Ligand
Encyclopedia
In coordination chemistry, a ligand is an ion
or molecule
(see also: functional group
) that binds to a central metal
atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron
pairs. The nature of metal-ligand bonding can range from covalent
to ionic. Furthermore, the metal-ligand bond order
can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known involving Lewis acidic "ligands."
Metal
and metalloid
s are bound to ligands in virtually all circumstances, although gaseous "naked" metal ions can be generated in high vacuum. Ligands in a complex dictate the reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox
. Ligand selection is a critical consideration in many practical areas, including bioinorganic
and medicinal chemistry
, homogeneous catalysis
, and environmental chemistry
.
Ligands are classified in many ways: their charge, their size (bulk), the identity of the coordinating atom(s), and the number of electrons donated to the metal (denticity
or hapticity
). The size of a ligand is indicated by its cone angle
.
reconciled formulas and isomers. He showed, among other things, that the formulas of many cobalt(III) and chromium(III) compounds can be understood if the metal has six ligands in an octahedral geometry. The first to use the term "ligand" were Alfred Stock
and Carl Somiesky, in relation to silicon chemistry. The theory allows one to understand the difference between coordinated and ionic chloride in the cobalt ammine
chlorides and to explain many of the previously inexplicable isomers. He resolved the first coordination complex called hexol
into optical isomers, overthrowing the theory that chirality
was necessarily associated with carbon compounds.
(Highest Occupied Molecular Orbital) can be mainly of ligands or metal character.
Ligands and metal ions can be ordered in many ways; one ranking system focuses on ligand 'hardness' (see also hard/soft acid/base theory
). Metal ions preferentially bind certain ligands. In general, 'soft' metal ions prefer weak field ligands, whereas 'hard' metal ions prefer strong field ligands. According to the molecular orbital theory, the HOMO of the ligand should have an energy that overlaps with the LUMO (Lowest Unoccupied Molecular Orbital) of the metal preferential. Metal ions bound to strong-field ligands follow the Aufbau principle
, whereas complexes bound to weak-field ligands follow Hund's rule.
Binding of the metal with the ligands results in a set of molecular orbitals, where the metal can be identified with a new HOMO and LUMO (the orbitals defining the properties and reactivity of the resulting complex) and a certain ordering of the 5 d-orbitals (which may be filled, or partially filled with electrons). In an octahedral environment, the 5 otherwise degenerate d-orbitals split in sets of 2 and 3 orbitals (for a more in depth explanation, see crystal field theory
).
The energy difference between these 2 sets of d-orbitals is called the splitting parameter, Δo. The magnitude of Δo is determined by the field-strength of the ligand: strong field ligands, by definition, increase Δo more than weak field ligands. Ligands can now be sorted according to the magnitude of Δo (see the table below). This ordering of ligands is almost invariable for all metal ions and is called spectrochemical series
.
For complexes with a tetrahedral surrounding, the d-orbitals again split into two sets, but this time in reverse order.
The energy difference between these 2 sets of d-orbitals is now called Δt. The magnitude of Δt is smaller than for Δo, because in a tetrahedral complex only 4 ligands influence the d-orbitals, whereas in an octahedral complex the d-orbitals are influenced by 6 ligands. When the coordination number
is neither octahedral nor tetrahedral, the splitting becomes correspondingly more complex. For the purposes of ranking ligands, however, the properties of the octahedral complexes and the resulting Δo has been of primary interest.
The arrangement of the d-orbitals on the central atom (as determined by the 'strength' of the ligand), has a strong effect on virtually all the properties of the resulting complexes. E.g. the energy differences in the d-orbitals has a strong effect in the optical absorption spectra of metal complexes. It turns out that valence electrons occupying orbitals with significant 3d-orbital character absorb in the 400-800 nm region of the spectrum (UV-visible range). The absorption of light (what we perceive as the color
) by these electrons (that is, excitation of electrons from one orbital to another orbital under influence of light) can be correlated to the ground state
of the metal complex, which reflects the bonding properties of the ligands. The relative change in (relative) energy of the d-orbitals as a function of the field-strength of the ligands is described in Tanabe-Sugano diagram
s.
In cases where the ligand has low energy LUMO, such orbitals also participate in the bonding. The metal-ligand bond can be further stabilised by a formal donation of electron density
back to the ligand in a process known as back-bonding. In this case a filled, central-atom-based orbital donates density into the LUMO of the (coordinated) ligand. Carbon monoxide is the preeminent example a ligand that engages metals via back-donation. Complementarily, ligands with low-energy filled orbitals of pi-symmetry can serve as pi-donor.
, ligands are classified as L and X (or combinations of the two). The classification scheme - the "CBC Method" for covalent bond classification - was popularized by M.L.H. Green
and "is based on the notion that there are three basic types [of ligands]... represented by the symbols L, X, and Z, which correspond respectively to 2-electron, 1-electron and 0-electron neutral ligands." L ligands from charge-neutral precursors and are represented by amines, phosphines, CO, N2, and alkene
s. X ligands typically are derived from anionic precursors such as chloride but includes ligands where salts of anion do not really exist such as hydride and alkyl. Thus, the complex IrCl(CO)(PPh3)2
is classified as an MXL3 complex, since CO and the two PPh3 ligands are classified as L's. The oxidative addition
of H2 to IrCl(CO)(PPh3)2 gives an 18e- ML3X3 product, IrClH2(CO)(PPh3)2. EDTA
4- is classified as an L2X4 ligand, as it features four anions and two neutral donor sites. Cp
is classified as an L2X ligand.
s on more than one atom. Ligands that bind via more than one atom are often termed chelating
. A ligand that binds through two sites is classified as bidentate, and three sites as tridentate. The "bite angle
" refers to the angle between the two bonds of a bidentate chelate. Chelating ligands are commonly formed by linking donor groups via organic linkers. A classic bidentate ligand is ethylenediamine, which is derived by the linking of two ammonia groups with an ethylene (-CH2CH2-) linker. A classic example of a polydentate ligand is the hexadentate chelating agent EDTA
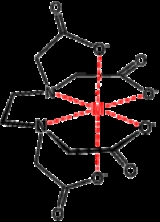
, which is able to bond through six sites, completely surrounding some metals. The number of times a polydentate ligand bind to a metal centre is symbolized with "κn", where "n" indicates the number sites by which a ligand attaches to a metal. EDTA4− , when it is hexidentate, binds as a κ6-ligand, the amines and the carboxylate oxygen atoms are not contiguous. In practice, the n value of a ligand is not indicated explicitly but rather assumed. The binding affinity of a chelating system depends on the chelating angle or bite angle
.
