Indole
Encyclopedia
Indole is an aromatic heterocyclic
organic compound
. It has a bicyclic structure, consisting of a six-membered benzene
ring fused to a five-membered nitrogen
-containing pyrrole
ring. Indole is a popular component of fragrances and the precursor to many pharmaceuticals. Compounds that contain an indole ring are called indoles. The indolic amino acid tryptophan
is the precursor of the neurotransmitter serotonin
.
at room temperature. Indole can be produced by bacteria
as a degradation product of the amino acid
tryptophan
. It occurs naturally in human feces
and has an intense fecal odor
. At very low concentrations, however, it has a flowery smell, and is a constituent of many flower scents (such as orange blossoms) and perfumes. It also occurs in coal tar
.
The corresponding substituent is called indolyl.
Indole undergoes electrophilic substitution
, mainly at position 3. Substituted indoles are structural elements of (and for some compounds the synthetic precursors for) the tryptophan-derived tryptamine
alkaloids like the neurotransmitter
serotonin
, and melatonin
.
Other indolic compounds include the plant hormone Auxin
(indolyl-3-acetic acid, IAA
), the anti-inflammatory drug indomethacin, the betablocker pindolol
, and the naturally occurring hallucinogen dimethyltryptamine
(N,N-DMT).
The name indole is a portmanteau
of the words indigo
and oleum
, since indole was first isolated by treatment of the indigo dye with oleum.
 Indole chemistry began to develop with the study of the dye indigo
Indole chemistry began to develop with the study of the dye indigo
. Indigo can be converted to isatin
and then to oxindole
. Then, in 1866, Adolf von Baeyer
reduced oxindole to indole using zinc
dust. In 1869, he proposed a formula for indole (left).
Certain indole derivatives were important dyestuffs until the end of the 19th century. In the 1930s, interest in indole intensified when it became known that the indole nucleus is present in many important alkaloid
s, as well as in tryptophan
and auxin
s, and it remains an active area of research today.
Illustrative of such large-scale syntheses, indole (and substituted derivatives) form via vapor-phase reaction of aniline
with ethylene glycol
in the presence of catalysts:
In general, reactions are conducted between 200 and 500 °C. Yields can be as high as 60%. Other precursors to indole include formyltoluidine, 2-ethylaniline, and 2-(2-nitrophenyl)ethanol, all of which undergo cyclizations. Many other methods have been developed that are applicable.
The Leimgruber-Batcho indole synthesis
is an efficient method of sythesizing indole and substituted indoles. Originally disclosed in a patent in 1976, this method is high-yielding and can generate substituted indoles. This method is especially popular in the pharmaceutical industry, where many pharmaceutical drugs
are made up of specifically substituted indoles.
One of the oldest and most reliable methods for synthesizing substituted indoles is the Fischer indole synthesis
, developed in 1883 by Emil Fischer
. Although the synthesis of indole itself is problematic using the Fischer indole synthesis, it is often used to generate indoles substituted in the 2- and/or 3-positions. Indole can still be synthesized, however, using the Fischer indole synthesis by reacting phenylhydrazine
with pyruvic acid
followed by decarboxylation
of the formed indole-2-carboxylic acid. This has also been accomplished in a one-pot synthesis using microwave irradiation.
s, indole is not basic
. The bonding situation is completely analogous to that in pyrrole
. Very strong acids such as hydrochloric acid
are required to protonate
indole. The protonated form has an pKa
of −3.6. The sensitivity of many indolic compounds (e.g., tryptamine
s) under acidic conditions is caused by this protonation.
is C-3, which is 1013 times more reactive than benzene
. For example, Vilsmeier-Haack
formylation
of indole will take place at room temperature exclusively at C-3. Since the pyrrollic ring is the most reactive portion of indole, electrophilic substitution of the carbocyclic (benzene) ring can take place only after N-1, C-2, and C-3 are substituted.
Gramine
, a useful synthetic intermediate, is produced via a Mannich reaction
of indole with dimethylamine
and formaldehyde
. It is the precursor to indole acetic acid and synthetic tryptophan.
