanism of the cooperativity in hemoglobin and its relation with the low-frequency resonance has been discussed.
because both gases compete for the same binding sites on hemoglobin, carbon monoxide binding preferentially in place of oxygen.
The binding of oxygen is affected by molecules such as carbon monoxide (CO) (for example, from tobacco smoking
, car exhaust, and incomplete combustion in furnaces). CO competes with oxygen at the heme binding site. Hemoglobin binding affinity for CO is 250 times greater than its affinity for oxygen, meaning that small amounts of CO dramatically reduce hemoglobin's ability to transport oxygen. Since Carbon Monoxide
is a colorless, odorless and tasteless gas, and poses a potentially fatal threat detectors
have become commercially available to warn of dangerous levels in residences. When hemoglobin combines with CO, it forms a very bright red compound called carboxyhemoglobin
, which may cause the skin of CO poisoning victims to appear pink in death, instead of white or blue. When inspired air contains CO levels as low as 0.02%, headache and nausea occur; if the CO concentration is increased to 0.1%, unconsciousness will follow. In heavy smokers, up to 20% of the oxygen-active sites can be blocked by CO.
In similar fashion, hemoglobin also has competitive binding affinity for cyanide
(CN-), sulfur monoxide
(SO), nitric oxide
(NO), and sulfide
(S2-), including hydrogen sulfide
(H2S). All of these bind to iron in heme without changing its oxidation state, but they nevertheless inhibit oxygen-binding, causing grave toxicity.
The iron atom in the heme group must initially be in the ferrous (Fe2+) oxidation state to support oxygen and other gases' binding and transport (it temporarily switches to ferric during the time oxygen is bound, as explained above). Initial oxidation to the ferric (Fe3+) state without oxygen converts hemoglobin into "hemiglobin" or methemoglobin , which cannot bind oxygen. Hemoglobin in normal red blood cells is protected by a reduction system to keep this from happening. Nitric oxide is capable of converting a small fraction of hemoglobin to methemoglobin in red blood cells. The latter reaction is a remnant activity of the more ancient nitric oxide dioxygenase
function of globins.
. Through the enzyme carbonic anhydrase
, carbon dioxide reacts with water to give carbonic acid
, which decomposes into bicarbonate
and proton
s:
 Hence blood with high carbon dioxide levels is also lower in pH
Hence blood with high carbon dioxide levels is also lower in pH
(more acid
ic). Hemoglobin can bind protons and carbon dioxide, which causes a conformational change in the protein and facilitates the release of oxygen. Protons bind at various places on the protein, while carbon dioxide binds at the α-amino group. Carbon dioxide binds to hemoglobin and forms carbaminohemoglobin
. This decrease in hemoglobin's affinity for oxygen by the binding of carbon dioxide and acid is known as the Bohr effect
(shifts the O2-saturation curve to the right). Conversely, when the carbon dioxide levels in the blood decrease (i.e., in the lung capillaries), carbon dioxide and protons are released from hemoglobin, increasing the oxygen affinity of the protein. A reduction in the total binding capacity of hemoglobin to oxygen (i.e. shifting the curve down, not just to the right) due to reduced pH is called the root effect
. This is seen in bony fish.
It is necessary for hemoglobin to release the oxygen that it binds; if not, there is no point in binding it. The sigmoidal curve of hemoglobin makes it efficient in binding (taking up O2 in lungs), and efficient in unloading (unloading O2 in tissues).
In people acclimated to high altitudes, the concentration of 2,3-Bisphosphoglycerate
(2,3-BPG) in the blood is increased, which allows these individuals to deliver a larger amount of oxygen to tissues under conditions of lower oxygen tension. This phenomenon, where molecule Y affects the binding of molecule X to a transport molecule Z, is called a heterotropic allosteric effect.
A variant hemoglobin, called fetal hemoglobin
(HbF, α2γ2), is found in the developing fetus
, and binds oxygen with greater affinity than adult hemoglobin. This means that the oxygen binding curve for fetal hemoglobin is left-shifted (i.e., a higher percentage of hemoglobin has oxygen bound to it at lower oxygen tension), in comparison to that of adult hemoglobin. As a result, fetal blood in the placenta
is able to take oxygen from maternal blood.
Hemoglobin also carries nitric oxide
in the globin part of the molecule. This improves oxygen delivery in the periphery and contributes to the control of respiration. NO binds reversibly to a specific cysteine residue in globin; the binding depends on the state (R or T) of the hemoglobin. The resulting S-nitrosylated hemoglobin influences various NO-related activities such as the control of vascular resistance, blood pressure and respiration. NO is not released in the cytoplasm of erythrocytes but transported by an anion exchanger called AE1 out of them.
A study was performed to examine the influence of the form of hemoglobin (Hb) on the partitioning of inhaled volatile organic compounds (VOCs) into [human and animal] blood. Benzene was the prototypic VOC used in the investigations for this research due to the similar properties it shares with many other VOCs. To be specific, this study analyses the influence of the water solubility of Hb on the partitioning coefficient (PC) of a VOC as compared to the influence of the “species” or form of Hb. The different forms of blood used include: human hemoglobin (HbA), rat Hb, and sickle-cell hemoglobin (HbS). Rat Hb contains little water and is in a quasi-crystalline form, found inside the red blood cells (RBC), meaning they are more hydrophobic than human Hb, which are water-soluble. Sickle-cell hemoglobin (HbS) is water-soluble, however it can become water-insoluble, forming hydrophobic polymers, when deoxygenated. The findings state that the benzene PC for rat Hb was much higher than human that for Hb; however, the tests that measured the PCs of the oxygenated and deoxygenated forms of HbA and HbS did not differ, indicating that the affinity of benzene was not affected by the water solubility of Hb.
are a part of the normal embryonic and fetal development, but may also be pathologic mutant forms of hemoglobin in a population
, caused by variations in genetics. Some well-known hemoglobin variants such as sickle-cell anemia are responsible for diseases, and are considered hemoglobinopathies. Other variants cause no detectable pathology
, and are thus considered non-pathological variants.
In the embryo
:
In the fetus
:
In adults:
Variant forms that cause disease:
s reach the end of their life due to aging or defects, they are broken down, the hemoglobin molecule is broken up and the iron gets recycled. When the porphyrin ring
is broken up, the fragments are normally secreted in the bile
by the liver
. This process also produces one molecule of carbon monoxide for every molecule of heme degraded. This is one of the few natural sources of carbon monoxide production in the human body, and is responsible for the normal blood levels of carbon monoxide even in people breathing pure air. The other major final product of heme degradation is bilirubin
. Increased levels of this chemical are detected in the blood if red cells are being destroyed more rapidly than usual. Improperly degraded hemoglobin protein or hemoglobin that has been released from the blood cells too rapidly can clog small blood vessels, especially the delicate blood filtering vessels of the kidney
s, causing kidney damage.
 Hemoglobin deficiency can be caused either by decreased amount of hemoglobin molecules, as in anemia
Hemoglobin deficiency can be caused either by decreased amount of hemoglobin molecules, as in anemia
, or by decreased ability of each molecule to bind oxygen at the same partial pressure of oxygen. Hemoglobinopathies
(genetic defects resulting in abnormal structure of the hemoglobin molecule) may cause both. In any case, hemoglobin deficiency decreases blood oxygen-carrying capacity. Hemoglobin deficiency is, in general, strictly distinguished from hypoxemia
, defined as decreased partial pressure
of oxygen in blood, although both are causes of hypoxia
(insufficient oxygen supply to tissues).
Other common causes of low hemoglobin include loss of blood, nutritional deficiency, bone marrow problems, chemotherapy, kidney failure, or abnormal hemoglobin (such as that of sickle-cell disease).
High hemoglobin levels may be caused by exposure to high altitudes, smoking, dehydration, or tumors.
The ability of each hemoglobin molecule to carry oxygen is normally modified by altered blood pH or CO2
, causing an altered oxygen-hemoglobin dissociation curve
. However, it can also be pathologically altered in, e.g., carbon monoxide poisoning
.
Decrease of hemoglobin, with or without an absolute decrease of red blood cells, leads to symptoms of anemia
. Anemia has many different causes, although iron deficiency
and its resultant iron deficiency anemia
are the most common causes in the Western world. As absence of iron decreases heme synthesis, red blood cells in iron deficiency anemia are hypochromic (lacking the red hemoglobin pigment) and microcytic (smaller than normal). Other anemias are rarer. In hemolysis
(accelerated breakdown of red blood cells), associated jaundice
is caused by the hemoglobin metabolite bilirubin, and the circulating hemoglobin can cause renal failure
.
Some mutations in the globin chain are associated with the hemoglobinopathies
, such as sickle-cell disease
and thalassemia
. Other mutations, as discussed at the beginning of the article, are benign and are referred to merely as hemoglobin variants
.
There is a group of genetic disorders, known as the porphyria
s that are characterized by errors in metabolic pathways of heme synthesis. King George III of the United Kingdom
was probably the most famous porphyria sufferer.
To a small extent, hemoglobin A slowly combines with glucose
at the terminal valine (an alpha aminoacid) of each β chain. The resulting molecule is often referred to as Hb A1c. As the concentration of glucose in the blood increases, the percentage of Hb A that turns into Hb A1c increases. In diabetics
whose glucose usually runs high, the percent Hb A1c also runs high. Because of the slow rate of Hb A combination with glucose, the Hb A1c percentage is representative of glucose level in the blood averaged over a longer time (the half-life of red blood cells, which is typically 50–55 days).
Glycosylated hemoglobin
is the form of hemoglobin to which glucose is bound. The binding of glucose to amino acids in the hemoglobin takes place spontaneously (without the help of an enzyme) in many proteins, and is not known to serve a useful purpose. However, the binding to hemoglobin does serve as a record for average blood glucose levels over the lifetime of red cells, which is approximately 120 days. The levels of glycosylated hemoglobin are therefore measured in order to monitor the long-term control of the chronic disease of type 2 diabetes mellitus (T2DM). Poor control of T2DM results in high levels of glycosylated hemoglobin in the red blood cells. The normal reference range is approximately 4–5.9 %. Though difficult to obtain, values less than 7 % are recommended for people with T2DM. Levels greater than 9 % are associated with poor control of the glycosylated hemoglobin, and levels greater than 12 % are associated with very poor control. Diabetics who keep their glycosylated hemoglobin levels close to 7 % have a much better chance of avoiding the complications that may accompany diabetes (than those whose levels are 8 % or higher).
Elevated levels of hemoglobin are associated with increased numbers or sizes of red blood cells, called polycythemia
. This elevation may be caused by congenital heart disease, cor pulmonale
, pulmonary fibrosis
, too much erythropoietin
, or polycythemia vera
.
Elevation in levels of hemoglobin were found in one study of the yogic practice of Yoga Nidra
(yogic sleep) for half an hour daily.
A recent study done in Puducherry, India, shows its importance in coronary artery disease.
s, usually as part of a complete blood count
. For example it is typically tested before or after blood donation
. Results are reported in g
/L
, g/dL or mol
/L. 1 g/dL equals about 0.6206 mmol/L. Normal levels are:
Normal values of hemoglobin in the 1st and 3rd trimesters of pregnant women must be at least 11 g/dL and at least 10.5 g/dL during the 2nd trimester.
Dehydration or hyperhydration can greatly influence measured hemoglobin levels. Albumin can indicate hydration status.
If the concentration is below normal, this is called anemia
. Anemias are classified by the size of red blood cells, the cells
that contain hemoglobin in vertebrates. The anemia is called "microcytic" if red cells are small, "macrocytic" if they are large, and "normocytic" otherwise.
Hematocrit
, the proportion of blood volume occupied by red blood cells, is typically about three times the hemoglobin level. For example, if the hemoglobin is measured at 17, that compares with a hematocrit of 51.
Laboratory hemoglobin test methods require a blood sample (arterial, venous, or capillary) and analysis on hematology analyzer and CO-oximeter. Additionally, a new noninvasive hemoglobin (SpHb) test method called Pulse CO-Oximetry is also available with comparable accuracy to invasive methods.
Long-term control of blood sugar
concentration can be measured by the concentration of Hb A1c. Measuring it directly would require many samples because blood sugar levels vary widely through the day. Hb A1c is the product of the irreversible reaction of hemoglobin A with glucose
. A higher glucose concentration
results in more Hb A1c. Because the reaction is slow, the Hb A1c proportion represents glucose level in blood averaged over the half-life of red blood cells, is typically 50–55 days. An Hb A1c proportion of 6.0% or less show good long-term glucose control, while values above 7.0% are elevated. This test is especially useful for diabetics.
The functional magnetic resonance imaging
(fMRI) machine uses the signal from deoxyhemoglobin, which is sensitive to magnetic fields since it is paramagnetic.
, protozoa
ns, and fungi all have hemoglobin-like proteins whose known and predicted roles include the reversible binding of gaseous ligand
s. Since many of these proteins contain globins and the heme moiety
(iron in a flat porphyrin support), they are often called hemoglobins, even if their overall tertiary structure is very different from that of vertebrate hemoglobin. In particular, the distinction of “myoglobin” and hemoglobin in lower animals is often impossible, because some of these organisms do not contain muscle
s. Or, they may have a recognizable separate circulatory system
but not one that deals with oxygen transport (for example, many insect
s and other arthropod
s). In all these groups, heme/globin-containing molecules (even monomeric globin ones) that deal with gas-binding are referred to as oxyhemoglobins. In addition to dealing with transport and sensing of oxygen, they may also deal with NO, CO2, sulfide compounds, and even O2 scavenging in environments that must be anaerobic. They may even deal with detoxification of chlorinated materials in a way analogous to heme-containing P450 enzymes and peroxidases.