Complexes of polydentate ligands are called chelate complexes. They tend to be more stable than complexes derived from monodentate
ligands. This enhanced stability, the chelate effect, is usually attributed to effects of entropy
, which favors the displacement of many ligands by one polydentate ligand. When the chelating ligand forms a large ring that at least partially surrounds the central atom and bonds to it, leaving the central atom at the centre of a large ring. The more rigid and the higher its denticity, the more inert will be the macrocyclic complex. Heme
is a good example: the iron
atom is at the centre of a porphyrin
macrocycle, being bound to four nitrogen atoms of the tetrapyrrole macrocycle. The very stable dimethylglyoximate complex of nickel is a synthetic macrocycle derived from the anion of dimethylglyoxime
.
and are sometimes called "inner sphere" ligands. "Outer-sphere" ligands are not directly attached to the metal, but are bonded, generally weakly, to the first coordination shell, affecting the inner sphere in subtle ways. The complex of the metal with the inner sphere ligands is then called a coordination complex, which can be neutral, cationic, or anionic. The complex, along with its counter ions (if required), is called a coordination compound.
, SCN− , which can attach at either the sulfur atom or the nitrogen atom. Such compounds give rise to linkage isomerism
. Polyfunctional ligands, see especially proteins, can bond to a metal center through different ligand atoms to form various isomers.
are ambidentate and thus are found to often bind to two or three metals simultaneously. Atoms that bridge metals are sometimes indicated with the prefix "μ" (mu). Most inorganic solids, are polymers by virtue of the presence of multiple bridging ligands.
s some ligands can bond to a metal center through the same atom but with a different number of lone pair
s. The bond order
of the metal ligand bond can be in part distinguished through the metal ligand bond angle (M-X-R). This bond angle is often referred to as being linear or bent with further discussion concerning the degree to which the angle is bent. For example, an imido ligand in the ionic form has three lone pairs. One lone pair is used as a sigma X donor, the other two lone pairs are available as L type pi donors. If both lone pairs are used in pi bonds then the M-N-R geometry is linear. However, if one or both these lone pairs is non-bonding then the M-N-R bond is bent and the extent of the bend speaks to how much pi bonding there may be. η1-Nitric oxide can coordinate to a metal center in linear or bent manner.
s that have partial contributions to the overall state.
. Of academic interest, bulky ligands stabilize unusual coordination sites, e.g. reactive coligands or low coordination numbers. Often bulky ligands are employed to simulate the steric protection afforded by proteins to metal-containing active sites. Of course excessive steric bulk can prevent the coordination of certain ligands.
Virtually every molecule and every ion can serve as a ligand for (or "coordinate to") metals. Monodentate ligands include virtually all anions and all simple Lewis bases. Thus, the halide
s and pseudohalides are important anionic ligands whereas ammonia
, carbon monoxide
, and water are particularly common charge-neutral ligands. Simple organic species are also very common, be they anionic (RO−
and RCO2− ) or neutral (R2O
, R2S
, R3− xNHx
, and R3P
). The steric properties of some ligands are evaluated in terms of their cone angles.
Beyond the classical Lewis bases and anions, all unsaturated molecules are also ligands, utilizing their π-electrons in forming the coordinate bond. Also, metals can bind to the σ bonds in for example silane
s, hydrocarbon
s, and dihydrogen (see also: agostic interaction
).
In complexes of non-innocent ligand
s, the ligand is bonded to metals via conventional bonds, but the ligand is also redox-active.
Note: The entries in the table are sorted by field strength, binding through the stated atom (i.e. as a terminal ligand), the 'strength' of the ligand changes when the ligand binds in an alternative binding mode (e.g. when it bridges between metals) or when the conformation of the ligand gets distorted (e.g. a linear ligand that is forced through steric interactions to bind in a non-linear fashion).
in which one ligand in a chemical compound is replaced by another ligand. In organometallic chemistry this can take place by associative substitution
or by dissociative substitution
.
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
or molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
(see also: functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
) that binds to a central metal
Metal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
pairs. The nature of metal-ligand bonding can range from covalent
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
to ionic. Furthermore, the metal-ligand bond order
Bond order
Bond order is the number of chemical bonds between a pair of atoms. For example, in diatomic nitrogen N≡N the bond order is 3, while in acetylene H−C≡C−H the bond order between the two carbon atoms is also 3, and the C−H bond order is 1. Bond order gives an indication to the stability of a bond....
can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known involving Lewis acidic "ligands."
Metal
Metal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
and metalloid
Metalloid
Metalloid is a term used in chemistry when classifying the chemical elements. On the basis of their general physical and chemical properties, each element can usually be classified as a metal or a nonmetal. However, some elements with intermediate or mixed properties can be harder to characterize...
s are bound to ligands in virtually all circumstances, although gaseous "naked" metal ions can be generated in high vacuum. Ligands in a complex dictate the reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
. Ligand selection is a critical consideration in many practical areas, including bioinorganic
Bioinorganic chemistry
Bioinorganic chemistry is a field that examines the role of metals in biology. Bioinorganic chemistry includes the study of both natural phenomena such as the behavior of metalloproteins as well artificially introduced metals, including those that are non-essential, in medicine and toxicology...
and medicinal chemistry
Medicinal chemistry
Medicinal chemistry and pharmaceutical chemistry are disciplines at the intersection of chemistry, especially synthetic organic chemistry, and pharmacology and various other biological specialties, where it is involved with design, chemical synthesis and development for market of pharmaceutical...
, homogeneous catalysis
Homogeneous catalysis
In chemistry, homogeneous catalysis is a sequence of reactions that involve a catalyst in the same phase as the reactants. Most commonly, a homogeneous catalyst is codissolved in a solvent with the reactants.-Acid catalysis:...
, and environmental chemistry
Environmental chemistry
Environmental chemistry is the scientific study of the chemical and biochemical phenomena that occur in natural places. It should not be confused with green chemistry, which seeks to reduce potential pollution at its source...
.
Ligands are classified in many ways: their charge, their size (bulk), the identity of the coordinating atom(s), and the number of electrons donated to the metal (denticity
Denticity
Denticity refers to the number of atoms in a single ligand that bind to a central atom in a coordination complex. In many cases, only one atom in the ligand binds to the metal, so the denticity equals one, and the ligand is said to be monodentate...
or hapticity
Hapticity
The term hapticity is used to describe how a group of contiguous atoms of a ligand are coordinated to a central atom. Hapticity of a ligand is indicated by the Greek character 'eta', η. A superscripted number following the η denotes the number of contiguous atoms of the ligand that are bound to...