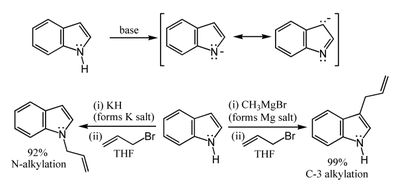
, so that very strong bases such as sodium hydride
or butyl lithium
and water-free conditions are required for complete deprotonation
. The resulting alkali metal derivatives can react in two ways. The more ionic
salts such as the sodium
or potassium
compounds tend to react with electrophile
s at nitrogen-1, whereas the more covalent
magnesium compounds (indole Grignard reagents
) and (especially) zinc
complexes tend to react at carbon-3 (see figure below). In analogous fashion, polar
aprotic solvent
s such as DMF
and DMSO
tend to favour attack at the nitrogen, whereas nonpolar solvents such as toluene
favour C-3 attack.
or lithium diisopropylamide
results in lithiation exclusively at the C-2 position. This strong nucleophile can then be used as such with other electrophiles.
Bergman and Venemalm developed a technique for lithiating the 2-position of unsubstituted indole.
Alan Katritzky also developed a technique for lithiating the 2-position of unsubstituted indole.
. Simple oxidants such as N-bromosuccinimide
will selectively oxidize indole 1 to oxindole
(4 and 5).
to form advanced strychnine
intermediates. In this case, the 2-aminofuran is the diene
, whereas the indole is the dienophile.
Indoles also undergo intramolecular [2+3] and [2+2] cycloadditions.
oil
, used in the perfume
industry, contains around 2.5% of indole. Since 1 kilogram of the natural oil requires processing several million jasmine blossoms and costs around $10,000, indole (among other things) is used in the manufacture of synthetic jasmine oil (which costs around $10/kg).
Heterocyclic compound
A heterocyclic compound is a cyclic compound which has atoms of at least two different elements as members of its ring. The counterparts of heterocyclic compounds are homocyclic compounds, the rings of which are made of a single element....
organic compound
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
. It has a bicyclic structure, consisting of a six-membered benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
ring fused to a five-membered nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
-containing pyrrole
Pyrrole
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4H4NH. It is a colourless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., N-methylpyrrole, C4H4NCH3...
ring. Indole is a popular component of fragrances and the precursor to many pharmaceuticals. Compounds that contain an indole ring are called indoles. The indolic amino acid tryptophan
Tryptophan
Tryptophan is one of the 20 standard amino acids, as well as an essential amino acid in the human diet. It is encoded in the standard genetic code as the codon UGG...
is the precursor of the neurotransmitter serotonin
Serotonin
Serotonin or 5-hydroxytryptamine is a monoamine neurotransmitter. Biochemically derived from tryptophan, serotonin is primarily found in the gastrointestinal tract, platelets, and in the central nervous system of animals including humans...
.
General properties and occurrence
Indole is a solidSolid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
at room temperature. Indole can be produced by bacteria
Bacteria
Bacteria are a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria have a wide range of shapes, ranging from spheres to rods and spirals...
as a degradation product of the amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
tryptophan
Tryptophan
Tryptophan is one of the 20 standard amino acids, as well as an essential amino acid in the human diet. It is encoded in the standard genetic code as the codon UGG...
. It occurs naturally in human feces
Feces
Feces, faeces, or fæces is a waste product from an animal's digestive tract expelled through the anus or cloaca during defecation.-Etymology:...
and has an intense fecal odor
Odor
An odor or odour is caused by one or more volatilized chemical compounds, generally at a very low concentration, that humans or other animals perceive by the sense of olfaction. Odors are also commonly called scents, which can refer to both pleasant and unpleasant odors...
. At very low concentrations, however, it has a flowery smell, and is a constituent of many flower scents (such as orange blossoms) and perfumes. It also occurs in coal tar
Coal tar
Coal tar is a brown or black liquid of extremely high viscosity, which smells of naphthalene and aromatic hydrocarbons. Coal tar is among the by-products when coal iscarbonized to make coke or gasified to make coal gas...
.
The corresponding substituent is called indolyl.
Indole undergoes electrophilic substitution
Electrophilic substitution
Electrophilic substitution reactions are chemical reactions in which an electrophile displaces a group in a compound, typically but not always hydrogen. Electrophilic aromatic substitution is characteristic of aromatic compounds and is an important way of introducing functional groups onto benzene...