 The structure of hemoglobins varies across species. Hemoglobin occurs in all kingdoms of organisms, but not in all organisms. Primitive species such as bacteria, protozoa, algae
The structure of hemoglobins varies across species. Hemoglobin occurs in all kingdoms of organisms, but not in all organisms. Primitive species such as bacteria, protozoa, algae
, and plant
s often have single-globin hemoglobins. Many nematode
worms, molluscs
, and crustacean
s contain very large multisubunit molecules, much larger than those in vertebrates. In particular, chimeric hemoglobins found in fungi and giant annelids may contain both globin and other types of proteins.
One of the most striking occurrences and uses of hemoglobin in organisms is in the giant tube worm
(Riftia pachyptila, also called Vestimentifera), which can reach 2.4 meters length and populates ocean volcanic vents. Instead of a digestive tract, these worms contain a population of bacteria constituting half the organism's weight. The bacteria react with H2S from the vent and O2 from the water to produce energy to make food from H2O and CO2. The worms end with a deep red fan-like structure ("plume"), which extends into the water and absorbs H2S and O2 for the bacteria, and CO2 for use as synthetic raw material similar to photosynthetic plants. The structures are bright-red due to their containing several extraordinarily complex hemoglobins that have up to 144 globin chains, each including associated heme structures. These hemoglobins are remarkable for being able to carry oxygen in the presence of sulfide, and even to carry sulfide, without being completely "poisoned" or inhibited by it as hemoglobins in most other species are.
: Found in the muscle tissue of many vertebrates, including humans, it gives muscle tissue a distinct red or dark gray color. It is very similar to hemoglobin in structure and sequence, but is not a tetramer; instead, it is a monomer that lacks cooperative binding
. It is used to store oxygen rather than transport it.
Hemocyanin
: The second most common oxygen-transporting protein found in nature, it is found in the blood of many arthropod
s and molluscs. Uses copper prosthetic groups instead of iron heme groups and is blue in color when oxygenated.
Hemerythrin
: Some marine invertebrates and a few species of annelid
use this iron-containing non-heme protein to carry oxygen in their blood. Appears pink/violet when oxygenated, clear when not.
Chlorocruorin
: Found in many annelids, it is very similar to erythrocruorin, but the heme group is significantly different in structure. Appears green when deoxygenated and red when oxygenated.
Vanabins
: Also known as vanadium
chromagens, they are found in the blood of sea squirts. There were once hypothesized to use the rare metal vanadium as an oxygen binding prosthetic group. However, although they do contain vanadium by preference, they apparently bind little oxygen, and thus have some other function, which has not been elucidated (sea squirts also contain some hemoglobin). They may act as toxins.
Erythrocruorin
: Found in many annelids, including earthworm
s, it is a giant free-floating blood protein containing many dozens—possibly hundreds—of iron- and heme-bearing protein subunits bound together into a single protein complex with a molecular mass greater than 3.5 million daltons.
Pinnaglobin: Only seen in the mollusc Pinna squamosa. Brown manganese-based porphyrin protein.
Leghemoglobin
: In leguminous plants, such as alfalfa or soybeans, the nitrogen fixing bacteria in the roots are protected from oxygen by this iron heme containing oxygen-binding protein. The specific enzyme protected is nitrogenase
, which is unable to reduce nitrogen gas in the presence of free oxygen.
Coboglobin
: A synthetic cobalt-based porphyrin. Coboprotein would appear colorless when oxygenated, but yellow when in veins.
neurons in the substantia nigra
, astrocyte
s in the cerebral cortex
and hippocampus
, and in all mature oligodendrocyte
s. It has been suggested that brain hemoglobin in these cell may enable the "storage of oxygen to provide a homeostatic mechanism in anoxic conditions, which is especially important for A9 DA neurons that have an elevated metabolism with a high requirement for energy production". It has been noted further that "A9 dopaminergic
neurons may be at particular risk since in addition to their high mitochondrial activity they are under intense oxidative stress caused by the production of hydrogen peroxide via autoxidation and/or monoamine oxidase (MAO)-mediated deamination of dopamine and the subsequent reaction of accessible ferrous iron to generate highly toxic hydroxyl radicals". This may explain the risk of these cells for degeneration in Parkinson's disease
. The presence of iron from hemoglobin in these cells also results in the post-mortem darkness of these cells, which is the origin of the Latin name, substantia nigra.
Outside the brain, hemoglobin has non-oxygen-carrying functions as an antioxidant
and a regulator of iron metabolism in macrophage
s, alveolar cells, and mesangial cell
s in the kidney.
 Historically, the color of blood was associated with rust, as ancient Romans
Historically, the color of blood was associated with rust, as ancient Romans
associated the planet Mars
with the god of war since Mars is orange-red. The color of Mars is due to the iron oxide
in the Martian soil, but the red in blood is not due to the iron in hemoglobin and its oxides, which is a common misconception. The red is due to the porphyrin
moiety
of hemoglobin to which the iron is bound, not the iron itself, although the ligation and redox state of the iron can influence the pi to pi* or n to pi* electronic transitions of the porphyrin and hence its optical characteristics.
.jpg) Artist Julian Voss-Andreae
Artist Julian Voss-Andreae
created a sculpture
called "Heart of Steel (Hemoglobin)" in 2005, based on the protein's backbone. The sculpture was made from glass and weathering steel. The intentional rusting of the initially shiny work of art mirrors hemoglobin's fundamental chemical reaction of oxygen binding to iron.
Rock band Placebo
recorded a song called "Haemoglobin
" with the lyrics "Haemoglobin is the key to a healthy heartbeat". French rap artist MC Solaar
also had a successful single titled "La Concubine de L'Hemoglobin" in 1994.
Hemoglobin variants:
Hemoglobin protein subunits (genes):
Hemoglobin compounds:
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
-containing oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
-transport metalloprotein
Metalloprotein
Metalloprotein is a generic term for a protein that contains a metal ion cofactor. Metalloproteins have many different functions in cells, such as enzymes, transport and storage proteins, and signal transduction proteins. Indeed, about one quarter to one third of all proteins require metals to...
in the red blood cell
Red blood cell
Red blood cells are the most common type of blood cell and the vertebrate organism's principal means of delivering oxygen to the body tissues via the blood flow through the circulatory system...
s of all vertebrate
Vertebrate
Vertebrates are animals that are members of the subphylum Vertebrata . Vertebrates are the largest group of chordates, with currently about 58,000 species described. Vertebrates include the jawless fishes, bony fishes, sharks and rays, amphibians, reptiles, mammals, and birds...
s, with the exception of the fish family Channichthyidae
Channichthyidae
The crocodile icefish or white-blooded fish are a family of perciform fish found in the cold waters around Antarctica and southern South America. Water temperature can drop below 0°C in the Antarctic sea but stays rather constant. There are sixteen known species of crocodile icefish...
, as well as the tissues of some invertebrate
Invertebrate
An invertebrate is an animal without a backbone. The group includes 97% of all animal species – all animals except those in the chordate subphylum Vertebrata .Invertebrates form a paraphyletic group...
s. Hemoglobin in the blood
Blood
Blood is a specialized bodily fluid in animals that delivers necessary substances such as nutrients and oxygen to the cells and transports metabolic waste products away from those same cells....
carries oxygen from the respiratory organs (lung
Lung
The lung is the essential respiration organ in many air-breathing animals, including most tetrapods, a few fish and a few snails. In mammals and the more complex life forms, the two lungs are located near the backbone on either side of the heart...
s or gill
Gill
A gill is a respiratory organ found in many aquatic organisms that extracts dissolved oxygen from water, afterward excreting carbon dioxide. The gills of some species such as hermit crabs have adapted to allow respiration on land provided they are kept moist...
s) to the rest of the body (i.e., the tissues) where it releases the oxygen to burn nutrients to provide energy to power the functions of the organism, and collects the resultant carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
to bring it back to the respiratory organs to be dispensed from the organism.
In mammal
Mammal
Mammals are members of a class of air-breathing vertebrate animals characterised by the possession of endothermy, hair, three middle ear bones, and mammary glands functional in mothers with young...
s, the protein makes up about 97% of the red blood cells' dry content, and around 35% of the total content (including water).
Hemoglobin has an oxygen binding capacity of 1.34 ml O2 per gram of hemoglobin, which increases the total blood oxygen capacity seventy-fold compared to dissolved oxygen in blood. The mammalian hemoglobin molecule can bind (carry) up to four oxygen molecules.
Hemoglobin is involved in the transport of other gases: it carries some of the body's respiratory carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
(about 10% of the total) as carbaminohemoglobin
Carbaminohemoglobin
Carbaminohemoglobin is a compound of hemoglobin and carbon dioxide, and is one of the forms in which carbon dioxide exists in the blood. When carbon dioxide binds to hemoglobin, carbaminohemoglobin is formed, lowering hemoglobin's affinity for oxygen via the Haldane effect...
, in which CO2 is bound to the globin protein. The molecule also carries the important regulatory molecule nitric oxide
Nitric oxide
Nitric oxide, also known as nitrogen monoxide, is a diatomic molecule with chemical formula NO. It is a free radical and is an important intermediate in the chemical industry...
bound to a globin protein thiol
Thiol
In organic chemistry, a thiol is an organosulfur compound that contains a carbon-bonded sulfhydryl group...
group, releasing it at the same time as oxygen.
Hemoglobin is also found outside red blood cells and their progenitor lines. Other cells that contain hemoglobin include the A9 dopaminergic
Dopaminergic
Dopaminergic means related to the neurotransmitter dopamine. For example, certain proteins such as the dopamine transporter , vesicular monoamine transporter 2 , and dopamine receptors can be classified as dopaminergic, and neurons which synthesize or contain dopamine and synapses with dopamine...
neurons in the substantia nigra
Substantia nigra
The substantia nigra is a brain structure located in the mesencephalon that plays an important role in reward, addiction, and movement. Substantia nigra is Latin for "black substance", as parts of the substantia nigra appear darker than neighboring areas due to high levels of melanin in...
, macrophage
Macrophage
Macrophages are cells produced by the differentiation of monocytes in tissues. Human macrophages are about in diameter. Monocytes and macrophages are phagocytes. Macrophages function in both non-specific defense as well as help initiate specific defense mechanisms of vertebrate animals...
s, alveolar cells, and mesangial cell
Mesangial cell
Mesangial cells are specialized cells around blood vessels in the kidneys, at the mesangium. They are specialized smooth muscle cells that function to regulate blood flow through the capillaries, usually divided into two types, each having a very distinct function and location:* Extraglomerular...
s in the kidney. In these tissues, hemoglobin has a non-oxygen-carrying function as an antioxidant
Antioxidant
An antioxidant is a molecule capable of inhibiting the oxidation of other molecules. Oxidation is a chemical reaction that transfers electrons or hydrogen from a substance to an oxidizing agent. Oxidation reactions can produce free radicals. In turn, these radicals can start chain reactions. When...
and a regulator of iron metabolism.
Hemoglobin and hemoglobin-like molecules are also found in many invertebrates, fungi, and plants. In these organisms, hemoglobins may carry oxygen, or they may act to transport and regulate other things such as carbon dioxide, nitric oxide, hydrogen sulfide and sulfide. A variant of the molecule, called leghemoglobin
Leghemoglobin
Leghemoglobin is a nitrogen or oxygen carrier, because naturally occurring oxygen and nitrogen interact similarly with this protein; and a hemoprotein found in the nitrogen-fixing root nodules of leguminous plants. But nitrogen is necessary for the cycle to occur...
, is used to scavenge oxygen, to keep it from poisoning anaerobic
Anaerobic respiration
Anaerobic respiration is a form of respiration using electron acceptors other than oxygen. Although oxygen is not used as the final electron acceptor, the process still uses a respiratory electron transport chain; it is respiration without oxygen...
systems, such as nitrogen-fixing nodules of leguminous plants.
Research history
The oxygen-carrying protein hemoglobin was discovered by Hünefeld in 1840. In 1851, Otto FunkeOtto Funke
Otto Funke was a German physiologist who was a native of Chemnitz. He studied at Leipzig and Heidelberg, and in 1852 became a lecturer of physiology at the University of Leipzig. In 1856 he became a professor of physiological chemistry in Leipzig, and in 1860 a professor of physiology and zoology...
published a series of articles in which he described growing hemoglobin crystals by successively diluting red blood cells with a solvent such as pure water, alcohol or ether, followed by slow evaporation of the solvent from the resulting protein solution. Hemoglobin's reversible oxygenation was described a few years later by Felix Hoppe-Seyler.
In 1959 Max Perutz
Max Perutz
Max Ferdinand Perutz, OM, CH, CBE, FRS was an Austrian-born British molecular biologist, who shared the 1962 Nobel Prize for Chemistry with John Kendrew, for their studies of the structures of hemoglobin and globular proteins...
determined the molecular structure of hemoglobin by X-ray crystallography
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
. This work resulted in his sharing with John Kendrew
John Kendrew
Sir John Cowdery Kendrew, CBE, FRS was an English biochemist and crystallographer who shared the 1962 Nobel Prize in Chemistry with Max Perutz; their group in the Cavendish Laboratory investigated the structure of heme-containing proteins.-Biography:He was born in Oxford, son of Wilford George...
the 1962 Nobel Prize in Chemistry
Nobel Prize in Chemistry
The Nobel Prize in Chemistry is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for outstanding contributions in chemistry, physics, literature,...
.
The role of hemoglobin in the blood was elucidated by physiologist Claude Bernard
Claude Bernard
Claude Bernard was a French physiologist. He was the first to define the term milieu intérieur . Historian of science I. Bernard Cohen of Harvard University called Bernard "one of the greatest of all men of science"...
.