). The size of a ligand is indicated by its cone angle
Ligand cone angle
The ligand cone angle is a measure of the size of a ligand. It is defined as the solid angle formed with the metal at the vertex and the hydrogen atoms at the perimeter of the cone . Tertiary phosphine ligands are commonly classified using this parameter, but the method can be applied to any...
.
History
The composition of coordination complexes have been known since the early 1800s, e.g. Prussian blue and copper vitriol. The key breakthrough occurred when Alfred WernerAlfred Werner
Alfred Werner was a Swiss chemist who was a student at ETH Zurich and a professor at the University of Zurich. He won the Nobel Prize in Chemistry in 1913 for proposing the octahedral configuration of transition metal complexes. Werner developed the basis for modern coordination chemistry...
reconciled formulas and isomers. He showed, among other things, that the formulas of many cobalt(III) and chromium(III) compounds can be understood if the metal has six ligands in an octahedral geometry. The first to use the term "ligand" were Alfred Stock
Alfred Stock
Alfred Stock was a German inorganic chemist. He did pioneering research on the hydrides of boron and silicon, coordination chemistry, mercury, and mercury poisoning...
and Carl Somiesky, in relation to silicon chemistry. The theory allows one to understand the difference between coordinated and ionic chloride in the cobalt ammine
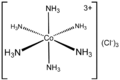
Ammine
In coordination chemistry, metal ammine complexes are metal complexes containing at least one ammonia ligand. "Ammine" is spelled this way due to historical reasons; in contrast, alkyl or aryl bearing ligands are spelled with a single "m"...
chlorides and to explain many of the previously inexplicable isomers. He resolved the first coordination complex called hexol
Hexol
Hexol is a cobalt compound that was first prepared by Alfred Werner in 1914 and represented the first non-carbon-containing chiral compound. The salt with the molecular formula of[Co3]3 was prepared starting from cobalt sulfate....
into optical isomers, overthrowing the theory that chirality
Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
was necessarily associated with carbon compounds.
Strong field and weak field ligands
In general, ligands are viewed as electron donors and the metals as electron acceptors. Bonding is often described using the formalisms of molecular orbital theory. The HOMOHOMO/LUMO
HOMO and LUMO are acronyms for highest occupied molecular orbital and lowest unoccupied molecular orbital, respectively. The energy difference between the HOMO and LUMO is termed the HOMO-LUMO gap...
(Highest Occupied Molecular Orbital) can be mainly of ligands or metal character.
Ligands and metal ions can be ordered in many ways; one ranking system focuses on ligand 'hardness' (see also hard/soft acid/base theory
HSAB theory
The HSAB concept is an acronym for 'hard and soft acids and bases. Also known as the Pearson acid base concept, HSAB is widely used in chemistry for explaining stability of compounds, reaction mechanisms and pathways....
). Metal ions preferentially bind certain ligands. In general, 'soft' metal ions prefer weak field ligands, whereas 'hard' metal ions prefer strong field ligands. According to the molecular orbital theory, the HOMO of the ligand should have an energy that overlaps with the LUMO (Lowest Unoccupied Molecular Orbital) of the metal preferential. Metal ions bound to strong-field ligands follow the Aufbau principle
Aufbau principle
The Aufbau principle is used to determine the electron configuration of an atom, molecule or ion. The principle postulates a hypothetical process in which an atom is "built up" by progressively adding electrons...
, whereas complexes bound to weak-field ligands follow Hund's rule.
Binding of the metal with the ligands results in a set of molecular orbitals, where the metal can be identified with a new HOMO and LUMO (the orbitals defining the properties and reactivity of the resulting complex) and a certain ordering of the 5 d-orbitals (which may be filled, or partially filled with electrons). In an octahedral environment, the 5 otherwise degenerate d-orbitals split in sets of 2 and 3 orbitals (for a more in depth explanation, see crystal field theory
Crystal field theory
Crystal field theory is a model that describes the electronic structure of transition metal compounds, all of which can be considered coordination complexes. CFT successfully accounts for some magnetic properties, colours, hydration enthalpies, and spinel structures of transition metal complexes,...
).
-
- 3 orbitals of low energy: dxy, dxz and dyz
- 2 of high energy: dz2 and dx2
− y2
The energy difference between these 2 sets of d-orbitals is called the splitting parameter, Δo. The magnitude of Δo is determined by the field-strength of the ligand: strong field ligands, by definition, increase Δo more than weak field ligands. Ligands can now be sorted according to the magnitude of Δo (see the table below). This ordering of ligands is almost invariable for all metal ions and is called spectrochemical series
Spectrochemical series
A spectrochemical series is a list of ligands ordered on ligand strength and a list of metal ions based on oxidation number, group and its identity...
.
For complexes with a tetrahedral surrounding, the d-orbitals again split into two sets, but this time in reverse order.
-
- 2 orbitals of low energy: dz2 and dx2
− y2 - 3 orbitals of high energy: dxy, dxz and dyz
- 2 orbitals of low energy: dz2 and dx2
The energy difference between these 2 sets of d-orbitals is now called Δt. The magnitude of Δt is smaller than for Δo, because in a tetrahedral complex only 4 ligands influence the d-orbitals, whereas in an octahedral complex the d-orbitals are influenced by 6 ligands. When the coordination number
Coordination number
In chemistry and crystallography, the coordination number of a central atom in a molecule or crystal is the number of its nearest neighbours. This number is determined somewhat differently for molecules and for crystals....
is neither octahedral nor tetrahedral, the splitting becomes correspondingly more complex. For the purposes of ranking ligands, however, the properties of the octahedral complexes and the resulting Δo has been of primary interest.
The arrangement of the d-orbitals on the central atom (as determined by the 'strength' of the ligand), has a strong effect on virtually all the properties of the resulting complexes. E.g. the energy differences in the d-orbitals has a strong effect in the optical absorption spectra of metal complexes. It turns out that valence electrons occupying orbitals with significant 3d-orbital character absorb in the 400-800 nm region of the spectrum (UV-visible range). The absorption of light (what we perceive as the color
Color
Color or colour is the visual perceptual property corresponding in humans to the categories called red, green, blue and others. Color derives from the spectrum of light interacting in the eye with the spectral sensitivities of the light receptors...