, mainly at position 3. Substituted indoles are structural elements of (and for some compounds the synthetic precursors for) the tryptophan-derived tryptamine
Tryptamine
Tryptamine is a monoamine alkaloid found in plants, fungi, and animals. It is based around the indole ring structure, and is chemically related to the amino acid tryptophan, from which its name is derived...
alkaloids like the neurotransmitter
Neurotransmitter
Neurotransmitters are endogenous chemicals that transmit signals from a neuron to a target cell across a synapse. Neurotransmitters are packaged into synaptic vesicles clustered beneath the membrane on the presynaptic side of a synapse, and are released into the synaptic cleft, where they bind to...
serotonin
Serotonin
Serotonin or 5-hydroxytryptamine is a monoamine neurotransmitter. Biochemically derived from tryptophan, serotonin is primarily found in the gastrointestinal tract, platelets, and in the central nervous system of animals including humans...
, and melatonin
Melatonin
Melatonin , also known chemically as N-acetyl-5-methoxytryptamine, is a naturally occurring compound found in animals, plants, and microbes...
.
Other indolic compounds include the plant hormone Auxin
Auxin
Auxins are a class of plant hormones with some morphogen-like characteristics. Auxins have a cardinal role in coordination of many growth and behavioral processes in the plant's life cycle and are essential for plant body development. Auxins and their role in plant growth were first described by...
(indolyl-3-acetic acid, IAA
Indole-3-acetic acid
Indole-3-acetic acid, also known as IAA, is a heterocyclic compound that is a phytohormone called auxin. This colourless solid is native plant compound, potent and the most important auxin...
), the anti-inflammatory drug indomethacin, the betablocker pindolol
Pindolol
Pindolol is a beta blocker....
, and the naturally occurring hallucinogen dimethyltryptamine
Dimethyltryptamine
N,N-Dimethyltryptamine is a naturally occurring psychedelic compound of the tryptamine family. DMT is found in several plants, and also in trace amounts in humans and other mammals, where it is originally derived from the essential amino acid tryptophan, and ultimately produced by the enzyme INMT...
(N,N-DMT).
The name indole is a portmanteau
Portmanteau word
A portmanteau or portmanteau word is a blend of two words or morphemes into one new word. A portmanteau word typically combines both sounds and meanings, as in smog, coined by blending smoke and fog. More generally, it may refer to any term or phrase that combines two or more meanings...
of the words indigo
Indigo dye
Indigo dye is an organic compound with a distinctive blue color . Historically, indigo was a natural dye extracted from plants, and this process was important economically because blue dyes were once rare. Nearly all indigo dye produced today — several thousand tons each year — is synthetic...
and oleum
Oleum
Oleum , or fuming sulfuric acid refers to a solution of various compositions of sulfur trioxide in sulfuric acid or sometimes more specifically to disulfuric acid ....
, since indole was first isolated by treatment of the indigo dye with oleum.
History
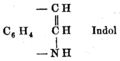
Indigo dye
Indigo dye is an organic compound with a distinctive blue color . Historically, indigo was a natural dye extracted from plants, and this process was important economically because blue dyes were once rare. Nearly all indigo dye produced today — several thousand tons each year — is synthetic...
. Indigo can be converted to isatin
Isatin
Isatin or 1H-indole-2,3-dione is an indole derivative. The compound was first obtained by Erdman and Laurent in 1841 as a product from the oxidation of indigo dye by nitric acid and chromic acids...
and then to oxindole
Oxindole
Oxindole is an aromatic heterocyclic organic compound. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing ring...
. Then, in 1866, Adolf von Baeyer
Adolf von Baeyer
Johann Friedrich Wilhelm Adolf von Baeyer was a German chemist who synthesized indigo, and was the 1905 recipient of the Nobel Prize in Chemistry. Born in Berlin, he initially studied mathematics and physics at Berlin University before moving to Heidelberg to study chemistry with Robert Bunsen...
reduced oxindole to indole using zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
dust. In 1869, he proposed a formula for indole (left).
