
Bohr effect
Encyclopedia
Hemoglobin
Hemoglobin is the iron-containing oxygen-transport metalloprotein in the red blood cells of all vertebrates, with the exception of the fish family Channichthyidae, as well as the tissues of some invertebrates...
first described in 1904 by the Danish physiologist Christian Bohr
Christian Bohr
Christian Harald Lauritz Peter Emil Bohr was a Danish physician, father of the physicist and Nobel laureate Niels Bohr, as well as the mathematician Harald Bohr and grandfather of another physicist and nobel laureate Aage Bohr...
(father of physicist Niels Bohr
Niels Bohr
Niels Henrik David Bohr was a Danish physicist who made foundational contributions to understanding atomic structure and quantum mechanics, for which he received the Nobel Prize in Physics in 1922. Bohr mentored and collaborated with many of the top physicists of the century at his institute in...
), which states that an increasing concentration of protons and/or carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
will reduce the oxygen affinity
Affinity
Affinity is a word used in a variety of fields, usually to indicate some kind of preference, relationship, or a potential or actual closeness between two entities.Articles dealing with various usages of the word: affinity include:-Commerce and law:...
of hemoglobin
Hemoglobin
Hemoglobin is the iron-containing oxygen-transport metalloprotein in the red blood cells of all vertebrates, with the exception of the fish family Channichthyidae, as well as the tissues of some invertebrates...
. Increasing blood carbon dioxide levels can lead to a decrease in pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
because of the chemical equilibrium
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
between protons and carbon dioxide.
Proton and oxygen coupling
In deoxyhemoglobin, the N-terminal amino groups of the α-subunits and the C-terminal histidineHistidine
Histidine Histidine, an essential amino acid, has a positively charged imidazole functional group. It is one of the 22 proteinogenic amino acids. Its codons are CAU and CAC. Histidine was first isolated by German physician Albrecht Kossel in 1896. Histidine is an essential amino acid in humans...
of the β-subunits participate in ion pairs
Ion-association
Ion-association is a chemical reaction whereby ions of opposite electrical charge come together in solution to form a distinct chemical entity. Ion-associates are classified according to the number of ions that associate with each other, and the nature of the interaction. The most important factor...
. The formation of ion pairs causes them to decrease in acidity. Thus, deoxyhemoglobin binds one proton for every two O2 released. In oxyhemoglobin, these ion pairings are absent and these groups increase in acidity. Consequentially, a proton is released for every two O2 bound. Specifically, this reciprocal coupling of protons and oxygen is the Bohr effect.
Effect of carbondioxide and hydrogen ions on the oxygen affinity of haemoglobin is known as Bohr effect.
Physiological role
This effect facilitates oxygen transport as hemoglobin binds to oxygen in the lungs, but then releases it in the tissues, particularly those tissues in most need of oxygen. When a tissue's metabolic rate increases, its carbon dioxide production increases. Carbon dioxide forms bicarbonateBicarbonate
In inorganic chemistry, bicarbonate is an intermediate form in the deprotonation of carbonic acid...
through the following reaction:
- CO2 + H2O
 H2CO3
H2CO3  H+ + HCO3−
H+ + HCO3−
Although the reaction usually proceeds very slowly, the enzyme family, carbonic anhydrase
Carbonic anhydrase
The carbonic anhydrases form a family of enzymes that catalyze the rapid interconversion of carbon dioxide and water to bicarbonate and protons , a reversible reaction that occurs rather slowly in the absence of a catalyst...
in red blood cells accelerates the formation of bicarbonate and protons. This causes the pH of tissues to decrease, and so, promotes the dissociation of oxygen from hemoglobin to the tissue, allowing the tissue to obtain enough oxygen to meet its demands. Conversely, in the lungs, where oxygen concentration is high, binding of oxygen causes hemoglobin to release protons, which combine with bicarbonate to drive off carbon dioxide in exhalation
Exhalation
Exhalation is the movement of air out of the bronchial tubes, through the airways, to the external environment during breathing....
. Since these two reactions are closely matched, there is little change in blood pH.