) by these electrons (that is, excitation of electrons from one orbital to another orbital under influence of light) can be correlated to the ground state
Ground state
The ground state of a quantum mechanical system is its lowest-energy state; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state...
of the metal complex, which reflects the bonding properties of the ligands. The relative change in (relative) energy of the d-orbitals as a function of the field-strength of the ligands is described in Tanabe-Sugano diagram
Tanabe-Sugano diagram
Tanabe-Sugano diagrams are used in coordination chemistry to predict absorptions in the UV and visible electromagnetic spectrum of coordination compounds. The results from a Tanabe-Sugano diagram analysis of a metal complex can also be compared to experimental spectroscopic data...
s.
In cases where the ligand has low energy LUMO, such orbitals also participate in the bonding. The metal-ligand bond can be further stabilised by a formal donation of electron density
Electron density
Electron density is the measure of the probability of an electron being present at a specific location.In molecules, regions of electron density are usually found around the atom, and its bonds...
back to the ligand in a process known as back-bonding. In this case a filled, central-atom-based orbital donates density into the LUMO of the (coordinated) ligand. Carbon monoxide is the preeminent example a ligand that engages metals via back-donation. Complementarily, ligands with low-energy filled orbitals of pi-symmetry can serve as pi-donor.

Classification of ligands as L and X
Especially in the area of organometallic chemistryOrganometallic chemistry
Organometallic chemistry is the study of chemical compounds containing bonds between carbon and a metal. Since many compounds without such bonds are chemically similar, an alternative may be compounds containing metal-element bonds of a largely covalent character...
, ligands are classified as L and X (or combinations of the two). The classification scheme - the "CBC Method" for covalent bond classification - was popularized by M.L.H. Green
Malcolm Green (chemist)
Malcolm Green also known as M. L. H. Green is a British Emeritus Professor of Inorganic Chemistry.Born in Eastleigh, Hampshire, he received his BSc degree from Acton Technical College in 1956 and his PhD from Imperial College of Science and Technology in 1959 under the supervision of Professor...
and "is based on the notion that there are three basic types [of ligands]... represented by the symbols L, X, and Z, which correspond respectively to 2-electron, 1-electron and 0-electron neutral ligands." L ligands from charge-neutral precursors and are represented by amines, phosphines, CO, N2, and alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s. X ligands typically are derived from anionic precursors such as chloride but includes ligands where salts of anion do not really exist such as hydride and alkyl. Thus, the complex IrCl(CO)(PPh3)2
Vaska's complex
Vaska's complex is the trivial name for the chemical compound trans-chlorocarbonylbisiridium, which has the formula IrCl[P3]2. This square planar diamagnetic organometallic complex consists of a central iridium atom bound to two mutually trans triphenylphosphine ligands, carbon monoxide, and a...
is classified as an MXL3 complex, since CO and the two PPh3 ligands are classified as L's. The oxidative addition
Oxidative addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre...
of H2 to IrCl(CO)(PPh3)2 gives an 18e- ML3X3 product, IrClH2(CO)(PPh3)2. EDTA
EDTA
Ethylenediaminetetraacetic acid, widely abbreviated as EDTA , is a polyamino carboxylic acid and a colourless, water-soluble solid. Its conjugate base is named ethylenediaminetetraacetate. It is widely used to dissolve limescale. Its usefulness arises because of its role as a hexadentate ligand...
4- is classified as an L2X4 ligand, as it features four anions and two neutral donor sites. Cp
Cyclopentadienyl
In organic chemistry, cyclopentadienyl is a cyclic group of atoms with the formula C5H5. Cyclopentadienyl are closely related to cyclopentadiene. Cyclopentadienyl have five carbon atoms bonded together in a pentagonal planar ring, all five of which are bonded to individual hydrogen atoms...
is classified as an L2X ligand.
Denticity
Denticity (represented by κ) refers to the number of times a ligand bonds to a metal through non-contiguous donor sites. Many ligands are capable of binding metal ions through multiple sites, usually because the ligands have lone pairLone pair
In chemistry, a lone pair is a valence electron pair without bonding or sharing with other atoms. They are found in the outermost electron shell of an atom, so lone pairs are a subset of a molecule's valence electrons...
s on more than one atom. Ligands that bind via more than one atom are often termed chelating
Chelation
Chelation is the formation or presence of two or more separate coordinate bonds between apolydentate ligand and a single central atom....
. A ligand that binds through two sites is classified as bidentate, and three sites as tridentate. The "bite angle
Bite angle
The bite angle is a geometric parameter used to classify chelating ligands in inorganic and organometallic chemistry. Together with ligand cone angle, this parameter is relevant to diphosphine ligands, which are used in industrial processes such as hydroformylation and hydrocyanation...
" refers to the angle between the two bonds of a bidentate chelate. Chelating ligands are commonly formed by linking donor groups via organic linkers. A classic bidentate ligand is ethylenediamine, which is derived by the linking of two ammonia groups with an ethylene (-CH2CH2-) linker. A classic example of a polydentate ligand is the hexadentate chelating agent EDTA
EDTA
Ethylenediaminetetraacetic acid, widely abbreviated as EDTA , is a polyamino carboxylic acid and a colourless, water-soluble solid. Its conjugate base is named ethylenediaminetetraacetate. It is widely used to dissolve limescale. Its usefulness arises because of its role as a hexadentate ligand...
, which is able to bond through six sites, completely surrounding some metals. The number of times a polydentate ligand bind to a metal centre is symbolized with "κn", where "n" indicates the number sites by which a ligand attaches to a metal. EDTA4
Bite angle
The bite angle is a geometric parameter used to classify chelating ligands in inorganic and organometallic chemistry. Together with ligand cone angle, this parameter is relevant to diphosphine ligands, which are used in industrial processes such as hydroformylation and hydrocyanation...
.
Complexes of polydentate ligands are called chelate complexes. They tend to be more stable than complexes derived from monodentate
Denticity
Denticity refers to the number of atoms in a single ligand that bind to a central atom in a coordination complex. In many cases, only one atom in the ligand binds to the metal, so the denticity equals one, and the ligand is said to be monodentate...
ligands. This enhanced stability, the chelate effect, is usually attributed to effects of entropy
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
, which favors the displacement of many ligands by one polydentate ligand. When the chelating ligand forms a large ring that at least partially surrounds the central atom and bonds to it, leaving the central atom at the centre of a large ring. The more rigid and the higher its denticity, the more inert will be the macrocyclic complex. Heme
Heme
A heme or haem is a prosthetic group that consists of an iron atom contained in the center of a large heterocyclic organic ring called a porphyrin. Not all porphyrins contain iron, but a substantial fraction of porphyrin-containing metalloproteins have heme as their prosthetic group; these are...
is a good example: the iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
atom is at the centre of a porphyrin
Porphyrin
Porphyrins are a group of organic compounds, many naturally occurring. One of the best-known porphyrins is heme, the pigment in red blood cells; heme is a cofactor of the protein hemoglobin. Porphyrins are heterocyclic macrocycles composed of four modified pyrrole subunits interconnected at...
macrocycle, being bound to four nitrogen atoms of the tetrapyrrole macrocycle. The very stable dimethylglyoximate complex of nickel is a synthetic macrocycle derived from the anion of dimethylglyoxime
Dimethylglyoxime
Dimethylglyoxime is a chemical compound described by the formula CH3CCCH3. This colourless solid is the dioxime derivative of the diketone diacetyl . DmgH2 is used in the analysis of palladium or nickel. Its coordination complexes are of theoretical interest as models for enzymes and as catalysts...