Certain indole derivatives were important dyestuffs until the end of the 19th century. In the 1930s, interest in indole intensified when it became known that the indole nucleus is present in many important alkaloid
Alkaloid
Alkaloids are a group of naturally occurring chemical compounds that contain mostly basic nitrogen atoms. This group also includes some related compounds with neutral and even weakly acidic properties. Also some synthetic compounds of similar structure are attributed to alkaloids...
s, as well as in tryptophan
Tryptophan
Tryptophan is one of the 20 standard amino acids, as well as an essential amino acid in the human diet. It is encoded in the standard genetic code as the codon UGG...
and auxin
Auxin
Auxins are a class of plant hormones with some morphogen-like characteristics. Auxins have a cardinal role in coordination of many growth and behavioral processes in the plant's life cycle and are essential for plant body development. Auxins and their role in plant growth were first described by...
s, and it remains an active area of research today.
Synthesis of indoles
Indole is a major constituent of coal-tar, and the 220–260 °C distillation fraction is the main industrial source of the material. Indole and its derivatives can also be synthesized by a variety of methods. The main industrial routes start from aniline.Illustrative of such large-scale syntheses, indole (and substituted derivatives) form via vapor-phase reaction of aniline
Aniline
Aniline, phenylamine or aminobenzene is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the prototypical aromatic amine. Being a precursor to many industrial chemicals, its main use is in the manufacture of precursors to polyurethane...
with ethylene glycol
Ethylene glycol
Ethylene glycol is an organic compound widely used as an automotive antifreeze and a precursor to polymers. In its pure form, it is an odorless, colorless, syrupy, sweet-tasting liquid...
in the presence of catalysts:
In general, reactions are conducted between 200 and 500 °C. Yields can be as high as 60%. Other precursors to indole include formyltoluidine, 2-ethylaniline, and 2-(2-nitrophenyl)ethanol, all of which undergo cyclizations. Many other methods have been developed that are applicable.
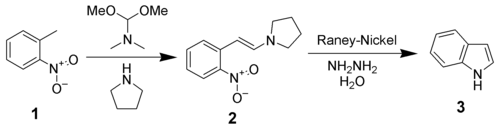
Leimgruber-Batcho indole synthesis
The Leimgruber-Batcho indole synthesis
Leimgruber-Batcho indole synthesis
The Leimgruber–Batcho indole synthesis is a series of organic reactions that produce indoles from o-nitrotoluenes 1. The first step is the formation of an enamine 2 using N,N-dimethylformamide dimethyl acetal and pyrrolidine...
is an efficient method of sythesizing indole and substituted indoles. Originally disclosed in a patent in 1976, this method is high-yielding and can generate substituted indoles. This method is especially popular in the pharmaceutical industry, where many pharmaceutical drugs
Medication
A pharmaceutical drug, also referred to as medicine, medication or medicament, can be loosely defined as any chemical substance intended for use in the medical diagnosis, cure, treatment, or prevention of disease.- Classification :...
are made up of specifically substituted indoles.
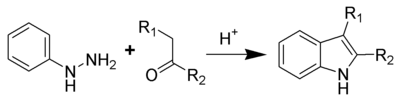
Fischer indole synthesis
One of the oldest and most reliable methods for synthesizing substituted indoles is the Fischer indole synthesis
Fischer indole synthesis
The Fischer indole synthesis isa chemical reaction that produces the aromatic heterocycle indole from a phenylhydrazine and an aldehyde or ketone under acidic conditions. The reaction was discovered in 1883 by Hermann Emil Fischer. Today antimigraine drugs of the triptan class are often...
, developed in 1883 by Emil Fischer
Hermann Emil Fischer
Hermann Emil Fischer, Emil Fischer was a German chemist and 1902 recipient of the Nobel Prize in Chemistry. He discovered the Fischer esterification. He developed the Fischer projection, a symbolic way of drawing asymmetric carbon atoms.-Early years:Fischer was born in Euskirchen, near Cologne,...
. Although the synthesis of indole itself is problematic using the Fischer indole synthesis, it is often used to generate indoles substituted in the 2- and/or 3-positions. Indole can still be synthesized, however, using the Fischer indole synthesis by reacting phenylhydrazine
Phenylhydrazine
Phenylhydrazine is the chemical compound with the formula C6H5NHNH2. Organic chemists abbreviate the compound as PhNHNH2.- Chemical properties :...
with pyruvic acid
Pyruvic acid
Pyruvic acid is an organic acid, a ketone, as well as the simplest of the alpha-keto acids. The carboxylate ion of pyruvic acid, CH3COCOO−, is known as pyruvate, and is a key intersection in several metabolic pathways....
followed by decarboxylation
Decarboxylation
Decarboxylation is a chemical reaction that releases carbon dioxide . Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is the first chemical step in photosynthesis, is called carbonation, the addition of CO2 to...
of the formed indole-2-carboxylic acid. This has also been accomplished in a one-pot synthesis using microwave irradiation.