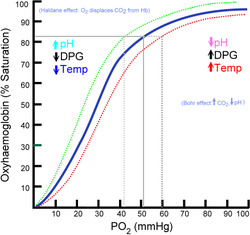
The dissociation curve
Dissociation curve
Dissociation curve may refer to:*Oxygen-haemoglobin dissociation curve, a graphical representation of oxygen release from haemoglobin*Melting curve analysis, a biochemical technique relying on heat-dependent dissociation between two DNA strands...
shifts to the right when carbon dioxide or hydrogen ion concentration is increased. This facilitates increased oxygen dumping. This mechanism allows for the body to adapt the problem of supplying more oxygen to tissues that need it the most. When muscles are undergoing strenuous activity, they generate CO2 and lactic acid
Lactic acid
Lactic acid, also known as milk acid, is a chemical compound that plays a role in various biochemical processes and was first isolated in 1780 by the Swedish chemist Carl Wilhelm Scheele. Lactic acid is a carboxylic acid with the chemical formula C3H6O3...
as products of cellular respiration
Cellular respiration
Cellular respiration is the set of the metabolic reactions and processes that take place in the cells of organisms to convert biochemical energy from nutrients into adenosine triphosphate , and then release waste products. The reactions involved in respiration are catabolic reactions that involve...
and lactic acid fermentation
Lactic acid fermentation
Lactic acid fermentation is a biological process by which sugars such as glucose, fructose, and sucrose, are converted into cellular energy and the metabolic byproduct lactate. It is an anaerobic fermentation reaction that occurs in some bacteria and animal cells, such as muscle cells, in the...
. In fact, muscles generate lactic acid so quickly that pH of the blood passing through the muscles will drop to around 7.2. As lactic acid releases its protons, pH decreases, which causes hemoglobin to release ~10% more oxygen.
Carbamates
Carbon dioxideCarbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
modulates O2 binding to hemoglobin directly by combining reversibly to N-terminal amino groups of blood proteins to form carbamates:
- R−NH2 + CO2
 R−NH−COO- + H+
R−NH−COO- + H+
Deoxyhemoglobin binds to CO2 more readily to form a carbamate
Carbamate
Carbamates are organic compounds derived from carbamic acid . A carbamate group, carbamate ester, and carbamic acids are functional groups that are inter-related structurally and often are interconverted chemically. Carbamate esters are also called urethanes.-Synthesis:Carbamic acids are derived...
than oxyhemoglobin. When CO2 concentration is high (as in the capillaries), the protons released by carbamate formation further promotes oxygen release.
Although the difference in CO2 binding between the oxy and deoxy states of hemoglobin accounts for only 5% of the total blood CO2, it is responsible for half of the CO2 transported by blood. This is because 10% of the total blood CO2 is lost through the lungs in each circulatory cycle.
Effects of cooperativity
The Bohr effect is dependent on cooperative interactions between the hemeHeme
A heme or haem is a prosthetic group that consists of an iron atom contained in the center of a large heterocyclic organic ring called a porphyrin. Not all porphyrins contain iron, but a substantial fraction of porphyrin-containing metalloproteins have heme as their prosthetic group; these are...
s of the hemoglobin tetramer. This is evidenced by the fact that myoglobin
Myoglobin
Myoglobin is an iron- and oxygen-binding protein found in the muscle tissue of vertebrates in general and in almost all mammals. It is related to hemoglobin, which is the iron- and oxygen-binding protein in blood, specifically in the red blood cells. The only time myoglobin is found in the...
, a monomer
Monomer
A monomer is an atom or a small molecule that may bind chemically to other monomers to form a polymer; the term "monomeric protein" may also be used to describe one of the proteins making up a multiprotein complex...
with no cooperativity
Cooperativity
Cooperativity is a phenomenon displayed by enzymes or receptors that have multiple binding sites where the affinity of the binding sites for a ligand is increased, positive cooperativity, or decreased, negative cooperativity, upon the binding of a ligand to a binding site...
, does not exhibit the Bohr effect. Hemoglobin mutants with weaker cooperativity may exhibit a reduced Bohr effect.
In the Hiroshima variant hemoglobinopathy
Hemoglobinopathy
Hemoglobinopathy is a kind of genetic defect that results in abnormal structure of one of the globin chains of the hemoglobin molecule. Hemoglobinopathies are inherited single-gene disorders; in most cases, they are inherited as autosomal co-dominant traits. Common hemoglobinopathies include...
, cooperativity in hemoglobin is reduced, and the Bohr effect is diminished. During periods of exercise, the mutant hemoglobin has a higher affinity for oxygen and tissue may suffer minor oxygen starvation.