The name hemoglobin is derived from the words heme
Heme
A heme or haem is a prosthetic group that consists of an iron atom contained in the center of a large heterocyclic organic ring called a porphyrin. Not all porphyrins contain iron, but a substantial fraction of porphyrin-containing metalloproteins have heme as their prosthetic group; these are...
and globin
Globin
Globins are a related family of proteins, which are thought to share a common ancestor. These proteins all incorporate the globin fold, a series of eight alpha helical segments. Two prominent members of this family include myoglobin and hemoglobin, which both bind the heme prosthetic group...
, reflecting the fact that each subunit
Protein subunit
In structural biology, a protein subunit or subunit protein is a single protein molecule that assembles with other protein molecules to form a protein complex: a multimeric or oligomeric protein. Many naturally occurring proteins and enzymes are multimeric...
of hemoglobin is a globular protein
Globular protein
Globular proteins, or spheroproteins are one of the two main protein classes, comprising "globe"-like proteins that are more or less soluble in aqueous solutions...
with an embedded heme
Heme
A heme or haem is a prosthetic group that consists of an iron atom contained in the center of a large heterocyclic organic ring called a porphyrin. Not all porphyrins contain iron, but a substantial fraction of porphyrin-containing metalloproteins have heme as their prosthetic group; these are...
group. Each heme group contains one iron atom, that can bind one oxygen molecule through ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
-induced dipole forces. The most common type of hemoglobin in mammals contains four such subunits.
Genetics
Hemoglobin consists mostly of protein (the "globin" chains) subunits, and these proteins, in turn, are folded chains of a large number of different amino acids called polypeptides. The amino acid sequence of any polypeptide created by a cell, is in turn determined by the stretches of DNA called genes. In all proteins, it is the amino acid sequence, which determines the protein's chemical properties and function.There is more than one hemoglobin gene. The amino acid sequences of the globin proteins in hemoglobins usually differ between species. These differences grow with evolutionary distance between species. For example, the most common hemoglobin sequences in humans and chimpanzees are nearly identical, differing by only one amino acid in both the alpha and the beta globin protein chains. These differences grow larger between less closely related species.
Even within a species, different variants of hemoglobin always exist, although one sequence is usually a "most common" one in each species. Mutations in the genes
Gênes
Gênes is the name of a département of the First French Empire in present Italy, named after the city of Genoa. It was formed in 1805, when Napoleon Bonaparte occupied the Republic of Genoa. Its capital was Genoa, and it was divided in the arrondissements of Genoa, Bobbio, Novi Ligure, Tortona and...
for the hemoglobin protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
in a species result in hemoglobin variants
Hemoglobin variants
Hemoglobin variants are mutant forms of hemoglobin in a population , caused by variations in genetics. Some well-known hemoglobin variants such as sickle-cell anemia are responsible for diseases, and are considered hemoglobinopathies...
. Many of these mutant forms of hemoglobin cause no disease. Some of these mutant forms of hemoglobin, however, cause a group of hereditary diseases termed the hemoglobinopathies
Hemoglobinopathy
Hemoglobinopathy is a kind of genetic defect that results in abnormal structure of one of the globin chains of the hemoglobin molecule. Hemoglobinopathies are inherited single-gene disorders; in most cases, they are inherited as autosomal co-dominant traits. Common hemoglobinopathies include...
. The best known hemoglobinopathy is sickle-cell disease
Sickle-cell disease
Sickle-cell disease , or sickle-cell anaemia or drepanocytosis, is an autosomal recessive genetic blood disorder with overdominance, characterized by red blood cells that assume an abnormal, rigid, sickle shape. Sickling decreases the cells' flexibility and results in a risk of various...
, which was the first human disease whose mechanism
Mechanism (biology)
In biology --and in science in general-- a mechanism is a complex object or, more generally, a process that produces a regular phenomenon. For example, natural selection is one of the mechanisms of biological evolution, other being genetic drift, biased mutation, and gene flow; competition,...
was understood at the molecular level. A (mostly) separate set of diseases called thalassemia
Thalassemia
Thalassemia is an inherited autosomal recessive blood disease that originated in the Mediterranean region. In thalassemia the genetic defect, which could be either mutation or deletion, results in reduced rate of synthesis or no synthesis of one of the globin chains that make up hemoglobin...
s involves underproduction of normal and sometimes abnormal hemoglobins, through problems and mutations in globin gene regulation. All these diseases produce anemia
Anemia
Anemia is a decrease in number of red blood cells or less than the normal quantity of hemoglobin in the blood. However, it can include decreased oxygen-binding ability of each hemoglobin molecule due to deformity or lack in numerical development as in some other types of hemoglobin...
.
Variations in hemoglobin amino acid sequences, as with other proteins, may be adaptive. For example, recent studies have suggested genetic variants in deer mice that help explain how deer mice that live in the mountains are able to survive in the thin air that accompanies high altitudes. A researcher from the University of Nebraska-Lincoln found mutations in four different genes that can account for differences between deer mice that live in lowland prairies versus the mountains. After examining wild mice captured from both highlands and lowlands, it was found that: the genes of the two breeds are “virtually identical–except for those that govern the oxygen-carrying capacity of their hemoglobin”. “The genetic difference enables highland mice to make more efficient use of their oxygen”, since less is available at higher altitudes, such as those in the mountains. Mammoth
Mammoth
A mammoth is any species of the extinct genus Mammuthus. These proboscideans are members of Elephantidae, the family of elephants and mammoths, and close relatives of modern elephants. They were often equipped with long curved tusks and, in northern species, a covering of long hair...
hemoglobin featured mutations that allowed for oxygen delivery at lower temperatures, thus enabling mammoths to migrate to higher latitudes during the Pleistocene
Pleistocene
The Pleistocene is the epoch from 2,588,000 to 11,700 years BP that spans the world's recent period of repeated glaciations. The name pleistocene is derived from the Greek and ....
.
Synthesis
Hemoglobin (Hb) is synthesized in a complex series of steps. The heme part is synthesized in a series of steps in the mitochondria and the cytosolCytosol
The cytosol or intracellular fluid is the liquid found inside cells, that is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondrion into compartments....
of immature red blood cells, while the globin
Globin
Globins are a related family of proteins, which are thought to share a common ancestor. These proteins all incorporate the globin fold, a series of eight alpha helical segments. Two prominent members of this family include myoglobin and hemoglobin, which both bind the heme prosthetic group...
protein parts are synthesized by ribosome
Ribosome
A ribosome is a component of cells that assembles the twenty specific amino acid molecules to form the particular protein molecule determined by the nucleotide sequence of an RNA molecule....
s in the cytosol. Production of Hb continues in the cell throughout its early development from the proerythroblast
Proerythroblast
A proerythroblast is the earliest of four stages in development of the normoblast.In histology, it is very difficult to distinguish it from the other "-blast" cells...
to the reticulocyte
Reticulocyte
Reticulocytes are immature red blood cells, typically composing about 1% of the red cells in the human body.Reticulocytes develop and mature in the red bone marrow and then circulate for about a day in the blood stream before developing into mature red blood cells. Like mature red blood cells,...
in the bone marrow
Bone marrow
Bone marrow is the flexible tissue found in the interior of bones. In humans, bone marrow in large bones produces new blood cells. On average, bone marrow constitutes 4% of the total body mass of humans; in adults weighing 65 kg , bone marrow accounts for approximately 2.6 kg...
. At this point, the nucleus
Cell nucleus
In cell biology, the nucleus is a membrane-enclosed organelle found in eukaryotic cells. It contains most of the cell's genetic material, organized as multiple long linear DNA molecules in complex with a large variety of proteins, such as histones, to form chromosomes. The genes within these...
is lost in mammalian red blood cells, but not in bird
Bird
Birds are feathered, winged, bipedal, endothermic , egg-laying, vertebrate animals. Around 10,000 living species and 188 families makes them the most speciose class of tetrapod vertebrates. They inhabit ecosystems across the globe, from the Arctic to the Antarctic. Extant birds range in size from...
s and many other species. Even after the loss of the nucleus in mammals, residual ribosomal RNA
Ribosomal RNA
Ribosomal ribonucleic acid is the RNA component of the ribosome, the enzyme that is the site of protein synthesis in all living cells. Ribosomal RNA provides a mechanism for decoding mRNA into amino acids and interacts with tRNAs during translation by providing peptidyl transferase activity...
allows further synthesis of Hb until the reticulocyte loses its RNA soon after entering the vasculature
Circulatory system
The circulatory system is an organ system that passes nutrients , gases, hormones, blood cells, etc...
(this hemoglobin-synthetic RNA in fact gives the reticulocyte its reticulated appearance and name).
Structure
Hemoglobin has a quaternary structureQuaternary structure
In biochemistry, quaternary structure is the arrangement of multiple folded protein or coiling protein molecules in a multi-subunit complex.-Description and examples:...
characteristic of many multi-subunit globular proteins. Most of the amino acids in hemoglobin form alpha helices, connected by short non-helical segments. Hydrogen bonds stabilize the helical sections inside this protein, causing attractions within the molecule, folding each polypeptide chain into a specific shape. Hemoglobin's quaternary structure comes from its four subunits in roughly a tetrahedral arrangement.
In most vertebrates, the hemoglobin molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
is an assembly of four globular protein
Globular protein
Globular proteins, or spheroproteins are one of the two main protein classes, comprising "globe"-like proteins that are more or less soluble in aqueous solutions...
subunits. Each subunit is composed of a protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
chain tightly associated with a non-protein heme
Heme
A heme or haem is a prosthetic group that consists of an iron atom contained in the center of a large heterocyclic organic ring called a porphyrin. Not all porphyrins contain iron, but a substantial fraction of porphyrin-containing metalloproteins have heme as their prosthetic group; these are...
group. Each protein chain arranges into a set of alpha-helix structural segments connected together in a globin fold
Globin fold
The globin fold is a common three-dimensional fold in proteins. This fold typically consists of eight alpha helices, although some proteins have additional helix extensions at their termini. The globin fold is found in its namesake proteins hemoglobin and myoglobin as well as in phycocyanin proteins...
arrangement, so called because this arrangement is the same folding motif used in other heme/globin proteins such as myoglobin
Myoglobin
Myoglobin is an iron- and oxygen-binding protein found in the muscle tissue of vertebrates in general and in almost all mammals. It is related to hemoglobin, which is the iron- and oxygen-binding protein in blood, specifically in the red blood cells. The only time myoglobin is found in the...
. This folding pattern contains a pocket that strongly binds the heme group.
A heme group consists of an iron (Fe) ion (charged atom) held in a heterocyclic
Heterocyclic compound
A heterocyclic compound is a cyclic compound which has atoms of at least two different elements as members of its ring. The counterparts of heterocyclic compounds are homocyclic compounds, the rings of which are made of a single element....
ring, known as a porphyrin
Porphyrin
Porphyrins are a group of organic compounds, many naturally occurring. One of the best-known porphyrins is heme, the pigment in red blood cells; heme is a cofactor of the protein hemoglobin. Porphyrins are heterocyclic macrocycles composed of four modified pyrrole subunits interconnected at...
. This porphyrin ring consists of four pyrrole molecules cyclically linked together (by methene bridges) with the iron ion bound in the center. The iron ion, which is the site of oxygen binding, coordinates with the four nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
s in the center of the ring, which all lie in one plane. The iron is bound strongly (covalently) to the globular protein via the imidazole
Imidazole
Imidazole is an organic compound with the formula C3H4N2. This aromatic heterocyclic is a diazole and is classified as an alkaloid. Imidazole refers to the parent compound, whereas imidazoles are a class of heterocycles with similar ring structure, but varying substituents...
ring of the F8 histidine
Histidine
Histidine Histidine, an essential amino acid, has a positively charged imidazole functional group. It is one of the 22 proteinogenic amino acids. Its codons are CAU and CAC. Histidine was first isolated by German physician Albrecht Kossel in 1896. Histidine is an essential amino acid in humans...
residue (also known as the proximal histidine) below the porphyrin ring. A sixth position can reversibly bind oxygen by a coordinate covalent bond
Coordinate covalent bond
A dipolar bond, also known as dative covalent bond or coordinate bond is a kind of 2-centre, 2-electron covalent bond in which the two electrons derive from the same atom. Typically, a dipolar bond is formed when a Lewis base donates a pair of electrons to a Lewis acid. This description of bonding...
, completing the octahedral group of six ligands. Oxygen binds in an "end-on bent" geometry where one oxygen atom binds Fe and the other protrudes at an angle. When oxygen is not bound, a very weakly bonded water molecule fills the site, forming a distorted octahedron
Octahedron
In geometry, an octahedron is a polyhedron with eight faces. A regular octahedron is a Platonic solid composed of eight equilateral triangles, four of which meet at each vertex....
.
Even though carbon dioxide is carried by hemoglobin, it does not compete with oxygen for the iron-binding positions, but is actually bound to the protein chains of the structure.
The iron ion may be either in the Fe2+ or in the Fe3+ state, but ferrihemoglobin (methemoglobin) (Fe3+) cannot bind oxygen. In binding, oxygen temporarily and reversibly oxidizes (Fe2+) to (Fe3+) while oxygen temporally turns into superoxide
Superoxide
A superoxide, also known by the obsolete name hyperoxide, is a compound that possesses the superoxide anion with the chemical formula O2−. The systematic name of the anion is dioxide. It is important as the product of the one-electron reduction of dioxygen O2, which occurs widely in nature...
, thus iron must exist in the +2 oxidation state to bind oxygen. If superoxide ion associated to Fe3+ is protonated the hemoglobin iron will remain oxidized and incapable to bind oxygen. In such cases, the enzyme methemoglobin reductase
Cytochrome b5 reductase
Cytochrome-b5 reductase is a NADH-dependent enzyme that converts methemoglobin to hemoglobin...
will be able to eventually reactivate methemoglobin by reducing the iron center.