Other indole-forming reactions
- Bartoli indole synthesisBartoli indole synthesisThe Bartoli indole synthesis is the chemical reaction of ortho-substituted nitroarenes with vinyl grignard reagents to form substituted indoles....
- Bischler-Möhlau indole synthesisBischler-Möhlau indole synthesisThe Bischler-Möhlau indole synthesis is a chemical reaction that forms a 2-aryl-indole from an α-bromo-acetophenone and excess aniline.In spite of its long history, this classical reaction has received relatively little attention in comparison with other methods for indole synthesis, perhaps owing...
- Fukuyama indole synthesisFukuyama indole synthesisThe Fukuyama indole synthesis is the chemical reaction of alkenylthioanilides to give 2,3-disubstituted indoles. Most commonly tributyltin hydride is utilized as the reducing agent, with azobisisobutyronitrile as a radical initiator....
- Gassman indole synthesisGassman indole synthesisThe Gassman indole synthesis is a series of chemical reactions used to synthesize substituted indoles from aniline.This is a one-pot chemical reaction, and none of the intermediates are isolated. R1 can be hydrogen or alkyl, while R2 works best with aryl, but can also be alkyl...
- Hemetsberger indole synthesisHemetsberger indole synthesisThe Hemetsberger indole synthesis is a chemical reaction that thermally decomposes a 3-aryl-2-azido-propenoic ester into an indole-2-carboxylic ester.Yields are typically above 70%...
- Larock indole synthesisLarock indole synthesisThe Larock indole synthesis is a chemical reaction used to synthesize indoles from ortho-iodoanilines and a disubstituted alkyne.An excess of alkyne, using palladium carbonate or palladium acetate and a base, and adding one equivalent of lithium chloride tend to give the best yields...
- Madelung synthesisMadelung synthesisThe Madelung synthesis is a chemical reaction that produces indoles by the intramolecular cyclization of N-phenylamides using strong base at high temperature....
- Nenitzescu indole synthesisNenitzescu indole synthesisThe Nenitzescu indole synthesis is a chemical reaction that forms 5-hydroxyindole derivatives from benzoquinone and β-aminocrotonic esters.-References:*Nenitzescu, C. D. Bull. Soc. Chim. Romania 1929, 11, 37....
- Reissert indole synthesisReissert indole synthesisThe Reissert indole synthesis is a series of chemical reactions designed to synthesize indole or substituted-indoles from ortho-nitrotoluene 1 and diethyl oxalate 2....
- Baeyer-Emmerling indole synthesisBaeyer-Emmerling indole synthesisThe Baeyer-Emmerling indole synthesis is a method for synthesizing indole from a ortho-nitrocinnamic acid and iron powder in strongly basic solution. This reaction was discovered by Adolf von Baeyer and A. Emmerling in 1869....
- In the Diels-Reese reaction dimethyl acetylenedicarboxylateDimethyl acetylenedicarboxylateDimethyl acetylenedicarboxylate is the organic compound with the formula CH3O2CC2CO2CH3. This ester, which exists as a liquid at room temperature, is highly electrophilic. As such, DMAD, as it is commonly called in the laboratory, is widely employed as a dienophile in cycloaddition reactions,...
reacts with diphenylhydrazineHydrazineHydrazine is an inorganic compound with the formula N2H4. It is a colourless flammable liquid with an ammonia-like odor. Hydrazine is highly toxic and dangerously unstable unless handled in solution. Approximately 260,000 tons are manufactured annually...
to an adduct, which in xyleneXyleneXylene encompasses three isomers of dimethylbenzene. The isomers are distinguished by the designations ortho- , meta- , and para- , which specify to which carbon atoms the two methyl groups are attached...
gives dimethyl indole-2,3-dicarboxylate and anilineAnilineAniline, phenylamine or aminobenzene is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the prototypical aromatic amine. Being a precursor to many industrial chemicals, its main use is in the manufacture of precursors to polyurethane...
