Self-ionization of water
Encyclopedia
The self-ionization of water (also autoionization of water, and autodissociation of water) is the chemical reaction in which a proton
is transferred from one water molecule to another, in pure water or an aqueous solution, to create the two ions, hydronium
, H3O+ and hydroxide
, OH−. It is an example of autoprotolysis
, and exemplifies the amphoteric nature of water.
of 0.055 µS
·cm−1. According to the theories of Svante Arrhenius
, this must be due to the presence of ions. The ions are produced by the self-ionization reaction
This equilibrium applies to pure water and any aqueous solution. The chemical equilibrium constant, Keq, for this reaction is given by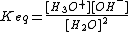
If the concentration of dissolved solutes is not very high, the concentration [H2O] can be taken as being constant at ca. 55.5M. The ionization constant, dissociation constant, self-ionization constant, or ionic product of water, symbolized by Kw is given by
where [H3O+] is the concentration of hydrogen or hydronium ion, and [OH−] is the concentration of hydroxide
ion. At 25 °C Kw is approximately equal to 1.0×10−14. Water molecules dissociate into equal amounts of H3O+ and OH−, so their concentrations are equal to ca. 1.0 × 10−7 mol dm−3. A solution in which the H3O+ and OH− concentrations equal each other is considered a neutral solution. Pure water is neutral, but most water samples contain impurities. If an impurity is an acid
or base
this will affect the concentrations of hydronium ion and hydoxide ion. Water samples which are exposed to air will absorb the acid carbon dioxide
and the concentration of H3O+ will increase. The concentration of OH- will decrease in such a way that the product [H3O+][OH-] remains constant.
The dependence of the water ionization on temperature and pressure has been investigated thoroughly. The value of pKw decreases as temperature increases from the melting point of ice to a minimum at ca. 250 °C, after which it increases up to the critical point
of ca. 374 °C. It decreases with increasing pressure.
With electrolyte
solutions, the value of pKw is dependent on ionic strength
of the electrolyte. Values for sodium chloride
are typical for a 1:1 electrolyte. With 1:2 electrolytes, MX2, pKw decreases with increasing ionic strength.
, D2O, self-ionizes less than normal water, H2O; oxygen forms a slightly stronger bond to deuterium
because the larger mass of deuterium difference results in a lower zero-point energy
, a quantum mechanical effect. The following table compares the values of pKw for H2O and D2O.
for the dissociation
depends on the activation energy
, ΔE‡. According to the Boltzmann distribution
the proportion of water molecules that have sufficient energy, due to thermal population, is given by

where k is the Boltzmann constant. Thus some dissociation can occur because sufficient thermal energy is available. The following sequence of events has been proposed on the basis of electric field fluctuations in liquid water. Random fluctuations in molecular motions occasionally (about once every 10 hours per water molecule) produce an electric field strong enough to break an oxygen-hydrogen bond, resulting in a hydroxide (OH−) and hydronium ion (H3O+); the proton of the hydronium ion travels along water molecules by the Grotthuss mechanism
and a change in the hydrogen bond network in the solvent isolates the two ions, which are stabilized by solvation. Within 1 picosecond
, however, a second reorganization of the hydrogen bond network allows rapid proton transfer down the electric potential difference and subsequent recombination of the ions. This timescale is consistent with the time it takes for hydrogen bonds to reorientate themselves in water.
Proton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
is transferred from one water molecule to another, in pure water or an aqueous solution, to create the two ions, hydronium
Hydronium
In chemistry, a hydronium ion is the cation , a type of oxonium ion produced by protonation of water. This cation is often used to represent the nature of the proton in aqueous solution, where the proton is highly solvated...
, H3O+ and hydroxide
Hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and a hydrogen atom held together by a covalent bond, and carrying a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, as a ligand, a nucleophile, and a...
, OH−. It is an example of autoprotolysis
Autoprotolysis
In autoprotolysis a proton is transferred between two identical molecules, one of which acts as a Brønsted acid, releasing a proton which is accepted by the other molecule acting as a Brønsted base. For example water undergoes autoprotolysis in the self-ionization of water reaction.Every solvent...
, and exemplifies the amphoteric nature of water.
Concentrations
Chemically pure water has an electrical conductivityConductivity (electrolytic)
The conductivity of an electrolyte solution is a measure of its ability to conduct electricity. The SI unit of conductivity is siemens per meter ....
of 0.055 µS
Siemens (unit)
The siemens is the SI derived unit of electric conductance and electric admittance. Conductance and admittance are the reciprocals of resistance and impedance respectively, hence one siemens is equal to the reciprocal of one ohm, and is sometimes referred to as the mho. In English, the term...
·cm−1. According to the theories of Svante Arrhenius
Svante Arrhenius
Svante August Arrhenius was a Swedish scientist, originally a physicist, but often referred to as a chemist, and one of the founders of the science of physical chemistry...
, this must be due to the presence of ions. The ions are produced by the self-ionization reaction
- H2O + H2O H3O+ + OH−
This equilibrium applies to pure water and any aqueous solution. The chemical equilibrium constant, Keq, for this reaction is given by
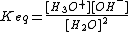
If the concentration of dissolved solutes is not very high, the concentration [H2O] can be taken as being constant at ca. 55.5M. The ionization constant, dissociation constant, self-ionization constant, or ionic product of water, symbolized by Kw is given by