In adult humans, the most common hemoglobin type is a tetramer (which contains 4 subunit proteins) called hemoglobin A, consisting of two α and two β subunits non-covalently bound, each made of 141 and 146 amino acid residues, respectively. This is denoted as α2β2. The subunits are structurally similar and about the same size. Each subunit has a molecular weight of about 17,000 daltons, for a total molecular weight of the tetramer of about 64,000 daltons (64,458 g/mol). Thus, 1 g/dL = 0.1551 mmol/L. Hemoglobin A is the most intensively studied of the hemoglobin molecules.
In human infants, the hemoglobin molecule is made up of 2 α chains and 2 gamma chains. The gamma chains are gradually replaced by β chains as the infant grows.
The four polypeptide chains are bound to each other by salt bridge
Salt bridge (protein)
Salt bridges fall into the broader category of noncovalent interactions. A salt bridge is actually a combination of two noncovalent interactions: hydrogen bonding and electrostatic interactions . This is most commonly observed to contribute stability to the entropically unfavorable folded...
s, hydrogen bond
Hydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
s, and the hydrophobic effect
Hydrophobic effect
The hydrophobic effect is the observed tendency of nonpolar substances to aggregate in aqueous solution and exclude water molecules. The name, literally meaning "water-fearing," describes the segregation and apparent repulsion between water and nonpolar substances...
. There are two kinds of contacts between the α and β chains: α1β1 and α1β2.
Oxygen saturation
In general, hemoglobin can be saturated with oxygen molecules (oxyhemoglobin), or desaturated with oxygen molecules (deoxyhemoglobin).Oxyhemoglobin
Oxyhemoglobin is formed during physiological respirationRespiration (physiology)
'In physiology, respiration is defined as the transport of oxygen from the outside air to the cells within tissues, and the transport of carbon dioxide in the opposite direction...
when oxygen binds to the heme component of the protein hemoglobin in red blood cells. This process occurs in the pulmonary capillaries adjacent to the alveoli of the lungs. The oxygen then travels through the blood stream to be dropped off at cells where it is utilized in glycolysis
Glycolysis
Glycolysis is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+...
and in the production of ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
by the process of oxidative phosphorylation
Oxidative phosphorylation
Oxidative phosphorylation is a metabolic pathway that uses energy released by the oxidation of nutrients to produce adenosine triphosphate . Although the many forms of life on earth use a range of different nutrients, almost all aerobic organisms carry out oxidative phosphorylation to produce ATP,...
. It does not, however, help to counteract a decrease in blood pH. Ventilation
Ventilation (physiology)
In respiratory physiology, ventilation is the rate at which gas enters or leaves the lung. It is categorized under the following definitions:-Sample values:...
, or breathing, may reverse this condition by removal of carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
, thus causing a shift up in pH.
Hemoglobin exists in two forms, a taut form (T) and a relaxed form (R). Various factors such as low pH, high CO2 and high 2,3 BPG at the level of the tissues favor the taut form, which has low oxygen affinity and releases oxygen in the tissues. Conversely, a high pH, low CO2, or low 2,3 BPG favors the relaxed form which can better bind oxygen.
Deoxygenated hemoglobin
Deoxygenated hemoglobin is the form of hemoglobin without the bound oxygen. The absorption spectra of oxyhemoglobin and deoxyhemoglobin differ. The oxyhemoglobin has significantly lower absorption of the 660 nm wavelengthWavelength
In physics, the wavelength of a sinusoidal wave is the spatial period of the wave—the distance over which the wave's shape repeats.It is usually determined by considering the distance between consecutive corresponding points of the same phase, such as crests, troughs, or zero crossings, and is a...
than deoxyhemoglobin, while at 940 nm its absorption is slightly higher. This difference is used for measurement of the amount of oxygen in patient's blood by an instrument called pulse oximeter
Pulse oximeter
A pulse oximeter is a medical device that indirectly monitors the oxygen saturation of a patient's blood and changes in blood volume in the skin, producing a photoplethysmograph. It is often attached to a medical monitor so staff can see a patient's oxygenation at all times...
. This difference also accounts for the presentation of cyanosis
Cyanosis
Cyanosis is the appearance of a blue or purple coloration of the skin or mucous membranes due to the tissues near the skin surface being low on oxygen. The onset of cyanosis is 2.5 g/dL of deoxyhemoglobin. The bluish color is more readily apparent in those with high hemoglobin counts than it is...
, the blue to purplish color that tissues develop during hypoxia
Hypoxia (medical)
Hypoxia, or hypoxiation, is a pathological condition in which the body as a whole or a region of the body is deprived of adequate oxygen supply. Variations in arterial oxygen concentrations can be part of the normal physiology, for example, during strenuous physical exercise...
.
Iron's oxidation state in oxyhemoglobin
Assigning oxygenated hemoglobin's oxidation state is difficult because oxyhemoglobin (Hb-O2), by experimental measurement, is diamagnetic (no net unpaired electrons), yet the low-energy electron configurations in both oxygen and iron are paramagneticParamagnetism
Paramagnetism is a form of magnetism whereby the paramagnetic material is only attracted when in the presence of an externally applied magnetic field. In contrast with this, diamagnetic materials are repulsive when placed in a magnetic field...
(suggesting at least one unpaired electron in the complex). The lowest-energy form of oxygen, and the lowest energy forms of the relevant oxidation states of iron, are these:
- Triplet oxygenTriplet oxygenTriplet oxygen is the ground state of the oxygen molecule. The electron configuration of the molecule has two unpaired electrons occupying two degenerate molecular orbitals...
, the lowest energy molecular oxygen species, has two unpaired electrons in antibonding π* molecular orbitals. - Iron(II) tends to exist in a high-spin configuration where unpaired electrons exist in Eg antibonding orbitals.
- Iron(III) has an odd number of electrons, and thus must have one or more unpaired electrons, in any energy state.
All of these structures are paramagnetic (have unpaired electrons), not diamagnetic. Thus, a non-intuitive (e.g., a higher-energy for at least one species) distribution of electrons in the combination of iron and oxygen must exist, in order to explain the observed diamagnetism and no unpaired electrons.
The three logical possibilities to produce diamagnetic (no net spin) Hb-O2 are:
- Low-spin Fe2+ binds to singlet oxygenSinglet oxygenSinglet oxygen is the common name used for the diamagnetic form of molecular oxygen , which is less stable than the normal triplet oxygen. Because of its unusual properties, singlet oxygen can persist for over an hour at room temperature, depending on the environment...
. Both low-spin iron and singlet oxygen are diamagnetic. However, the singlet form of oxygen is the higher-energy form of the molecule. - Low-spin Fe3+ binds to .O2- (the superoxideSuperoxideA superoxide, also known by the obsolete name hyperoxide, is a compound that possesses the superoxide anion with the chemical formula O2−. The systematic name of the anion is dioxide. It is important as the product of the one-electron reduction of dioxygen O2, which occurs widely in nature...
ion) and the two unpaired electrons couple antiferromagnetically, giving diamagnetic properties. - Low-spin Fe4+ binds to peroxide, O22-. Both are diamagnetic.
Direct experimental data:
- X-ray photoelectron spectroscopyX-ray photoelectron spectroscopyX-ray photoelectron spectroscopy is a quantitative spectroscopic technique that measures the elemental composition, empirical formula, chemical state and electronic state of the elements that exist within a material...
suggests iron has an oxidation state of approximately 3.2 - infrared stretching frequenciesInfrared spectroscopyInfrared spectroscopy is the spectroscopy that deals with the infrared region of the electromagnetic spectrum, that is light with a longer wavelength and lower frequency than visible light. It covers a range of techniques, mostly based on absorption spectroscopy. As with all spectroscopic...
of the O-O bond suggests a bond length fitting with superoxide (a bond order of about 1.6, with superoxide being 1.5). - X-ray Absorption Near Edge StructuresXANESX-ray Absorption Near Edge Structure , also known as Near edge X-ray absorption fine structure is a type of absorption spectroscopy. NEXAFS also at times used the abbreviation EXAFS....
at the iron K-edge. The energy shift of 5 eV between Deoxyhemoglobin and Oxyhemoglobin, as for all the Methemoglobin species, strongly suggests an actual local charge closer to Fe3+ than Fe2+.
Thus, the nearest formal oxidation state of iron in Hb-O2 is the +3 state, with oxygen in the -1 state (as superoxide .O2-). The diamagnetism in this configuration arises from the single unpaired electron on superoxide aligning antiferromagnetically from the single unpaired electron on iron, to give no net spin to the entire configuration, in accordance with diamagnetic oxyhemoglobin from experiment.
The second choice of the three logical possibilities above for diamagnetic oxyhemoglobin being found correct by experiment, is not surprising: singlet oxygen (possibility #1) and large separations of charge (possibility #3) are both unfavorably high-energy states. Iron's shift to a higher oxidation state in Hb-O2 decreases the atom's size, and allows it into the plane of the porphyrin ring, pulling on the coordinated histidine residue and initiating the allosteric changes seen in the globulins.
Early postulates by bio-inorganic chemists claimed that possibility #1 (above) was correct and that iron should exist in oxidation state II. This seemed particularly likely since the iron oxidation state III as methemoglobin, when not accompanied by superoxide .O2- to "hold" the oxidation electron, was known to render hemoglobin incapable of binding normal triplet O2 as it occurs in the air. It was thus assumed that iron remained as Fe(II) when oxygen gas was bound in the lungs. The iron chemistry in this previous classical model was elegant, but the required presence of the required diamagnetic high-energy singlet oxygen was never explained. It was classically argued that the binding of an oxygen molecule placed high-spin iron(II) in an octahedral field of strong-field ligands; this change in field would increase the crystal field splitting energy
Crystal field theory
Crystal field theory is a model that describes the electronic structure of transition metal compounds, all of which can be considered coordination complexes. CFT successfully accounts for some magnetic properties, colours, hydration enthalpies, and spinel structures of transition metal complexes,...
, causing iron's electrons to pair into the low-spin configuration, which would be diamagnetic in Fe(II). This forced low-spin pairing is indeed thought to happen in iron when oxygen binds, but is not enough to explain iron's change in size. Extraction of an additional electron from iron by oxygen is required to explain both iron's smaller size and observed increased oxidation state, and oxygen's weaker bond.
It should be noted that the assignment of a whole-number oxidation state is a formalism, as the covalent bonds are not required to have perfect bond orders involving whole electron-transfer. Thus, all three models for paramagnetic Hb-O2 may contribute to some small degree (by resonance) to the actual electronic configuration of Hb-O2. However, the model of iron in Hb-O2 being Fe(III) is more correct than the classical idea that it remains Fe(II).
Binding for ligands other than oxygen
Besides the oxygen ligandLigand (biochemistry)
In biochemistry and pharmacology, a ligand is a substance that forms a complex with a biomolecule to serve a biological purpose. In a narrower sense, it is a signal triggering molecule, binding to a site on a target protein.The binding occurs by intermolecular forces, such as ionic bonds, hydrogen...
, which binds to hemoglobin in a cooperative
Cooperative binding
In biochemistry, a macromolecule exhibits cooperative binding if its affinity for its ligand changes with the amount of ligand already bound.Cooperative binding is a special case of allostery. Cooperative binding requires that the macromolecule have more than one binding site, since cooperativity...
manner, hemoglobin ligands also include competitive inhibitors
Competitive inhibition
Competitive inhibition is a form of enzyme inhibition where binding of the inhibitor to the active site on the enzyme prevents binding of the substrate and vice versa.-Mechanism:...
such as carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
(CO) and allosteric
Allosteric regulation
In biochemistry, allosteric regulation is the regulation of an enzyme or other protein by binding an effector molecule at the protein's allosteric site . Effectors that enhance the protein's activity are referred to as allosteric activators, whereas those that decrease the protein's activity are...
ligands such as carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
(CO2) and nitric oxide
Nitric oxide
Nitric oxide, also known as nitrogen monoxide, is a diatomic molecule with chemical formula NO. It is a free radical and is an important intermediate in the chemical industry...
(NO). The carbon dioxide is bound to amino groups of the globin proteins as carbaminohemoglobin
Carbaminohemoglobin
Carbaminohemoglobin is a compound of hemoglobin and carbon dioxide, and is one of the forms in which carbon dioxide exists in the blood. When carbon dioxide binds to hemoglobin, carbaminohemoglobin is formed, lowering hemoglobin's affinity for oxygen via the Haldane effect...
, and is thought to account for about 10% of carbon dioxide transport in mammals. Nitric oxide is bound to specific thiol groups in the globin protein to form an S-nitrosothiol which dissociates into free nitric oxide and thiol again, as the hemoglobin releases oxygen from its heme site. This nitric oxide transport to peripheral tissues is hypothesized to assist oxygen transport in tissues, by releasing vasodilatory nitric oxide to tissues in which oxygen levels are low.
Cooperative

In the tetrameric form of normal adult hemoglobin, the binding of oxygen is, thus, a cooperative
Cooperative binding
In biochemistry, a macromolecule exhibits cooperative binding if its affinity for its ligand changes with the amount of ligand already bound.Cooperative binding is a special case of allostery. Cooperative binding requires that the macromolecule have more than one binding site, since cooperativity...
process. The binding affinity of hemoglobin for oxygen is increased by the oxygen saturation of the molecule, with the first oxygens bound influencing the shape of the binding sites for the next oxygens, in a way favorable for binding. This positive cooperative binding is achieved through steric
Steric effects
Steric effects arise from the fact that each atom within a molecule occupies a certain amount of space. If atoms are brought too close together, there is an associated cost in energy due to overlapping electron clouds , and this may affect the molecule's preferred shape and reactivity.-Steric...
conformational changes of the hemoglobin protein complex as discussed above; i.e., when one subunit protein in hemoglobin becomes oxygenated, a conformational or structural change in the whole complex is initiated, causing the other subunits to gain an increased affinity for oxygen. As a consequence, the oxygen binding curve of hemoglobin is sigmoidal
Sigmoid function
Many natural processes, including those of complex system learning curves, exhibit a progression from small beginnings that accelerates and approaches a climax over time. When a detailed description is lacking, a sigmoid function is often used. A sigmoid curve is produced by a mathematical...