. With other solvents, other products are formed: with glacial acetic acid a pyrazolonePyrazolonePyrazolone, a five-membered-ring lactam, is a derivative of pyrazole that has an additional keto group.It has a molecular formula of C3H4N2O.Examples of derivatives include:* Ampyrone* Metamizole* Phenazone-External links:* *...
, and with pyridinePyridinePyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
a quinolineQuinolineQuinoline is a heterocyclic aromatic organic compound. It has the formula C9H7N and is a colourless hygroscopic liquid with a strong odour. Aged samples, if exposed to light, become yellow and later brown...
.
Basicity
Unlike most amineAmine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s, indole is not basic
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
. The bonding situation is completely analogous to that in pyrrole
Pyrrole
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4H4NH. It is a colourless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., N-methylpyrrole, C4H4NCH3...
. Very strong acids such as hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
are required to protonate
Protonation
In chemistry, protonation is the addition of a proton to an atom, molecule, or ion. Some classic examples include*the protonation of water by sulfuric acid:*the protonation of isobutene in the formation of a carbocation:2C=CH2 + HBF4 → 3C+ + BF4−*the protonation of ammonia in the...
indole. The protonated form has an pKa
Acid dissociation constant
An acid dissociation constant, Ka, is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction known as dissociation in the context of acid-base reactions...
of −3.6. The sensitivity of many indolic compounds (e.g., tryptamine
Tryptamine
Tryptamine is a monoamine alkaloid found in plants, fungi, and animals. It is based around the indole ring structure, and is chemically related to the amino acid tryptophan, from which its name is derived...
s) under acidic conditions is caused by this protonation.
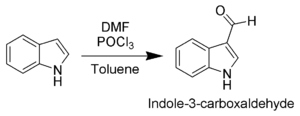
Electrophilic substitution
The most reactive position on indole for electrophilic aromatic substitutionElectrophilic aromatic substitution
Electrophilic aromatic substitution EAS is an organic reaction in which an atom, usually hydrogen, appended to an aromatic system is replaced by an electrophile...
is C-3, which is 1013 times more reactive than benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
. For example, Vilsmeier-Haack
Vilsmeier-Haack reaction
The Vilsmeier–Haack reaction is the chemical reaction of a substituted amide with phosphorus oxychloride and an electron-rich arene to produce an aryl aldehyde or ketone . The reaction is named after Anton Vilsmeier and Albrecht Haack...
formylation
Formylation reaction
A formylation reaction in organic chemistry is the catch-all name for any organic reaction in which an organic compound is functionalized with a formyl group .Aromatic formylation reactions via electrophilic aromatic substitution include:...
of indole will take place at room temperature exclusively at C-3. Since the pyrrollic ring is the most reactive portion of indole, electrophilic substitution of the carbocyclic (benzene) ring can take place only after N-1, C-2, and C-3 are substituted.
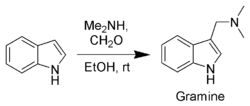
Gramine
Gramine
Gramine is a naturally occurring indole alkaloid present in several plant species. Gramine may play a defensive role in these plants, since it is toxic to many organisms.-Occurrence:...
, a useful synthetic intermediate, is produced via a Mannich reaction
Mannich reaction
The Mannich reaction is an organic reaction which consists of an amino alkylation of an acidic proton placed next to a carbonyl functional group with formaldehyde and ammonia or any primary or secondary amine. The final product is a β-amino-carbonyl compound also known as a Mannich base...
of indole with dimethylamine
Dimethylamine
Dimethylamine is an organic compound with the formula 2NH. This secondary amine is a colorless, flammable liquified gas with an ammonia-like odor. Dimethylamine is generally encountered as a solution in water at concentrations up to around 40%...
and formaldehyde
Formaldehyde
Formaldehyde is an organic compound with the formula CH2O. It is the simplest aldehyde, hence its systematic name methanal.Formaldehyde is a colorless gas with a characteristic pungent odor. It is an important precursor to many other chemical compounds, especially for polymers...
. It is the precursor to indole acetic acid and synthetic tryptophan.
Nitrogen-H acidity and organometallic indole anion complexes
The N-H center has a pKa of 21 in DMSODimethyl sulfoxide
Dimethyl sulfoxide is an organosulfur compound with the formula 2SO. This colorless liquid is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water...