.
Hapticity
Hapticity (represented by η) refers to the number of contiguous atoms that comprise a donor site and attach to a metal center. Butadiene forms both η2 and η4 complexes depending on the number of carbon atoms that are bonded to the metal.Outer-sphere ligands
In coordination chemistry, the ligands that are directly bonded to the metal (that is, share electrons), form part of the first coordination sphereCoordination sphere
In coordination chemistry, the coordination sphere refers to a central atom or ion and an array of molecules or anions, the ligands, around.Molecules that are attached noncovalently to the ligands are called the second coordination sphere....
and are sometimes called "inner sphere" ligands. "Outer-sphere" ligands are not directly attached to the metal, but are bonded, generally weakly, to the first coordination shell, affecting the inner sphere in subtle ways. The complex of the metal with the inner sphere ligands is then called a coordination complex, which can be neutral, cationic, or anionic. The complex, along with its counter ions (if required), is called a coordination compound.
Trans-spanning ligands
Trans-spanning ligands are bidentate ligands that can span coordination positions on opposite sides of a coordination complex.Ambidentate ligand
Unlike polydentate ligands, ambidentate ligands can attach to the central atom in two places but not both. A good example of this is thiocyanateThiocyanate
Thiocyanate is the anion [SCN]−. It is the conjugate base of thiocyanic acid. Common derivatives include the colourless salts potassium thiocyanate and sodium thiocyanate. Organic compounds containing the functional group SCN are also called thiocyanates...
, SCN
Linkage isomerism
Linkage isomerism is the existence of co-ordination compounds that have the same composition differing with the connectivity of the metal to a ligand.Typical ligands that give rise to linkage isomers are:*thiocyanate, SCN-*selenocyanate, SeCN-*nitrite, NO2-...
. Polyfunctional ligands, see especially proteins, can bond to a metal center through different ligand atoms to form various isomers.
Bridging ligand
A bridging ligand links two or more metal center. Virtually all inorganic solids with simple formulas are coordination polymers, consisting of metal centres linked by bridging ligands. This group of materials includes all anhydrous binary metal halides and pseudohalides. Bridging ligands also persist in solution. Polyatomic ligands such as carbonateCarbonate
In chemistry, a carbonate is a salt of carbonic acid, characterized by the presence of the carbonate ion, . The name may also mean an ester of carbonic acid, an organic compound containing the carbonate group C2....
are ambidentate and thus are found to often bind to two or three metals simultaneously. Atoms that bridge metals are sometimes indicated with the prefix "μ" (mu). Most inorganic solids, are polymers by virtue of the presence of multiple bridging ligands.
Metal ligand multiple bond
Metal ligand multiple bondMetal ligand multiple bond
In Chemistry, a metal–ligand multiple bond describes the interaction of certain ligands with a metal with a bond order greater than one. Coordination complexes featuring multiply bonded ligands are of both scholarly and practical interest. Transition metal carbene complexes catalyze the olefin...
s some ligands can bond to a metal center through the same atom but with a different number of lone pair
Lone pair
In chemistry, a lone pair is a valence electron pair without bonding or sharing with other atoms. They are found in the outermost electron shell of an atom, so lone pairs are a subset of a molecule's valence electrons...
s. The bond order
Bond order
Bond order is the number of chemical bonds between a pair of atoms. For example, in diatomic nitrogen N≡N the bond order is 3, while in acetylene H−C≡C−H the bond order between the two carbon atoms is also 3, and the C−H bond order is 1. Bond order gives an indication to the stability of a bond....
of the metal ligand bond can be in part distinguished through the metal ligand bond angle (M-X-R). This bond angle is often referred to as being linear or bent with further discussion concerning the degree to which the angle is bent. For example, an imido ligand in the ionic form has three lone pairs. One lone pair is used as a sigma X donor, the other two lone pairs are available as L type pi donors. If both lone pairs are used in pi bonds then the M-N-R geometry is linear. However, if one or both these lone pairs is non-bonding then the M-N-R bond is bent and the extent of the bend speaks to how much pi bonding there may be. η1-Nitric oxide can coordinate to a metal center in linear or bent manner.
Non-innocent ligand
Non-innocent ligands bond with metals in such a manner that the distribution of electron density between the metal center and ligand is unclear. Describing the bonding of noninnocent ligands often involves writing multiple resonance formResonance (chemistry)
In chemistry, resonance or mesomerism is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by one single Lewis formula...
s that have partial contributions to the overall state.
Bulky ligands
Bulky ligands are used to control the steric properties of a metal center. They are used for many reasons, both practical and academic. On the practical side, they influence the selectivity of metal catalysts, e.g. in hydroformylationHydroformylation
Hydroformylation, also known as oxo synthesis or oxo process, is an important industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group and a hydrogen atom to a carbon-carbon double bond...
. Of academic interest, bulky ligands stabilize unusual coordination sites, e.g. reactive coligands or low coordination numbers. Often bulky ligands are employed to simulate the steric protection afforded by proteins to metal-containing active sites. Of course excessive steric bulk can prevent the coordination of certain ligands.
Chiral ligands
Chiral ligands are useful for inducing asymmetry within the coordination sphere. Often the ligand is employed as an optically pure group. In some cases, e.g. secondary amines, the asymmetry arises upon coordination. Chiral ligands are essential components of asymmetric homogeneous catalysis.Common ligands
- See nomenclature.
Virtually every molecule and every ion can serve as a ligand for (or "coordinate to") metals. Monodentate ligands include virtually all anions and all simple Lewis bases. Thus, the halide
Halide
A halide is a binary compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative than the halogen, to make a fluoride, chloride, bromide, iodide, or astatide compound. Many salts are halides...
s and pseudohalides are important anionic ligands whereas ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
, carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
, and water are particularly common charge-neutral ligands. Simple organic species are also very common, be they anionic (RO
Alkoxide
An alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They can be written as RO−, where R is the organic substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands...
and RCO2
Ether
Ethers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"...