where [H3O+] is the concentration of hydrogen or hydronium ion, and [OH−] is the concentration of hydroxide
Hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and a hydrogen atom held together by a covalent bond, and carrying a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, as a ligand, a nucleophile, and a...
ion. At 25 °C Kw is approximately equal to 1.0×10−14. Water molecules dissociate into equal amounts of H3O+ and OH−, so their concentrations are equal to ca. 1.0 × 10−7 mol dm−3. A solution in which the H3O+ and OH− concentrations equal each other is considered a neutral solution. Pure water is neutral, but most water samples contain impurities. If an impurity is an acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
or base
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
this will affect the concentrations of hydronium ion and hydoxide ion. Water samples which are exposed to air will absorb the acid carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
and the concentration of H3O+ will increase. The concentration of OH- will decrease in such a way that the product [H3O+][OH-] remains constant.
Dependence on temperature, pressure and ionic strength
 |
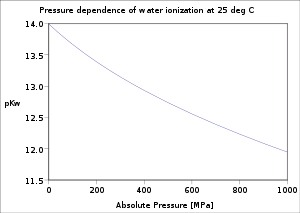 |
The dependence of the water ionization on temperature and pressure has been investigated thoroughly. The value of pKw decreases as temperature increases from the melting point of ice to a minimum at ca. 250 °C, after which it increases up to the critical point
Critical point (thermodynamics)
In physical chemistry, thermodynamics, chemistry and condensed matter physics, a critical point, also called a critical state, specifies the conditions at which a phase boundary ceases to exist...
of ca. 374 °C. It decreases with increasing pressure.
With electrolyte
Electrolyte
In chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
solutions, the value of pKw is dependent on ionic strength
Ionic strength
The ionic strength of a solution is a measure of the concentration of ions in that solution. Ionic compounds, when dissolved in water, dissociate into ions. The total electrolyte concentration in solution will affect important properties such as the dissociation or the solubility of different salts...
of the electrolyte. Values for sodium chloride
Sodium chloride
Sodium chloride, also known as salt, common salt, table salt or halite, is an inorganic compound with the formula NaCl. Sodium chloride is the salt most responsible for the salinity of the ocean and of the extracellular fluid of many multicellular organisms...
are typical for a 1:1 electrolyte. With 1:2 electrolytes, MX2, pKw decreases with increasing ionic strength.
Isotope effects
Heavy waterHeavy water
Heavy water is water highly enriched in the hydrogen isotope deuterium; e.g., heavy water used in CANDU reactors is 99.75% enriched by hydrogen atom-fraction...
, D2O, self-ionizes less than normal water, H2O; oxygen forms a slightly stronger bond to deuterium
Deuterium
Deuterium, also called heavy hydrogen, is one of two stable isotopes of hydrogen. It has a natural abundance in Earth's oceans of about one atom in of hydrogen . Deuterium accounts for approximately 0.0156% of all naturally occurring hydrogen in Earth's oceans, while the most common isotope ...
because the larger mass of deuterium difference results in a lower zero-point energy
Zero-point energy
Zero-point energy is the lowest possible energy that a quantum mechanical physical system may have; it is the energy of its ground state. All quantum mechanical systems undergo fluctuations even in their ground state and have an associated zero-point energy, a consequence of their wave-like nature...
, a quantum mechanical effect. The following table compares the values of pKw for H2O and D2O.
| T/°C | 10 | 20 | 25 | 30 | 40 | 50 |
|---|---|---|---|---|---|---|
| H2O | 14.535 | 14.167 | 13.997 | 13.830 | 13.535 | 13.262 |
| D2O | 15.439 | 15.049 | 14.869 | 14.699 | 14.385 | 14.103 |
Mechanism
The rate of reactionReaction rate
The reaction rate or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a reaction takes place...
for the dissociation
- H2O → H+ + OH-
depends on the activation energy
Activation energy
In chemistry, activation energy is a term introduced in 1889 by the Swedish scientist Svante Arrhenius that is defined as the energy that must be overcome in order for a chemical reaction to occur. Activation energy may also be defined as the minimum energy required to start a chemical reaction...
, ΔE‡. According to the Boltzmann distribution
Boltzmann distribution
In chemistry, physics, and mathematics, the Boltzmann distribution is a certain distribution function or probability measure for the distribution of the states of a system. It underpins the concept of the canonical ensemble, providing its underlying distribution...
the proportion of water molecules that have sufficient energy, due to thermal population, is given by

where k is the Boltzmann constant. Thus some dissociation can occur because sufficient thermal energy is available. The following sequence of events has been proposed on the basis of electric field fluctuations in liquid water. Random fluctuations in molecular motions occasionally (about once every 10 hours per water molecule) produce an electric field strong enough to break an oxygen-hydrogen bond, resulting in a hydroxide (OH−) and hydronium ion (H3O+); the proton of the hydronium ion travels along water molecules by the Grotthuss mechanism
Grotthuss mechanism
The Grotthuss mechanism is the mechanism by which an 'excess' proton or protonic defect diffuses through the hydrogen bond network of water molecules or other hydrogen-bonded liquids through the formation or cleavage of covalent bonds....
and a change in the hydrogen bond network in the solvent isolates the two ions, which are stabilized by solvation. Within 1 picosecond
Picosecond
A picosecond is 10−12 of a second. That is one trillionth, or one millionth of one millionth of a second, or 0.000 000 000 001 seconds. A picosecond is to one second as one second is to 31,700 years....
, however, a second reorganization of the hydrogen bond network allows rapid proton transfer down the electric potential difference and subsequent recombination of the ions. This timescale is consistent with the time it takes for hydrogen bonds to reorientate themselves in water.
External links
- General Chemistry—Autoionization of Water