, so that very strong bases such as sodium hydride
Sodium hydride
Sodium hydride is the chemical compound with the empirical formula NaH. It is primarily used as a strong base in organic synthesis. NaH is representative of the saline hydrides, meaning it is a salt-like hydride, composed of Na+ and H− ions, in contrast to the more molecular hydrides such as...
or butyl lithium
N-Butyllithium
n-Butyllithium is an organolithium reagent. It is widely used as a polymerization initiator in the production of elastomers such as polybutadiene or styrene-butadiene-styrene...
and water-free conditions are required for complete deprotonation
Deprotonation
Deprotonation is the removal of a proton from a molecule, forming the conjugate base.The relative ability of a molecule to give up a proton is measured by its pKa value. A low pKa value indicates that the compound is acidic and will easily give up its proton to a base...
. The resulting alkali metal derivatives can react in two ways. The more ionic
Ionic bond
An ionic bond is a type of chemical bond formed through an electrostatic attraction between two oppositely charged ions. Ionic bonds are formed between a cation, which is usually a metal, and an anion, which is usually a nonmetal. Pure ionic bonding cannot exist: all ionic compounds have some...
salts such as the sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
or potassium
Potassium
Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
compounds tend to react with electrophile
Electrophile
In general electrophiles are positively charged species that are attracted to an electron rich centre. In chemistry, an electrophile is a reagent attracted to electrons that participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile...
s at nitrogen-1, whereas the more covalent
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
magnesium compounds (indole Grignard reagents
Grignard reaction
The Grignard reaction is an organometallic chemical reaction in which alkyl- or aryl-magnesium halides add to a carbonyl group in an aldehyde or ketone. This reaction is an important tool for the formation of carbon–carbon bonds...
) and (especially) zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
complexes tend to react at carbon-3 (see figure below). In analogous fashion, polar
Chemical polarity
In chemistry, polarity refers to a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Molecular polarity is dependent on the difference in...
aprotic solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
s such as DMF
Dimethylformamide
Dimethylformamide is an organic compound with the formula 2NCH. Commonly abbreviated as DMF , this colourless liquid is miscible with water and the majority of organic liquids. DMF is a common solvent for chemical reactions...
and DMSO
Dimethyl sulfoxide
Dimethyl sulfoxide is an organosulfur compound with the formula 2SO. This colorless liquid is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water...
tend to favour attack at the nitrogen, whereas nonpolar solvents such as toluene
Toluene
Toluene, formerly known as toluol, is a clear, water-insoluble liquid with the typical smell of paint thinners. It is a mono-substituted benzene derivative, i.e., one in which a single hydrogen atom from the benzene molecule has been replaced by a univalent group, in this case CH3.It is an aromatic...
favour C-3 attack.
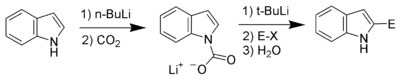
Carbon acidity and C-2 lithiation
After the N-H proton, the hydrogen at C-2 is the next most acidic proton on indole. Reaction of N-protected indoles with butyl lithiumN-Butyllithium
n-Butyllithium is an organolithium reagent. It is widely used as a polymerization initiator in the production of elastomers such as polybutadiene or styrene-butadiene-styrene...
or lithium diisopropylamide
Lithium diisopropylamide
Lithium diisopropylamide is the chemical compound with the formula [2CH]2NLi. Generally abbreviated LDA, it is a strong base used in organic chemistry for the deprotonation of weakly acidic compounds. The reagent has been widely accepted because it is soluble in non-polar organic solvents and it...
results in lithiation exclusively at the C-2 position. This strong nucleophile can then be used as such with other electrophiles.
Bergman and Venemalm developed a technique for lithiating the 2-position of unsubstituted indole.
Alan Katritzky also developed a technique for lithiating the 2-position of unsubstituted indole.
Oxidation of indole
Due to the electron-rich nature of indole, it is easily oxidizedRedox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
. Simple oxidants such as N-bromosuccinimide
N-Bromosuccinimide
N-Bromosuccinimide or NBS is a chemical reagent which is used in radical substitution and electrophilic addition reactions in organic chemistry. NBS can be considered a convenient source of cationic bromine.-Preparation:...
will selectively oxidize indole 1 to oxindole
Oxindole
Oxindole is an aromatic heterocyclic organic compound. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing ring...