, or S-shaped, as opposed to the normal hyperbolic
Hyperbolic function
In mathematics, hyperbolic functions are analogs of the ordinary trigonometric, or circular, functions. The basic hyperbolic functions are the hyperbolic sine "sinh" , and the hyperbolic cosine "cosh" , from which are derived the hyperbolic tangent "tanh" and so on.Just as the points form a...
curve associated with noncooperative binding.
The dynamic mechanism of the cooperativity in hemoglobin and its relation with the low-frequency resonance has been discussed.
Competitive
Hemoglobin's oxygen-binding capacity is decreased in the presence of carbon monoxideCarbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
because both gases compete for the same binding sites on hemoglobin, carbon monoxide binding preferentially in place of oxygen.
The binding of oxygen is affected by molecules such as carbon monoxide (CO) (for example, from tobacco smoking
Tobacco smoking
Tobacco smoking is the practice where tobacco is burned and the resulting smoke is inhaled. The practice may have begun as early as 5000–3000 BCE. Tobacco was introduced to Eurasia in the late 16th century where it followed common trade routes...
, car exhaust, and incomplete combustion in furnaces). CO competes with oxygen at the heme binding site. Hemoglobin binding affinity for CO is 250 times greater than its affinity for oxygen, meaning that small amounts of CO dramatically reduce hemoglobin's ability to transport oxygen. Since Carbon Monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
is a colorless, odorless and tasteless gas, and poses a potentially fatal threat detectors
Carbon monoxide detector
A carbon monoxide detector or CO detector is a device that detects the presence of the carbon monoxide gas in order to prevent carbon monoxide poisoning. CO is a colorless and odorless compound produced by incomplete combustion. It is often referred to as the "silent killer" because it is...
have become commercially available to warn of dangerous levels in residences. When hemoglobin combines with CO, it forms a very bright red compound called carboxyhemoglobin
Carboxyhemoglobin
Carboxyhemoglobin is a stable complex of carbon monoxide and hemoglobin that forms in red blood cells when carbon monoxide is inhaled or produced in normal metabolism. Large quantities of it hinder delivery of oxygen to the body...
, which may cause the skin of CO poisoning victims to appear pink in death, instead of white or blue. When inspired air contains CO levels as low as 0.02%, headache and nausea occur; if the CO concentration is increased to 0.1%, unconsciousness will follow. In heavy smokers, up to 20% of the oxygen-active sites can be blocked by CO.
In similar fashion, hemoglobin also has competitive binding affinity for cyanide
Cyanide
A cyanide is a chemical compound that contains the cyano group, -C≡N, which consists of a carbon atom triple-bonded to a nitrogen atom. Cyanides most commonly refer to salts of the anion CN−. Most cyanides are highly toxic....
(CN-), sulfur monoxide
Sulfur monoxide
Sulfur monoxide is an inorganic compound with formula . It is only found as a dilute gas phase. When concentrated or condensed, it converts to S2O2 . It has been detected in space but is rarely encountered intact otherwise.-Structure and bonding:The SO molecule has a triplet ground state similar...
(SO), nitric oxide
Nitric oxide
Nitric oxide, also known as nitrogen monoxide, is a diatomic molecule with chemical formula NO. It is a free radical and is an important intermediate in the chemical industry...
(NO), and sulfide
Sulfide
A sulfide is an anion of sulfur in its lowest oxidation state of 2-. Sulfide is also a slightly archaic term for thioethers, a common type of organosulfur compound that are well known for their bad odors.- Properties :...
(S2-), including hydrogen sulfide
Hydrogen sulfide
Hydrogen sulfide is the chemical compound with the formula . It is a colorless, very poisonous, flammable gas with the characteristic foul odor of expired eggs perceptible at concentrations as low as 0.00047 parts per million...
(H2S). All of these bind to iron in heme without changing its oxidation state, but they nevertheless inhibit oxygen-binding, causing grave toxicity.
The iron atom in the heme group must initially be in the ferrous (Fe2+) oxidation state to support oxygen and other gases' binding and transport (it temporarily switches to ferric during the time oxygen is bound, as explained above). Initial oxidation to the ferric (Fe3+) state without oxygen converts hemoglobin into "hemiglobin" or methemoglobin , which cannot bind oxygen. Hemoglobin in normal red blood cells is protected by a reduction system to keep this from happening. Nitric oxide is capable of converting a small fraction of hemoglobin to methemoglobin in red blood cells. The latter reaction is a remnant activity of the more ancient nitric oxide dioxygenase
Nitric oxide dioxygenase
Nitric oxide dioxygenase is an enzyme that catalyzes the conversion of toxic nitric oxide to nitrate . The net reaction for the reaction catalyzed by nitric oxide dioxygenase is shown below:* 2NO + 2O2 + NADH → 2NO3- + NAD+ + H+...
function of globins.
Allosteric
Carbon dioxide occupies a different binding site on the hemoglobin. Carbon dioxide is more readily dissolved in deoxygenated blood, facilitating its removal from the body after the oxygen has been released to tissues undergoing metabolism. This increased affinity for carbon dioxide by the venous blood is known as the Haldane effectHaldane effect
The Haldane effect is a property of hemoglobin first described by the Scottish physician John Scott Haldane. Deoxygenation of the blood increases its ability to carry carbon dioxide; this property is the Haldane effect. Conversely, oxygenated blood has a reduced capacity for carbon...
. Through the enzyme carbonic anhydrase
Carbonic anhydrase
The carbonic anhydrases form a family of enzymes that catalyze the rapid interconversion of carbon dioxide and water to bicarbonate and protons , a reversible reaction that occurs rather slowly in the absence of a catalyst...
, carbon dioxide reacts with water to give carbonic acid
Carbonic acid
Carbonic acid is the inorganic compound with the formula H2CO3 . It is also a name sometimes given to solutions of carbon dioxide in water, because such solutions contain small amounts of H2CO3. Carbonic acid forms two kinds of salts, the carbonates and the bicarbonates...
, which decomposes into bicarbonate
Bicarbonate
In inorganic chemistry, bicarbonate is an intermediate form in the deprotonation of carbonic acid...
and proton
Proton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
s:
- CO2 + H2O → H2CO3 → HCO3- + H+

PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
(more acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
ic). Hemoglobin can bind protons and carbon dioxide, which causes a conformational change in the protein and facilitates the release of oxygen. Protons bind at various places on the protein, while carbon dioxide binds at the α-amino group. Carbon dioxide binds to hemoglobin and forms carbaminohemoglobin
Carbaminohemoglobin
Carbaminohemoglobin is a compound of hemoglobin and carbon dioxide, and is one of the forms in which carbon dioxide exists in the blood. When carbon dioxide binds to hemoglobin, carbaminohemoglobin is formed, lowering hemoglobin's affinity for oxygen via the Haldane effect...
. This decrease in hemoglobin's affinity for oxygen by the binding of carbon dioxide and acid is known as the Bohr effect
Bohr effect
Bohr effect is a property of hemoglobin first described in 1904 by the Danish physiologist Christian Bohr , which states that an increasing concentration of protons and/or carbon dioxide will reduce the oxygen affinity of hemoglobin...
(shifts the O2-saturation curve to the right). Conversely, when the carbon dioxide levels in the blood decrease (i.e., in the lung capillaries), carbon dioxide and protons are released from hemoglobin, increasing the oxygen affinity of the protein. A reduction in the total binding capacity of hemoglobin to oxygen (i.e. shifting the curve down, not just to the right) due to reduced pH is called the root effect
Root effect
The Root Effect is a physiological phenomenon that occurs in fish hemoglobin, named after its discoverer R. W. Root. It is the phenomenon where an increased proton or carbon dioxide concentration lowers hemoglobin's affinity and carrying capacity for oxygen . The Root effect is to be...
. This is seen in bony fish.
It is necessary for hemoglobin to release the oxygen that it binds; if not, there is no point in binding it. The sigmoidal curve of hemoglobin makes it efficient in binding (taking up O2 in lungs), and efficient in unloading (unloading O2 in tissues).
In people acclimated to high altitudes, the concentration of 2,3-Bisphosphoglycerate
2,3-Bisphosphoglycerate
2,3-Bisphosphoglyceric acid is a three-carbon isomer of the glycolytic intermediate 1,3-bisphosphoglyceric acid . 2,3-BPG is present in human red blood cells at approximately 5 mmol/L...
(2,3-BPG) in the blood is increased, which allows these individuals to deliver a larger amount of oxygen to tissues under conditions of lower oxygen tension. This phenomenon, where molecule Y affects the binding of molecule X to a transport molecule Z, is called a heterotropic allosteric effect.
A variant hemoglobin, called fetal hemoglobin
Fetal hemoglobin
Fetal hemoglobin, or foetal haemoglobin, is the main oxygen transport protein in the fetus during the last seven months of development in the uterus and in the newborn until roughly 6 months old...
(HbF, α2γ2), is found in the developing fetus
Fetus
A fetus is a developing mammal or other viviparous vertebrate after the embryonic stage and before birth.In humans, the fetal stage of prenatal development starts at the beginning of the 11th week in gestational age, which is the 9th week after fertilization.-Etymology and spelling variations:The...
, and binds oxygen with greater affinity than adult hemoglobin. This means that the oxygen binding curve for fetal hemoglobin is left-shifted (i.e., a higher percentage of hemoglobin has oxygen bound to it at lower oxygen tension), in comparison to that of adult hemoglobin. As a result, fetal blood in the placenta
Placenta
The placenta is an organ that connects the developing fetus to the uterine wall to allow nutrient uptake, waste elimination, and gas exchange via the mother's blood supply. "True" placentas are a defining characteristic of eutherian or "placental" mammals, but are also found in some snakes and...
is able to take oxygen from maternal blood.
Hemoglobin also carries nitric oxide
Nitric oxide
Nitric oxide, also known as nitrogen monoxide, is a diatomic molecule with chemical formula NO. It is a free radical and is an important intermediate in the chemical industry...
in the globin part of the molecule. This improves oxygen delivery in the periphery and contributes to the control of respiration. NO binds reversibly to a specific cysteine residue in globin; the binding depends on the state (R or T) of the hemoglobin. The resulting S-nitrosylated hemoglobin influences various NO-related activities such as the control of vascular resistance, blood pressure and respiration. NO is not released in the cytoplasm of erythrocytes but transported by an anion exchanger called AE1 out of them.
A study was performed to examine the influence of the form of hemoglobin (Hb) on the partitioning of inhaled volatile organic compounds (VOCs) into [human and animal] blood. Benzene was the prototypic VOC used in the investigations for this research due to the similar properties it shares with many other VOCs. To be specific, this study analyses the influence of the water solubility of Hb on the partitioning coefficient (PC) of a VOC as compared to the influence of the “species” or form of Hb. The different forms of blood used include: human hemoglobin (HbA), rat Hb, and sickle-cell hemoglobin (HbS). Rat Hb contains little water and is in a quasi-crystalline form, found inside the red blood cells (RBC), meaning they are more hydrophobic than human Hb, which are water-soluble. Sickle-cell hemoglobin (HbS) is water-soluble, however it can become water-insoluble, forming hydrophobic polymers, when deoxygenated. The findings state that the benzene PC for rat Hb was much higher than human that for Hb; however, the tests that measured the PCs of the oxygenated and deoxygenated forms of HbA and HbS did not differ, indicating that the affinity of benzene was not affected by the water solubility of Hb.
Types in humans
Hemoglobin variantsHemoglobin variants
Hemoglobin variants are mutant forms of hemoglobin in a population , caused by variations in genetics. Some well-known hemoglobin variants such as sickle-cell anemia are responsible for diseases, and are considered hemoglobinopathies...
are a part of the normal embryonic and fetal development, but may also be pathologic mutant forms of hemoglobin in a population
Population
A population is all the organisms that both belong to the same group or species and live in the same geographical area. The area that is used to define a sexual population is such that inter-breeding is possible between any pair within the area and more probable than cross-breeding with individuals...
, caused by variations in genetics. Some well-known hemoglobin variants such as sickle-cell anemia are responsible for diseases, and are considered hemoglobinopathies. Other variants cause no detectable pathology
Pathology
Pathology is the precise study and diagnosis of disease. The word pathology is from Ancient Greek , pathos, "feeling, suffering"; and , -logia, "the study of". Pathologization, to pathologize, refers to the process of defining a condition or behavior as pathological, e.g. pathological gambling....
, and are thus considered non-pathological variants.
In the embryo
Embryo
An embryo is a multicellular diploid eukaryote in its earliest stage of development, from the time of first cell division until birth, hatching, or germination...
:
- Gower 1 (ζ2ε2)
- Gower 2 (α2ε2)
- Hemoglobin Portland (ζ2γ2)
In the fetus
Fetus
A fetus is a developing mammal or other viviparous vertebrate after the embryonic stage and before birth.In humans, the fetal stage of prenatal development starts at the beginning of the 11th week in gestational age, which is the 9th week after fertilization.-Etymology and spelling variations:The...