, R2S
Thioether
A thioether is a functional group in organosulfur chemistry with the connectivity C-S-C as shown on right. Like many other sulfur-containing compounds, volatile thioethers have foul odors. A thioether is similar to an ether except that it contains a sulfur atom in place of the oxygen...
, R3
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
, and R3P
Phosphine
Phosphine is the compound with the chemical formula PH3. It is a colorless, flammable, toxic gas. Pure phosphine is odourless, but technical grade samples have a highly unpleasant odor like garlic or rotting fish, due to the presence of substituted phosphine and diphosphine...
). The steric properties of some ligands are evaluated in terms of their cone angles.
Beyond the classical Lewis bases and anions, all unsaturated molecules are also ligands, utilizing their π-electrons in forming the coordinate bond. Also, metals can bind to the σ bonds in for example silane
Silane
Silane is a toxic, extremely flammable chemical compound with chemical formula SiH4. In 1857, the German chemists and Friedrich Woehler discovered silane among the products formed by the action of hydrochloric acid on aluminum silicide, which they had previously prepared...
s, hydrocarbon
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
s, and dihydrogen (see also: agostic interaction
Agostic interaction
Agostic interaction is a term in organometallic chemistry for the interaction of a coordinately-unsaturated transition metal with a C-H bond, when the two electrons involved in the C-H bond enter the empty d-orbital of a transition metal, resulting in a three-center two-electron bond. Many...
).
In complexes of non-innocent ligand
Non-innocent ligand
In chemistry, a non-innocent ligand is a ligand in a metal complex where the oxidation state is unclear. Typically, complexes containing non-innocent ligands are redox active at mild potentials...
s, the ligand is bonded to metals via conventional bonds, but the ligand is also redox-active.
Examples of common ligands (by field strength)
In the following table the ligands are sorted by field strength (weak field ligands first):| Ligand | formula (bonding atom(s) in bold) | Charge | Most common denticity | Remark(s) |
|---|---|---|---|---|
| Iodide Iodide An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. This page is for the iodide ion and its salts. For information on organoiodides, see organohalides. In everyday life, iodide is most commonly encountered as a component of iodized salt,... (iodo) |
I |
monoanionic | monodentate Denticity Denticity refers to the number of atoms in a single ligand that bind to a central atom in a coordination complex. In many cases, only one atom in the ligand binds to the metal, so the denticity equals one, and the ligand is said to be monodentate... |
|
| Bromide Bromide A bromide is a chemical compound containing bromide ion, that is bromine atom with effective charge of −1. The class name can include ionic compounds such as caesium bromide or covalent compounds such as sulfur dibromide.-Natural occurrence:... (bromo) |
Br |
monoanionic | monodentate | |
| Sulfide Sulfide A sulfide is an anion of sulfur in its lowest oxidation state of 2-. Sulfide is also a slightly archaic term for thioethers, a common type of organosulfur compound that are well known for their bad odors.- Properties :... (thio or less commonly "bridging thiolate") |
S2 |
dianionic | monodentate (M=S), or bidentate bridging (M-S-M') | |
| Thiocyanate Thiocyanate Thiocyanate is the anion [SCN]−. It is the conjugate base of thiocyanic acid. Common derivatives include the colourless salts potassium thiocyanate and sodium thiocyanate. Organic compounds containing the functional group SCN are also called thiocyanates... (S-thiocyanato) |
S-CN |
monoanionic | monodentate | ambidentate (see also isothiocyanate, below) |
| Chloride Chloride The chloride ion is formed when the element chlorine, a halogen, picks up one electron to form an anion Cl−. The salts of hydrochloric acid HCl contain chloride ions and can also be called chlorides. The chloride ion, and its salts such as sodium chloride, are very soluble in water... (chloro) |
Cl |
monoanionic | monodentate | also found bridging |
| Nitrate Nitrate The nitrate ion is a polyatomic ion with the molecular formula NO and a molecular mass of 62.0049 g/mol. It is the conjugate base of nitric acid, consisting of one central nitrogen atom surrounded by three identically-bonded oxygen atoms in a trigonal planar arrangement. The nitrate ion carries a... (nitrato) |
O-NO2 |
monoanionic | monodentate | |
| Azide Azide Azide is the anion with the formula N3−. It is the conjugate base of hydrazoic acid. N3− is a linear anion that is isoelectronic with CO2 and N2O. Per valence bond theory, azide can be described by several resonance structures, an important one being N−=N+=N−... (azido) |
N-N2 |
monoanionic | monodentate | |
| Fluoride Fluoride Fluoride is the anion F−, the reduced form of fluorine when as an ion and when bonded to another element. Both organofluorine compounds and inorganic fluorine containing compounds are called fluorides. Fluoride, like other halides, is a monovalent ion . Its compounds often have properties that are... (fluoro) |
F |
monoanionic | monodentate | |
| Hydroxide Hydroxide Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and a hydrogen atom held together by a covalent bond, and carrying a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, as a ligand, a nucleophile, and a... (hydroxo) |
O-H |
monoanionic | monodentate | often found as a bridging ligand |
| Oxalate Oxalate Oxalate , is the dianion with formula C2O42− also written 22−. Either name is often used for derivatives, such as disodium oxalate, 2C2O42−, or an ester of oxalic acid Oxalate (IUPAC: ethanedioate), is the dianion with formula C2O42− also written (COO)22−. Either... (oxalato) |
[O-C(=O)-C(=O)-O]2 |
dianionic | bidentate | |
| Water (aqua) | H-O-H | neutral | monodentate | monodentate |
| Nitrite Nitrite The nitrite ion has the chemical formula NO2−. The anion is symmetric with equal N-O bond lengths and a O-N-O bond angle of ca. 120°. On protonation the unstable weak acid nitrous acid is produced. Nitrite can be oxidised or reduced, with product somewhat dependent on the oxidizing/reducing agent... (nitrito) |
O-N-O |
monoanionic | monodentate | ambidentate (see also nitro) |
| Isothiocyanate Isothiocyanate Isothiocyanate is the chemical group –N=C=S, formed by substituting sulfur for oxygen in the isocyanate group. Many natural isothiocyanates from plants are produced by enzymatic conversion of metabolites called glucosinolates. These natural isothiocyanates, such as allyl isothiocyanate, are also... (isothiocyanato) |
N=C=S |
monoanionic | monodentate | ambidentate (see also thiocyanate, above) |