(4 and 5).
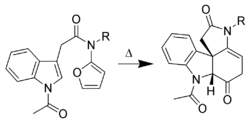
Cycloadditions of indole
Only the C-2 to C-3 pi-bond of indole is capable of cycloaddition reactions. Intermolecular cycloadditions are not favorable, whereas intramolecular variants are often high-yielding. For example, Padwa et al. have developed this Diels-Alder reactionDiels-Alder reaction
The Diels–Alder reaction is an organic chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon...
to form advanced strychnine
Strychnine
Strychnine is a highly toxic , colorless crystalline alkaloid used as a pesticide, particularly for killing small vertebrates such as birds and rodents. Strychnine causes muscular convulsions and eventually death through asphyxia or sheer exhaustion...
intermediates. In this case, the 2-aminofuran is the diene
Diene
In organic chemistry a diene or diolefin is a hydrocarbon that contains two carbon double bonds.Conjugated dienes are functional groups, with a general formula of CnH2n-2. Dienes and alkynes are functional isomers...
, whereas the indole is the dienophile.
Indoles also undergo intramolecular [2+3] and [2+2] cycloadditions.
Applications
Natural jasmineJasmine
Jasminum , commonly known as jasmines, is a genus of shrubs and vines in the olive family . It contains around 200 species native to tropical and warm temperate regions of the Old World...
oil
Essential oil
An essential oil is a concentrated hydrophobic liquid containing volatile aroma compounds from plants. Essential oils are also known as volatile oils, ethereal oils or aetherolea, or simply as the "oil of" the plant from which they were extracted, such as oil of clove...
, used in the perfume
Perfume
Perfume is a mixture of fragrant essential oils and/or aroma compounds, fixatives, and solvents used to give the human body, animals, objects, and living spaces "a pleasant scent"...
industry, contains around 2.5% of indole. Since 1 kilogram of the natural oil requires processing several million jasmine blossoms and costs around $10,000, indole (among other things) is used in the manufacture of synthetic jasmine oil (which costs around $10/kg).
See also
- IsoindoleIsoindoleIsoindole in heterocyclic chemistry is a benzo fused pyrrole. The compound is an isomer of indole and its reduced form is an isoindoline.In solution its tautomer without full aromaticity over the whole ring system is predominant:...
- Martinet dioxindole synthesisMartinet dioxindole synthesisThe Martinet dioxindole synthesis is a chemical reaction used to synthesize dioxindoles 3 from anilines and esters of mesoxalic acid 1.-Reaction mechanism:...
- SkatoleSkatoleSkatole or 3-methylindole is a mildly toxic white crystalline organic compound belonging to the indole family. It occurs naturally in feces and coal tar, and has a strong fecal odor...
(3-methylindole) - Stollé synthesisStollé synthesisThe Stollé synthesis is a series of chemical reactions that produce oxindoles from anilines and α-haloacid chlorides . The first step is an amide coupling, while the second step is a Friedel-Crafts reaction. An improved procedure has been developed....
- TryptamineTryptamineTryptamine is a monoamine alkaloid found in plants, fungi, and animals. It is based around the indole ring structure, and is chemically related to the amino acid tryptophan, from which its name is derived...
s
General references
- Indoles Part One, W. J. Houlihan (ed.), Wiley Interscience, New York, 1972.
- Joule, J., In Science of Synthesis, Thomas, E. J., Ed.; Thieme: Stuttgart, (2000); Vol. 10, p. 361. ISBN 3-13-112241-2 (GTV); ISBN 0-86577-949-X (TNY).
See also
- Indole-3-butyric acidIndole-3-butyric acidIndole-3-butyric acid is a white to light-yellow crystalline solid, with the molecular formula C12H13NO2. It melts at 125 °C in atmospheric pressure and decomposes before boiling.-Plant hormone:...
- Indole testIndole testThe indole test is a biochemical test performed on bacterial species to determine the ability of the organism to split indole from the amino acid tryptophan...
– biochemical test for bacterial identification
External links
- Synthesis of indoles (overview of recent methods)
- Synthesis and propierties of indoles at chemsynthesis.com