:
- Hemoglobin F (α2γ2)
In adults:
- Hemoglobin AHemoglobin AHemoglobin A or adult hemoglobin is the most common human hemoglobin tetramer, comprising over 97% of the total red cell hemoglobin. It consists of two alpha chains and two beta chains ....
(α2β2) - The most common with a normal amount over 95% - Hemoglobin A2Hemoglobin A2Hemoglobin A2 is a normal variant of hemoglobin A that consists of two alpha and two delta chains and is found in small quantity in normal human blood. Hemoglobin A2 may be increased in beta thalassemia or to people who are heterozygous to beta thalassemia gene....
(α2δ2) - δ chain synthesis begins late in the third trimester and in adults, it has a normal range of 1.5-3.5% - Hemoglobin F (α2γ2) - In adults Hemoglobin F is restricted to a limited population of red cells called F-cells. However, the level of Hb F can be elevated in persons with sickle-cell disease and beta-thalassemiaBeta-thalassemiaBeta-thalassemias are a group of inherited blood disorders caused by reduced or absent synthesis of the beta chains of hemoglobin resulting in variable phenotypes ranging from severe anemia to clinically asymptomatic individuals. The total annual incidence of symptomatic individuals is estimated...
.
Variant forms that cause disease:
- Hemoglobin H (β4) - A variant form of hemoglobin, formed by a tetramer of β chains, which may be present in variants of α thalassemiaThalassemiaThalassemia is an inherited autosomal recessive blood disease that originated in the Mediterranean region. In thalassemia the genetic defect, which could be either mutation or deletion, results in reduced rate of synthesis or no synthesis of one of the globin chains that make up hemoglobin...
. - Hemoglobin BartsHemoglobin BartsHemoglobin Barts consists of four gamma chains. It is moderately insoluble, and therefore accumulates in the red blood cells. It has an extremely high affinity for oxygen, resulting in almost no oxygen delivery to the tissues. It is produced in the disease alpha-thalassemia and in the most severe...
(γ4) - A variant form of hemoglobin, formed by a tetramer of γ chains, which may be present in variants of α thalassemiaThalassemiaThalassemia is an inherited autosomal recessive blood disease that originated in the Mediterranean region. In thalassemia the genetic defect, which could be either mutation or deletion, results in reduced rate of synthesis or no synthesis of one of the globin chains that make up hemoglobin...
. - Hemoglobin S (α2βS2) - A variant form of hemoglobin found in people with sickle cell disease. There is a variation in the β-chain gene, causing a change in the properties of hemoglobin, which results in sickling of red blood cells.
- Hemoglobin CHemoglobin CHemoglobin C is an abnormal hemoglobin with substitution of a lysine residue for a glutamic acid residue at the 6th position of the β-globin chain.-Clinical significance:...
(α2βC2) - Another variant due to a variation in the β-chain gene. This variant causes a mild chronic hemolytic anemiaHemolytic anemiaHemolytic anemia is a form of anemia due to hemolysis, the abnormal breakdown of red blood cells , either in the blood vessels or elsewhere in the human body . It has numerous possible causes, ranging from relatively harmless to life-threatening...
. - Hemoglobin E (α2βE2) - Another variant due to a variation in the β-chain gene. This variant causes a mild chronic hemolytic anemiaHemolytic anemiaHemolytic anemia is a form of anemia due to hemolysis, the abnormal breakdown of red blood cells , either in the blood vessels or elsewhere in the human body . It has numerous possible causes, ranging from relatively harmless to life-threatening...
. - Hemoglobin AS - A heterozygous form causing Sickle cell traitSickle cell traitSickle cell trait describes a condition in which a person has one abnormal allele of the hemoglobin beta gene , but does not display the severe symptoms of sickle cell disease that occur in a person who has two copies of that allele...
with one adult gene and one sickle cell disease gene - Hemoglobin SC disease - Another heterozygous form with one sickle gene and another encoding Hemoglobin CHemoglobin CHemoglobin C is an abnormal hemoglobin with substitution of a lysine residue for a glutamic acid residue at the 6th position of the β-globin chain.-Clinical significance:...
.
Degradation in vertebrate animals
When red cellRed blood cell
Red blood cells are the most common type of blood cell and the vertebrate organism's principal means of delivering oxygen to the body tissues via the blood flow through the circulatory system...
s reach the end of their life due to aging or defects, they are broken down, the hemoglobin molecule is broken up and the iron gets recycled. When the porphyrin ring
is broken up, the fragments are normally secreted in the bile
Bile
Bile or gall is a bitter-tasting, dark green to yellowish brown fluid, produced by the liver of most vertebrates, that aids the process of digestion of lipids in the small intestine. In many species, bile is stored in the gallbladder and upon eating is discharged into the duodenum...
by the liver
Liver
The liver is a vital organ present in vertebrates and some other animals. It has a wide range of functions, including detoxification, protein synthesis, and production of biochemicals necessary for digestion...
. This process also produces one molecule of carbon monoxide for every molecule of heme degraded. This is one of the few natural sources of carbon monoxide production in the human body, and is responsible for the normal blood levels of carbon monoxide even in people breathing pure air. The other major final product of heme degradation is bilirubin
Bilirubin
Bilirubin is the yellow breakdown product of normal heme catabolism. Heme is found in hemoglobin, a principal component of red blood cells. Bilirubin is excreted in bile and urine, and elevated levels may indicate certain diseases...
. Increased levels of this chemical are detected in the blood if red cells are being destroyed more rapidly than usual. Improperly degraded hemoglobin protein or hemoglobin that has been released from the blood cells too rapidly can clog small blood vessels, especially the delicate blood filtering vessels of the kidney
Kidney
The kidneys, organs with several functions, serve essential regulatory roles in most animals, including vertebrates and some invertebrates. They are essential in the urinary system and also serve homeostatic functions such as the regulation of electrolytes, maintenance of acid–base balance, and...
s, causing kidney damage.
Role in disease

Anemia
Anemia is a decrease in number of red blood cells or less than the normal quantity of hemoglobin in the blood. However, it can include decreased oxygen-binding ability of each hemoglobin molecule due to deformity or lack in numerical development as in some other types of hemoglobin...
, or by decreased ability of each molecule to bind oxygen at the same partial pressure of oxygen. Hemoglobinopathies
Hemoglobinopathy
Hemoglobinopathy is a kind of genetic defect that results in abnormal structure of one of the globin chains of the hemoglobin molecule. Hemoglobinopathies are inherited single-gene disorders; in most cases, they are inherited as autosomal co-dominant traits. Common hemoglobinopathies include...
(genetic defects resulting in abnormal structure of the hemoglobin molecule) may cause both. In any case, hemoglobin deficiency decreases blood oxygen-carrying capacity. Hemoglobin deficiency is, in general, strictly distinguished from hypoxemia
Hypoxemia
Hypoxemia is generally defined as decreased partial pressure of oxygen in blood, sometimes specifically as less than or causing hemoglobin oxygen saturation of less than 90%.-Distinction from anemia and hypoxia:...
, defined as decreased partial pressure
Partial pressure
In a mixture of ideal gases, each gas has a partial pressure which is the pressure which the gas would have if it alone occupied the volume. The total pressure of a gas mixture is the sum of the partial pressures of each individual gas in the mixture....
of oxygen in blood, although both are causes of hypoxia
Hypoxia (medical)
Hypoxia, or hypoxiation, is a pathological condition in which the body as a whole or a region of the body is deprived of adequate oxygen supply. Variations in arterial oxygen concentrations can be part of the normal physiology, for example, during strenuous physical exercise...
(insufficient oxygen supply to tissues).
Other common causes of low hemoglobin include loss of blood, nutritional deficiency, bone marrow problems, chemotherapy, kidney failure, or abnormal hemoglobin (such as that of sickle-cell disease).
High hemoglobin levels may be caused by exposure to high altitudes, smoking, dehydration, or tumors.
The ability of each hemoglobin molecule to carry oxygen is normally modified by altered blood pH or CO2
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
, causing an altered oxygen-hemoglobin dissociation curve
Oxygen-haemoglobin dissociation curve
The oxygen–haemoglobin dissociation curve plots the proportion of haemoglobin in its saturated form on the vertical axis against the prevailing oxygen tension on the horizontal axis. The oxyhaemoglobin dissociation curve is an important tool for understanding how our blood carries and releases...
. However, it can also be pathologically altered in, e.g., carbon monoxide poisoning
Carbon monoxide poisoning
Carbon monoxide poisoning occurs after enough inhalation of carbon monoxide . Carbon monoxide is a toxic gas, but, being colorless, odorless, tasteless, and initially non-irritating, it is very difficult for people to detect...
.
Decrease of hemoglobin, with or without an absolute decrease of red blood cells, leads to symptoms of anemia
Anemia
Anemia is a decrease in number of red blood cells or less than the normal quantity of hemoglobin in the blood. However, it can include decreased oxygen-binding ability of each hemoglobin molecule due to deformity or lack in numerical development as in some other types of hemoglobin...
. Anemia has many different causes, although iron deficiency
Iron deficiency (medicine)
Iron deficiency is one of the most common of the nutritional deficiencies. Iron is present in all cells in the human body, and has several vital functions...
and its resultant iron deficiency anemia
Iron deficiency anemia
Iron-deficiency anemia is a common anemia that occurs when iron loss occurs, and/or the dietary intake or absorption of iron is insufficient...
are the most common causes in the Western world. As absence of iron decreases heme synthesis, red blood cells in iron deficiency anemia are hypochromic (lacking the red hemoglobin pigment) and microcytic (smaller than normal). Other anemias are rarer. In hemolysis
Hemolysis
Hemolysis —from the Greek meaning "blood" and meaning a "loosing", "setting free" or "releasing"—is the rupturing of erythrocytes and the release of their contents into surrounding fluid...
(accelerated breakdown of red blood cells), associated jaundice
Jaundice
Jaundice is a yellowish pigmentation of the skin, the conjunctival membranes over the sclerae , and other mucous membranes caused by hyperbilirubinemia . This hyperbilirubinemia subsequently causes increased levels of bilirubin in the extracellular fluid...
is caused by the hemoglobin metabolite bilirubin, and the circulating hemoglobin can cause renal failure
Renal failure
Renal failure or kidney failure describes a medical condition in which the kidneys fail to adequately filter toxins and waste products from the blood...
.
Some mutations in the globin chain are associated with the hemoglobinopathies
Hemoglobinopathy
Hemoglobinopathy is a kind of genetic defect that results in abnormal structure of one of the globin chains of the hemoglobin molecule. Hemoglobinopathies are inherited single-gene disorders; in most cases, they are inherited as autosomal co-dominant traits. Common hemoglobinopathies include...
, such as sickle-cell disease
Sickle-cell disease
Sickle-cell disease , or sickle-cell anaemia or drepanocytosis, is an autosomal recessive genetic blood disorder with overdominance, characterized by red blood cells that assume an abnormal, rigid, sickle shape. Sickling decreases the cells' flexibility and results in a risk of various...
and thalassemia
Thalassemia
Thalassemia is an inherited autosomal recessive blood disease that originated in the Mediterranean region. In thalassemia the genetic defect, which could be either mutation or deletion, results in reduced rate of synthesis or no synthesis of one of the globin chains that make up hemoglobin...
. Other mutations, as discussed at the beginning of the article, are benign and are referred to merely as hemoglobin variants
Hemoglobin variants
Hemoglobin variants are mutant forms of hemoglobin in a population , caused by variations in genetics. Some well-known hemoglobin variants such as sickle-cell anemia are responsible for diseases, and are considered hemoglobinopathies...
.
There is a group of genetic disorders, known as the porphyria
Porphyria
Porphyrias are a group of inherited or acquired disorders of certain enzymes in the heme bio-synthetic pathway . They are broadly classified as acute porphyrias and cutaneous porphyrias, based on the site of the overproduction and accumulation of the porphyrins...
s that are characterized by errors in metabolic pathways of heme synthesis. King George III of the United Kingdom
George III of the United Kingdom
George III was King of Great Britain and King of Ireland from 25 October 1760 until the union of these two countries on 1 January 1801, after which he was King of the United Kingdom of Great Britain and Ireland until his death...
was probably the most famous porphyria sufferer.
To a small extent, hemoglobin A slowly combines with glucose
Glucose
Glucose is a simple sugar and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate...
at the terminal valine (an alpha aminoacid) of each β chain. The resulting molecule is often referred to as Hb A1c. As the concentration of glucose in the blood increases, the percentage of Hb A that turns into Hb A1c increases. In diabetics
Diabetes mellitus
Diabetes mellitus, often simply referred to as diabetes, is a group of metabolic diseases in which a person has high blood sugar, either because the body does not produce enough insulin, or because cells do not respond to the insulin that is produced...
whose glucose usually runs high, the percent Hb A1c also runs high. Because of the slow rate of Hb A combination with glucose, the Hb A1c percentage is representative of glucose level in the blood averaged over a longer time (the half-life of red blood cells, which is typically 50–55 days).
Glycosylated hemoglobin
Glycosylated hemoglobin
Glycated hemoglobin is a form of hemoglobin that is measured primarily to identify the average plasma glucose concentration over prolonged periods of time. It is formed in a non-enzymatic glycation pathway by hemoglobin's exposure to plasma glucose...
is the form of hemoglobin to which glucose is bound. The binding of glucose to amino acids in the hemoglobin takes place spontaneously (without the help of an enzyme) in many proteins, and is not known to serve a useful purpose. However, the binding to hemoglobin does serve as a record for average blood glucose levels over the lifetime of red cells, which is approximately 120 days. The levels of glycosylated hemoglobin are therefore measured in order to monitor the long-term control of the chronic disease of type 2 diabetes mellitus (T2DM). Poor control of T2DM results in high levels of glycosylated hemoglobin in the red blood cells. The normal reference range is approximately 4–5.9 %. Though difficult to obtain, values less than 7 % are recommended for people with T2DM. Levels greater than 9 % are associated with poor control of the glycosylated hemoglobin, and levels greater than 12 % are associated with very poor control. Diabetics who keep their glycosylated hemoglobin levels close to 7 % have a much better chance of avoiding the complications that may accompany diabetes (than those whose levels are 8 % or higher).