| Acetonitrile Acetonitrile Acetonitrile is the chemical compound with formula . This colourless liquid is the simplest organic nitrile. It is produced mainly as a byproduct of acrylonitrile manufacture... (acetonitrilo) |
CH3CN | neutral | monodentate | |
| Pyridine Pyridine Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom... |
C5H5N | neutral | monodentate | |
| Ammonia Ammonia Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or... (ammine or less commonly "ammino") |
NH3 | neutral | monodentate | |
| Ethylenediamine | en | neutral | bidentate | |
| 2,2'-Bipyridine 2,2'-Bipyridine 2,2'-Bipyridine is a organic compound with the formula . This colorless solid, commonly abbreviated bipy or bpy , is an important isomer of the bipyridine family. It is a bidentate chelating ligand, forming complexes with many transition metals... |
bipy | neutral | bidentate | easily reduced to its (radical) anion or even to its dianion |
| 1,10-Phenanthroline Phenanthroline Phenanthroline is a heterocyclic organic compound. As a bidentate ligand in coordination chemistry, it forms strong complexes with most metal ions... |
phen | neutral | bidentate | |
| Nitrite Nitrite The nitrite ion has the chemical formula NO2−. The anion is symmetric with equal N-O bond lengths and a O-N-O bond angle of ca. 120°. On protonation the unstable weak acid nitrous acid is produced. Nitrite can be oxidised or reduced, with product somewhat dependent on the oxidizing/reducing agent... (nitro) |
N-O2 |
monoanionic | monodentate | ambidentate (see also nitrito) |
| Triphenylphosphine Triphenylphosphine Triphenylphosphine is a common organophosphorus compound with the formula P3 - often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature... |
PPh3 | neutral | monodentate | |
| Cyanide Cyanide A cyanide is a chemical compound that contains the cyano group, -C≡N, which consists of a carbon atom triple-bonded to a nitrogen atom. Cyanides most commonly refer to salts of the anion CN−. Most cyanides are highly toxic.... (cyano) |
CN |
monoanionic | monodentate | can bridge between metals (both metals bound to C, or one to C and one to N) |
| Carbon monoxide Carbon monoxide Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal... (carbonyl) |
CO | neutral | monodentate | can bridge between metals (both metals bound to C) |
Note: The entries in the table are sorted by field strength, binding through the stated atom (i.e. as a terminal ligand), the 'strength' of the ligand changes when the ligand binds in an alternative binding mode (e.g. when it bridges between metals) or when the conformation of the ligand gets distorted (e.g. a linear ligand that is forced through steric interactions to bind in a non-linear fashion).
Other general encountered ligands (alphabetical)
In this table other common ligands are listed in alphabetical order.| Ligand | formula (bonding atom(s) in bold) | Charge | Most common denticity | Remark(s) |
|---|---|---|---|---|
| Acetylacetonate Acetylacetone Acetylacetone is an organic compound that famously exists in two tautomeric forms that rapidly interconvert. The less stable tautomer is a diketone formally named pentane-2,4-dione. The more common tautomer is the enol form. The pair of tautomers rapidly interconvert and are treated as a single... (Acac) |
CH3-C(O)-CH2-C(O)-CH3 | monoanionic | bidentate | In general bidentate, bound through both oxygens, but sometimes bound through the central carbon only, see also analogous ketimine analogues |
| Alkene Alkene In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond... s |
R2C=CR2 | neutral | compounds with a C-C double bond | |
| Benzene Benzene Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6.... |
C6H6 | neutral | and other arenes | |
| 1,2-Bis(diphenylphosphino)ethane 1,2-Bis(diphenylphosphino)ethane 1,2-Bisethane is a commonly used bidentate ligand in coordination chemistry. Dppe is almost invariably chelated, although there are examples of unidentate and of bridging behavior.-Preparation:... (dppe) |
Ph2PC2H4PPh2 | neutral | bidentate | |
| 1,1-Bis(diphenylphosphino)methane 1,1-Bis(diphenylphosphino)methane 1,1-Bismethane , is an organophosphorus compound with the formula CH22. Dppm, a white, crystalline powder, is used in inorganic and organometallic chemistry as a ligand... (dppm) |
C25H22P2 | neutral | Can bond to 2 metal atoms at once, forming dimers | |
| Corrole Corrole A corrole is an aromatic organic chemical, whose structure is in the form of the corrin ring which is also present in cobalamin . The ring consists of nineteen carbon atoms, with four nitrogen atoms in the core of the molecule.... s |
tetradentate | |||
| Crown ether Crown ether Crown ethers are cyclic chemical compounds that consist of a ring containing several ether groups. The most common crown ethers are oligomers of ethylene oxide, the repeating unit being ethyleneoxy, i.e., -CH2CH2O-. Important members of this series are the tetramer , the pentamer , and the hexamer... s |
neutral | primarily for alkali and alkaline earth metal cations | ||
| 2,2,2-crypt Cryptand Cryptands are a family of synthetic bi- and polycyclic multidentate ligands for a variety of cations. The Nobel Prize for Chemistry in 1987 was given to Donald J. Cram, Jean-Marie Lehn, and Charles J. Pedersen for their efforts in discovering and determining uses of cryptands and crown ethers,... |
hexadentate | primarily for alkali and alkaline earth metal cations | ||
| Cryptates | neutral | |||
| Cyclopentadienyl Cyclopentadienyl complex A cyclopentadienyl complex is a metal complex with one or more cyclopentadienyl groups . Based on the type of bonding between the metals and the cyclopentadienyl]] moieties, cyclopentadienyl complexes are classified into the following three categories: a) π-complexes, b) σ-complexes, and c) ionic... (Cp) |
[C5H5] |
monoanionic | Although monoanionic, by the nature of its occupied MO's, it is capable of acting as a tridentate ligand. | |
| Diethylenetriamine (dien) | C4H13N3 | neutral | tridentate | related to TACN, but not constrained to facial complexation |
| Dimethylglyoximate Dimethylglyoxime Dimethylglyoxime is a chemical compound described by the formula CH3CCCH3. This colourless solid is the dioxime derivative of the diketone diacetyl . DmgH2 is used in the analysis of palladium or nickel. Its coordination complexes are of theoretical interest as models for enzymes and as catalysts... (dmgH |
monoanionic | |||
| Ethylenediaminetetraacetate EDTA Ethylenediaminetetraacetic acid, widely abbreviated as EDTA , is a polyamino carboxylic acid and a colourless, water-soluble solid. Its conjugate base is named ethylenediaminetetraacetate. It is widely used to dissolve limescale. Its usefulness arises because of its role as a hexadentate ligand... (EDTA) |
tetra-anionic | hexadentate | actual ligand is the tetra-anion | |