Elevated levels of hemoglobin are associated with increased numbers or sizes of red blood cells, called polycythemia
Polycythemia
Polycythemia is a disease state in which the proportion of blood volume that is occupied by red blood cells increases...
. This elevation may be caused by congenital heart disease, cor pulmonale
Cor pulmonale
Cor pulmonale or pulmonary heart disease is enlargement of the right ventricle of the heart as a response to increased resistance or high blood pressure in the lungs ....
, pulmonary fibrosis
Pulmonary fibrosis
Pulmonary fibrosis is the formation or development of excess fibrous connective tissue in the lungs. It is also described as "scarring of the lung".-Symptoms:Symptoms of pulmonary fibrosis are mainly:...
, too much erythropoietin
Erythropoietin
Erythropoietin, or its alternatives erythropoetin or erthropoyetin or EPO, is a glycoprotein hormone that controls erythropoiesis, or red blood cell production...
, or polycythemia vera
Polycythemia vera
Polycythemia vera is a blood disorder in which the bone marrow makes too many red blood cells. It may also result in the overproduction of white blood cells and platelets. Most of the health concerns associated with polycythemia vera are caused by the blood being thicker as a result of the...
.
Elevation in levels of hemoglobin were found in one study of the yogic practice of Yoga Nidra
Yoga Nidra
Yoga-nidra or "yogi sleep" is a sleep-like state which yogis report to experience during their meditations.The practice of yoga relaxation has been found to reduce tension and anxiety. The autonomic symptoms of high anxiety such as headache, giddiness, chest pain, palpitations, sweating, abdominal...
(yogic sleep) for half an hour daily.
A recent study done in Puducherry, India, shows its importance in coronary artery disease.
Diagnostic uses
Hemoglobin concentration measurement is among the most commonly performed blood testBlood test
A blood test is a laboratory analysis performed on a blood sample that is usually extracted from a vein in the arm using a needle, or via fingerprick....
s, usually as part of a complete blood count
Complete blood count
A complete blood count , also known as full blood count or full blood exam or blood panel, is a test panel requested by a doctor or other medical professional that gives information about the cells in a patient's blood...
. For example it is typically tested before or after blood donation
Blood donation
A blood donation occurs when a person voluntarily has blood drawn and used for transfusions or made into medications by a process called fractionation....
. Results are reported in g
Gram
The gram is a metric system unit of mass....
/L
Litre
pic|200px|right|thumb|One litre is equivalent to this cubeEach side is 10 cm1 litre water = 1 kilogram water The litre is a metric system unit of volume equal to 1 cubic decimetre , to 1,000 cubic centimetres , and to 1/1,000 cubic metre...
, g/dL or mol
Mole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
/L. 1 g/dL equals about 0.6206 mmol/L. Normal levels are:
- Men: 13.8 to 18.0 g/dL (138 to 182 g/L, or 8.56 to 11.3 mmol/L)
- Women: 12.1 to 15.1 g/dL (121 to 151 g/L, or 7.51 to 9.37 mmol/L)
- Children: 11 to 16 g/dL (111 to 160 g/L, or 6.83 to 9.93 mmol/L)
- Pregnant women: 11 to 12 g/dL (110 to 120 g/L, or 6.83 to 7.45 mmol/L)
Normal values of hemoglobin in the 1st and 3rd trimesters of pregnant women must be at least 11 g/dL and at least 10.5 g/dL during the 2nd trimester.
Dehydration or hyperhydration can greatly influence measured hemoglobin levels. Albumin can indicate hydration status.
If the concentration is below normal, this is called anemia
Anemia
Anemia is a decrease in number of red blood cells or less than the normal quantity of hemoglobin in the blood. However, it can include decreased oxygen-binding ability of each hemoglobin molecule due to deformity or lack in numerical development as in some other types of hemoglobin...
. Anemias are classified by the size of red blood cells, the cells
Cell (biology)
The cell is the basic structural and functional unit of all known living organisms. It is the smallest unit of life that is classified as a living thing, and is often called the building block of life. The Alberts text discusses how the "cellular building blocks" move to shape developing embryos....
that contain hemoglobin in vertebrates. The anemia is called "microcytic" if red cells are small, "macrocytic" if they are large, and "normocytic" otherwise.
Hematocrit
Hematocrit
The hematocrit or packed cell volume or erythrocyte volume fraction is the percentage of the concentration of red blood cells in blood. It is normally about 45% for men and 40% for women...
, the proportion of blood volume occupied by red blood cells, is typically about three times the hemoglobin level. For example, if the hemoglobin is measured at 17, that compares with a hematocrit of 51.
Laboratory hemoglobin test methods require a blood sample (arterial, venous, or capillary) and analysis on hematology analyzer and CO-oximeter. Additionally, a new noninvasive hemoglobin (SpHb) test method called Pulse CO-Oximetry is also available with comparable accuracy to invasive methods.
Long-term control of blood sugar
Blood sugar
The blood sugar concentration or blood glucose level is the amount of glucose present in the blood of a human or animal. Normally in mammals, the body maintains the blood glucose level at a reference range between about 3.6 and 5.8 mM , or 64.8 and 104.4 mg/dL...
concentration can be measured by the concentration of Hb A1c. Measuring it directly would require many samples because blood sugar levels vary widely through the day. Hb A1c is the product of the irreversible reaction of hemoglobin A with glucose
Glucose
Glucose is a simple sugar and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate...
. A higher glucose concentration
Concentration
In chemistry, concentration is defined as the abundance of a constituent divided by the total volume of a mixture. Four types can be distinguished: mass concentration, molar concentration, number concentration, and volume concentration...
results in more Hb A1c. Because the reaction is slow, the Hb A1c proportion represents glucose level in blood averaged over the half-life of red blood cells, is typically 50–55 days. An Hb A1c proportion of 6.0% or less show good long-term glucose control, while values above 7.0% are elevated. This test is especially useful for diabetics.
The functional magnetic resonance imaging
Functional magnetic resonance imaging
Functional magnetic resonance imaging or functional MRI is a type of specialized MRI scan used to measure the hemodynamic response related to neural activity in the brain or spinal cord of humans or other animals. It is one of the most recently developed forms of neuroimaging...
(fMRI) machine uses the signal from deoxyhemoglobin, which is sensitive to magnetic fields since it is paramagnetic.
Analogues in non-vertebrate organisms
A variety of oxygen-transport and -binding proteins exist in organisms throughout the animal and plant kingdoms. Organisms including bacteriaBacteria
Bacteria are a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria have a wide range of shapes, ranging from spheres to rods and spirals...
, protozoa
Protozoa
Protozoa are a diverse group of single-cells eukaryotic organisms, many of which are motile. Throughout history, protozoa have been defined as single-cell protists with animal-like behavior, e.g., movement...
ns, and fungi all have hemoglobin-like proteins whose known and predicted roles include the reversible binding of gaseous ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
s. Since many of these proteins contain globins and the heme moiety
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
(iron in a flat porphyrin support), they are often called hemoglobins, even if their overall tertiary structure is very different from that of vertebrate hemoglobin. In particular, the distinction of “myoglobin” and hemoglobin in lower animals is often impossible, because some of these organisms do not contain muscle
Muscle
Muscle is a contractile tissue of animals and is derived from the mesodermal layer of embryonic germ cells. Muscle cells contain contractile filaments that move past each other and change the size of the cell. They are classified as skeletal, cardiac, or smooth muscles. Their function is to...
s. Or, they may have a recognizable separate circulatory system
Circulatory system
The circulatory system is an organ system that passes nutrients , gases, hormones, blood cells, etc...
but not one that deals with oxygen transport (for example, many insect
Insect
Insects are a class of living creatures within the arthropods that have a chitinous exoskeleton, a three-part body , three pairs of jointed legs, compound eyes, and two antennae...
s and other arthropod
Arthropod
An arthropod is an invertebrate animal having an exoskeleton , a segmented body, and jointed appendages. Arthropods are members of the phylum Arthropoda , and include the insects, arachnids, crustaceans, and others...
s). In all these groups, heme/globin-containing molecules (even monomeric globin ones) that deal with gas-binding are referred to as oxyhemoglobins. In addition to dealing with transport and sensing of oxygen, they may also deal with NO, CO2, sulfide compounds, and even O2 scavenging in environments that must be anaerobic. They may even deal with detoxification of chlorinated materials in a way analogous to heme-containing P450 enzymes and peroxidases.

Algae
Algae are a large and diverse group of simple, typically autotrophic organisms, ranging from unicellular to multicellular forms, such as the giant kelps that grow to 65 meters in length. They are photosynthetic like plants, and "simple" because their tissues are not organized into the many...
, and plant
Plant
Plants are living organisms belonging to the kingdom Plantae. Precise definitions of the kingdom vary, but as the term is used here, plants include familiar organisms such as trees, flowers, herbs, bushes, grasses, vines, ferns, mosses, and green algae. The group is also called green plants or...
s often have single-globin hemoglobins. Many nematode
Nematode
The nematodes or roundworms are the most diverse phylum of pseudocoelomates, and one of the most diverse of all animals. Nematode species are very difficult to distinguish; over 28,000 have been described, of which over 16,000 are parasitic. It has been estimated that the total number of nematode...
worms, molluscs
Mollusca
The Mollusca , common name molluscs or mollusksSpelled mollusks in the USA, see reasons given in Rosenberg's ; for the spelling mollusc see the reasons given by , is a large phylum of invertebrate animals. There are around 85,000 recognized extant species of molluscs. Mollusca is the largest...
, and crustacean
Crustacean
Crustaceans form a very large group of arthropods, usually treated as a subphylum, which includes such familiar animals as crabs, lobsters, crayfish, shrimp, krill and barnacles. The 50,000 described species range in size from Stygotantulus stocki at , to the Japanese spider crab with a leg span...
s contain very large multisubunit molecules, much larger than those in vertebrates. In particular, chimeric hemoglobins found in fungi and giant annelids may contain both globin and other types of proteins.
One of the most striking occurrences and uses of hemoglobin in organisms is in the giant tube worm
Giant tube worm
Giant tube worms, Riftia pachyptila, are marine invertebrates in the phylum Annelida related to tube worms commonly found in the intertidal and pelagic zones...
(Riftia pachyptila, also called Vestimentifera), which can reach 2.4 meters length and populates ocean volcanic vents. Instead of a digestive tract, these worms contain a population of bacteria constituting half the organism's weight. The bacteria react with H2S from the vent and O2 from the water to produce energy to make food from H2O and CO2. The worms end with a deep red fan-like structure ("plume"), which extends into the water and absorbs H2S and O2 for the bacteria, and CO2 for use as synthetic raw material similar to photosynthetic plants. The structures are bright-red due to their containing several extraordinarily complex hemoglobins that have up to 144 globin chains, each including associated heme structures. These hemoglobins are remarkable for being able to carry oxygen in the presence of sulfide, and even to carry sulfide, without being completely "poisoned" or inhibited by it as hemoglobins in most other species are.
Other oxygen-binding proteins
MyoglobinMyoglobin
Myoglobin is an iron- and oxygen-binding protein found in the muscle tissue of vertebrates in general and in almost all mammals. It is related to hemoglobin, which is the iron- and oxygen-binding protein in blood, specifically in the red blood cells. The only time myoglobin is found in the...
: Found in the muscle tissue of many vertebrates, including humans, it gives muscle tissue a distinct red or dark gray color. It is very similar to hemoglobin in structure and sequence, but is not a tetramer; instead, it is a monomer that lacks cooperative binding
Cooperative binding
In biochemistry, a macromolecule exhibits cooperative binding if its affinity for its ligand changes with the amount of ligand already bound.Cooperative binding is a special case of allostery. Cooperative binding requires that the macromolecule have more than one binding site, since cooperativity...
. It is used to store oxygen rather than transport it.
Hemocyanin
Hemocyanin
Hemocyanins are respiratory proteins in the form of metalloproteins containing two copper atoms that reversibly bind a single oxygen molecule . Oxygenation causes a color change between the colorless Cu deoxygenated form and the blue Cu oxygenated form...
: The second most common oxygen-transporting protein found in nature, it is found in the blood of many arthropod
Arthropod
An arthropod is an invertebrate animal having an exoskeleton , a segmented body, and jointed appendages. Arthropods are members of the phylum Arthropoda , and include the insects, arachnids, crustaceans, and others...
s and molluscs. Uses copper prosthetic groups instead of iron heme groups and is blue in color when oxygenated.
Hemerythrin
Hemerythrin
Hemerythrin is an oligomeric protein responsible for oxygen transport in the marine invertebrate phyla of sipunculids, priapulids, brachiopods, and in a single annelid worm, magelona. Recently, hemerythrin was discovered in methanotrophic bacterium Methylococcus capsulatus...
: Some marine invertebrates and a few species of annelid
Annelid
The annelids , formally called Annelida , are a large phylum of segmented worms, with over 17,000 modern species including ragworms, earthworms and leeches...
use this iron-containing non-heme protein to carry oxygen in their blood. Appears pink/violet when oxygenated, clear when not.
Chlorocruorin
Chlorocruorin
Chlorocruorin is an oxygen-binding hemeprotein present in the blood plasma of many annelids, particularly certain marine polychaetes. Its affinity for oxygen is weaker than that of most hemoglobins...
: Found in many annelids, it is very similar to erythrocruorin, but the heme group is significantly different in structure. Appears green when deoxygenated and red when oxygenated.
Vanabins
Vanabins
Vanabins are a specific group of vanadium-binding metalloproteins. Vanabins are found almost exclusively in the blood cells, or vanadocytes of some ascidians and tunicates . The vanabins extracted from tunicate vanadocytes are often called hemovanadins...
: Also known as vanadium
Vanadium
Vanadium is a chemical element with the symbol V and atomic number 23. It is a hard, silvery gray, ductile and malleable transition metal. The formation of an oxide layer stabilizes the metal against oxidation. The element is found only in chemically combined form in nature...
chromagens, they are found in the blood of sea squirts. There were once hypothesized to use the rare metal vanadium as an oxygen binding prosthetic group. However, although they do contain vanadium by preference, they apparently bind little oxygen, and thus have some other function, which has not been elucidated (sea squirts also contain some hemoglobin). They may act as toxins.
Erythrocruorin
Erythrocruorin
Erythrocruorin is a large oxygen-carrying protein, whose molecular mass is greater than 3.5 million Daltons. It is related to the similar chlorocruorin. It is found in many annelids.- References :*, PNAS June 20, 2000. Accessed July 17, 2007....
: Found in many annelids, including earthworm
Earthworm
Earthworm is the common name for the largest members of Oligochaeta in the phylum Annelida. In classical systems they were placed in the order Opisthopora, on the basis of the male pores opening posterior to the female pores, even though the internal male segments are anterior to the female...
s, it is a giant free-floating blood protein containing many dozens—possibly hundreds—of iron- and heme-bearing protein subunits bound together into a single protein complex with a molecular mass greater than 3.5 million daltons.
Pinnaglobin: Only seen in the mollusc Pinna squamosa. Brown manganese-based porphyrin protein.
Leghemoglobin
Leghemoglobin
Leghemoglobin is a nitrogen or oxygen carrier, because naturally occurring oxygen and nitrogen interact similarly with this protein; and a hemoprotein found in the nitrogen-fixing root nodules of leguminous plants. But nitrogen is necessary for the cycle to occur...
: In leguminous plants, such as alfalfa or soybeans, the nitrogen fixing bacteria in the roots are protected from oxygen by this iron heme containing oxygen-binding protein. The specific enzyme protected is nitrogenase
Nitrogenase
Nitrogenases are enzymes used by some organisms to fix atmospheric nitrogen gas . It is the only known family of enzymes that accomplish this process. Dinitrogen is quite inert because of the strength of its N-N triple bond...
, which is unable to reduce nitrogen gas in the presence of free oxygen.
Coboglobin
Coboglobin
A coboglobin is a synthetic compound, a metalloprotein chemically similar to hemoglobin or myoglobin but using the metal cobalt instead of iron . Just like hemoglobin and myoglobin, the coboglobins are able to reversibly bind molecular oxygen at the metal atom...
: A synthetic cobalt-based porphyrin. Coboprotein would appear colorless when oxygenated, but yellow when in veins.
Presence in nonerythroid cells
Some nonerythroid cells (i.e., cells other than the red blood cell line) contain hemoglobin. In the brain, these include the A9 dopaminergicDopaminergic
Dopaminergic means related to the neurotransmitter dopamine. For example, certain proteins such as the dopamine transporter , vesicular monoamine transporter 2 , and dopamine receptors can be classified as dopaminergic, and neurons which synthesize or contain dopamine and synapses with dopamine...
neurons in the substantia nigra
Substantia nigra
The substantia nigra is a brain structure located in the mesencephalon that plays an important role in reward, addiction, and movement. Substantia nigra is Latin for "black substance", as parts of the substantia nigra appear darker than neighboring areas due to high levels of melanin in...
, astrocyte
Astrocyte
Astrocytes , also known collectively as astroglia, are characteristic star-shaped glial cells in the brain and spinal cord...
s in the cerebral cortex
Cerebral cortex
The cerebral cortex is a sheet of neural tissue that is outermost to the cerebrum of the mammalian brain. It plays a key role in memory, attention, perceptual awareness, thought, language, and consciousness. It is constituted of up to six horizontal layers, each of which has a different...
and hippocampus
Hippocampus
The hippocampus is a major component of the brains of humans and other vertebrates. It belongs to the limbic system and plays important roles in the consolidation of information from short-term memory to long-term memory and spatial navigation. Humans and other mammals have two hippocampi, one in...
, and in all mature oligodendrocyte
Oligodendrocyte
Oligodendrocytes , or oligodendroglia , are a type of brain cell. They are a variety of neuroglia. Their main function is the insulation of axons in the central nervous system of some vertebrates...
s. It has been suggested that brain hemoglobin in these cell may enable the "storage of oxygen to provide a homeostatic mechanism in anoxic conditions, which is especially important for A9 DA neurons that have an elevated metabolism with a high requirement for energy production". It has been noted further that "A9 dopaminergic
Dopaminergic
Dopaminergic means related to the neurotransmitter dopamine. For example, certain proteins such as the dopamine transporter , vesicular monoamine transporter 2 , and dopamine receptors can be classified as dopaminergic, and neurons which synthesize or contain dopamine and synapses with dopamine...
neurons may be at particular risk since in addition to their high mitochondrial activity they are under intense oxidative stress caused by the production of hydrogen peroxide via autoxidation and/or monoamine oxidase (MAO)-mediated deamination of dopamine and the subsequent reaction of accessible ferrous iron to generate highly toxic hydroxyl radicals". This may explain the risk of these cells for degeneration in Parkinson's disease
Parkinson's disease
Parkinson's disease is a degenerative disorder of the central nervous system...
. The presence of iron from hemoglobin in these cells also results in the post-mortem darkness of these cells, which is the origin of the Latin name, substantia nigra.
Outside the brain, hemoglobin has non-oxygen-carrying functions as an antioxidant
Antioxidant
An antioxidant is a molecule capable of inhibiting the oxidation of other molecules. Oxidation is a chemical reaction that transfers electrons or hydrogen from a substance to an oxidizing agent. Oxidation reactions can produce free radicals. In turn, these radicals can start chain reactions. When...
and a regulator of iron metabolism in macrophage
Macrophage
Macrophages are cells produced by the differentiation of monocytes in tissues. Human macrophages are about in diameter. Monocytes and macrophages are phagocytes. Macrophages function in both non-specific defense as well as help initiate specific defense mechanisms of vertebrate animals...
s, alveolar cells, and mesangial cell
Mesangial cell
Mesangial cells are specialized cells around blood vessels in the kidneys, at the mesangium. They are specialized smooth muscle cells that function to regulate blood flow through the capillaries, usually divided into two types, each having a very distinct function and location:* Extraglomerular...
s in the kidney.
In history, art and music

Ancient Rome
Ancient Rome was a thriving civilization that grew on the Italian Peninsula as early as the 8th century BC. Located along the Mediterranean Sea and centered on the city of Rome, it expanded to one of the largest empires in the ancient world....
associated the planet Mars
Mars
Mars is the fourth planet from the Sun in the Solar System. The planet is named after the Roman god of war, Mars. It is often described as the "Red Planet", as the iron oxide prevalent on its surface gives it a reddish appearance...
with the god of war since Mars is orange-red. The color of Mars is due to the iron oxide
Iron oxide
Iron oxides are chemical compounds composed of iron and oxygen. All together, there are sixteen known iron oxides and oxyhydroxides.Iron oxides and oxide-hydroxides are widespread in nature, play an important role in many geological and biological processes, and are widely utilized by humans, e.g.,...
in the Martian soil, but the red in blood is not due to the iron in hemoglobin and its oxides, which is a common misconception. The red is due to the porphyrin
Porphyrin
Porphyrins are a group of organic compounds, many naturally occurring. One of the best-known porphyrins is heme, the pigment in red blood cells; heme is a cofactor of the protein hemoglobin. Porphyrins are heterocyclic macrocycles composed of four modified pyrrole subunits interconnected at...
moiety
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
of hemoglobin to which the iron is bound, not the iron itself, although the ligation and redox state of the iron can influence the pi to pi* or n to pi* electronic transitions of the porphyrin and hence its optical characteristics.
.jpg)
Julian Voss-Andreae
Julian Voss-Andreae is a German sculptor living and working in the U.S.Voss-Andreae started out as a painter and later studied experimental physics at the universities of Berlin, Edinburgh and Vienna...
created a sculpture
Sculpture
Sculpture is three-dimensional artwork created by shaping or combining hard materials—typically stone such as marble—or metal, glass, or wood. Softer materials can also be used, such as clay, textiles, plastics, polymers and softer metals...
called "Heart of Steel (Hemoglobin)" in 2005, based on the protein's backbone. The sculpture was made from glass and weathering steel. The intentional rusting of the initially shiny work of art mirrors hemoglobin's fundamental chemical reaction of oxygen binding to iron.
Rock band Placebo
Placebo (band)
Placebo are a British rock band from London, England, formed in 1994 by singer and guitarist Brian Molko and bass guitarist Stefan Olsdal. The band was joined by drummer Robert Schultzberg, who was later replaced by Steve Hewitt after conflicts with Molko. Hewitt left the band in October 2007 and...
recorded a song called "Haemoglobin
Black Market Music
Black Market Music is the third studio album by British alternative rock band Placebo, released in 2000 by Hut Records.- Background :Black Market Music took nine months to record, the longest that the band have ever spent recording an album to date, and is dedicated to the memory of Scott...
" with the lyrics "Haemoglobin is the key to a healthy heartbeat". French rap artist MC Solaar
MC Solaar
MC Solaar is a francophone hip hop and rap artist. He is one of the most internationally popular and influential French rappers....
also had a successful single titled "La Concubine de L'Hemoglobin" in 1994.
See also
- ChlorophyllChlorophyllChlorophyll is a green pigment found in almost all plants, algae, and cyanobacteria. Its name is derived from the Greek words χλωρος, chloros and φύλλον, phyllon . Chlorophyll is an extremely important biomolecule, critical in photosynthesis, which allows plants to obtain energy from light...
- Globin foldGlobin foldThe globin fold is a common three-dimensional fold in proteins. This fold typically consists of eight alpha helices, although some proteins have additional helix extensions at their termini. The globin fold is found in its namesake proteins hemoglobin and myoglobin as well as in phycocyanin proteins...
- HemocyaninHemocyaninHemocyanins are respiratory proteins in the form of metalloproteins containing two copper atoms that reversibly bind a single oxygen molecule . Oxygenation causes a color change between the colorless Cu deoxygenated form and the blue Cu oxygenated form...
- HemoproteinHemoproteinA hemeprotein , or heme protein, is a metalloprotein containing a heme prosthetic group- an organic compound that allows a protein to carry out a function that it cannot do alone....
- Sickle-cell diseaseSickle-cell diseaseSickle-cell disease , or sickle-cell anaemia or drepanocytosis, is an autosomal recessive genetic blood disorder with overdominance, characterized by red blood cells that assume an abnormal, rigid, sickle shape. Sickling decreases the cells' flexibility and results in a risk of various...
- Complete Blood CountComplete blood countA complete blood count , also known as full blood count or full blood exam or blood panel, is a test panel requested by a doctor or other medical professional that gives information about the cells in a patient's blood...
- HemoglobinometerHemoglobinometerA hemoglobinometer is a medical measuring device of hemoglobin blood concentration. It can operate by spectrophotometric measurement of hemoglobin concentration. Portable hemoglobinometers provide easy and convenient measurement of hematological variables, especially in areas where no clinic labs...
Hemoglobin variants:
- Hb A1C
- Hemoglobin A2Hemoglobin A2Hemoglobin A2 is a normal variant of hemoglobin A that consists of two alpha and two delta chains and is found in small quantity in normal human blood. Hemoglobin A2 may be increased in beta thalassemia or to people who are heterozygous to beta thalassemia gene....
- Hemoglobin CHemoglobin CHemoglobin C is an abnormal hemoglobin with substitution of a lysine residue for a glutamic acid residue at the 6th position of the β-globin chain.-Clinical significance:...
- Hemoglobin F
Hemoglobin protein subunits (genes):
- Alpha globin 1HBA1Hemoglobin, alpha 1, also known as HBA1, is a human gene encoding the hemoglobin protein.-Interactions:Hemoglobin, alpha 1 has been shown to interact with HBB. Interactions between the N-terminal amino groups of the alpha-subunits and the C-terminal histidine of the β-subunits participate in ion...
- Beta globinHBBBeta globin Beta globin Beta globin (HBB, β-globinprotin that, along with alpha globin (HBA), makes up the most common form of hemoglobin in adult humans. The normal adult hemoglobin tetramer consists of two alpha chains and two beta chains.-Gene locus:...
- Delta globinHBDHemoglobin subunit delta is a protein that in humans is encoded by the HBD gene.-Further reading:...
Hemoglobin compounds:
- CarbaminohemoglobinCarbaminohemoglobinCarbaminohemoglobin is a compound of hemoglobin and carbon dioxide, and is one of the forms in which carbon dioxide exists in the blood. When carbon dioxide binds to hemoglobin, carbaminohemoglobin is formed, lowering hemoglobin's affinity for oxygen via the Haldane effect...
(with carbon dioxideCarbon dioxideCarbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
, colored blue) - CarboxyhemoglobinCarboxyhemoglobinCarboxyhemoglobin is a stable complex of carbon monoxide and hemoglobin that forms in red blood cells when carbon monoxide is inhaled or produced in normal metabolism. Large quantities of it hinder delivery of oxygen to the body...
(with carbon monoxideCarbon monoxideCarbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
, colored cherry-red) - Oxyhemoglobin (with diatomic oxygenOxygenOxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
, colored blood-red)