| Ethylenediaminetriacetate | trianionic | pentadentate | actual ligand is the trianion | |
| glycinate Glycine Glycine is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its 'side chain', glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG cf. the genetic code.Glycine is a colourless, sweet-tasting crystalline solid... (Glycinato) |
monoanionic | bidentate | other α-amino acid anions are comparable (but chiral) | |
| Heme Heme A heme or haem is a prosthetic group that consists of an iron atom contained in the center of a large heterocyclic organic ring called a porphyrin. Not all porphyrins contain iron, but a substantial fraction of porphyrin-containing metalloproteins have heme as their prosthetic group; these are... |
dianionic | tetradentate | macrocyclic ligand | |
| Nitrosyl | NO+ | cationic | bent (1e) and linear (3e) bonding mode | |
| Oxo | O | dianion | monodentate | sometimes bridging |
| Pyrazine Pyrazine Pyrazine is a heterocyclic aromatic organic compound with the chemical formula C4H4N2.Pyrazine is a symmetrical molecule with point group D2h. Derivatives like phenazine are well known for their antitumor, antibiotic and diuretic activity. Pyrazine is less basic in nature than pyridine, pyridazine... |
N2C4H4 | neutral | ditopic | sometimes bridging |
| Scorpionate ligand Scorpionate ligand The term scorpionate ligand refers to a tridentate ligand which would bind to a metal in a fac manner. The most popular class of scorpionates are the hydrotrisborates or Tp ligands. These were also the first to become popular. These ligands first appeared in journals in 1966 from the then... |
tridentate | |||
| Sulfite Sulfite Sulfites are compounds that contain the sulfite ion SO. The sulfite ion is the conjugate base of bisulfite. Although the acid itself is elusive, its salts are widely used.-Structure:... |
monoanionic | monodentate | ambidentate | |
| 2,2',5',2-Terpyridine Terpyridine Terpyridine is a heterocyclic compound derived from pyridine. This colourless solid is used as a ligand in coordination chemistry.-Synthesis:... (terpy) |
neutral | tridentate | meridional bonding only | |
| Triazacyclononane (tacn) | (C2H4)3(NR)3 | neutral | tridentate | macrocyclic ligand see also the N,N',N"-trimethylated analogue |
| Tricyclohexylphosphine Tricyclohexylphosphine Tricyclohexylphosphine is the tertiary phosphine with the formula P3. Commonly used as a ligand in organometallic chemistry, it is often abbreviated to PCy3, where Cy stands for cyclohexyl... |
(C6H11)3P or (PCy3) | neutral | monodentate | |
| Triethylenetetramine (trien) | neutral | tetradentate | ||
| Trimethylphosphine Trimethylphosphine Trimethylphosphine is the organophosphorus compound with the formula P3, commonly abbreviated PMe3. This colorless liquid has a strongly unpleasant odour, which is characteristic of alkylphosphines. It is a pyramidal molecule with C3v symmetry, similar to ammonia and phosphine . As a ligand, its... |
PMe3 | neutral | monodentate | |
| Tri(o-tolyl)phosphine | P(o-tolyl)3 | neutral | monodentate | |
| Tris(2-aminoethyl)amine Tris(2-aminoethyl)amine Trisamine is the organic compound with the formula N3. This colourless liquid is soluble in water and is highly basic, consisting of a tertiary amine center and three pendant primary amine groups... (tren) |
(NH2CH2CH2)3N | neutral | tetradentate | |
| Tris(2-diphenylphosphineethyl)amine (np3) | neutral | tetradentate | ||
| Terpyridine Terpyridine Terpyridine is a heterocyclic compound derived from pyridine. This colourless solid is used as a ligand in coordination chemistry.-Synthesis:... |
C15H11N3 | neutral | tridentate | |
| Tropylium | C7H7+ | cationic | ||
| Carbon dioxide Carbon dioxide Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom... |
CO2 | see transition metal carbon dioxide complex |
Ligand exchange
Ligand exchange (also ligand substitution) is a type of chemical reactionChemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
in which one ligand in a chemical compound is replaced by another ligand. In organometallic chemistry this can take place by associative substitution
Associative substitution
Associative substitution describes a pathway by which compounds interchange ligands. The terminology is typically applied to coordination and organometallic complexes, but resembles the Sn2 mechanism in organic chemistry. The opposite pathway is dissociative substitution, being analogous to Sn1...
or by dissociative substitution
Dissociative substitution
Dissociative substitution describes a pathway by which compounds interchange ligands. The term is typically applied to coordination and organometallic complexes, but resembles the Sn1 mechanism in organic chemistry. The opposite pathway is associative substitution, being analogous to Sn2 pathway...
.
Pronunciation
Pronounced ˈlɪgənd with the first syllable sounding like the word "lithium" or 'laɪgənd with the first syllable sounding like the word "lie".See also
- Crystal field theoryCrystal field theoryCrystal field theory is a model that describes the electronic structure of transition metal compounds, all of which can be considered coordination complexes. CFT successfully accounts for some magnetic properties, colours, hydration enthalpies, and spinel structures of transition metal complexes,...
- Ligand dependent pathwayLigand dependent pathwayThere are two types of pathway for subsititution of ligands in a complex. The ligand dependent pathway is the one whereby the chemical properties of the ligand affect the rate of substitution. Alternatively, there is the ligand independent pathway, which is where the ligand does not have an...
- Ligand field theoryLigand field theoryLigand field theory describes the bonding, orbital arrangement, and other characteristics of coordination complexes. It represents an application of molecular orbital theory to transition metal complexes. A transition metal ion has nine valence atomic orbitals, five d, one s, and three p orbitals...
- Coordination chemistry
- Inorganic chemistryInorganic chemistryInorganic chemistry is the branch of chemistry concerned with the properties and behavior of inorganic compounds. This field covers all chemical compounds except the myriad organic compounds , which are the subjects of organic chemistry...
- Tanabe-Sugano diagramTanabe-Sugano diagramTanabe-Sugano diagrams are used in coordination chemistry to predict absorptions in the UV and visible electromagnetic spectrum of coordination compounds. The results from a Tanabe-Sugano diagram analysis of a metal complex can also be compared to experimental spectroscopic data...
- Spectrochemical seriesSpectrochemical seriesA spectrochemical series is a list of ligands ordered on ligand strength and a list of metal ions based on oxidation number, group and its identity...

