
Diels-Alder reaction
Encyclopedia
The Diels–Alder reaction is an organic chemical reaction (specifically, a cycloaddition
) between a conjugated diene
and a substituted alkene
, commonly termed the dienophile, to form a substituted cyclohexene
system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon
. Some of the Diels–Alder reactions are reversible; the decomposition reaction of the cyclic system is then called the retro-Diels–Alder. For example, retro-Diels–Alder compounds are commonly observed when a Diels–Alder product is analyzed via mass spectrometry
.
Otto Paul Hermann Diels
and Kurt Alder
first documented the novel reaction in 1928 for which they were awarded the Nobel Prize in Chemistry
in 1950 for their work on the eponymous reaction.
The Diels–Alder reaction is generally considered the "Mona Lisa" of reactions in organic chemistry since it requires very little energy to create a cyclohexene
ring, which is useful in many other organic reactions.
, which has a smaller volume than either the starting materials or the product. It is an associative type of reaction, and it is sped up by very high pressures. Diels–Alder is an example of a pericyclic reaction.
Some free-radical versions of this reaction have been observed, though these are not Diels–Alder reactions since the stereochemistry
at the carbons is scrambled. These are step-wise reactions of the free radicals which form the new bonds in at least two steps. An example of this type of reaction is the reaction of selenobenzophenone with a 1,3-diene (See: thioketones).
component in the Diels–Alder reaction can be open-chain or cyclic and it can have many different kinds of substituent
s. There is only one limitation: it must be able to exist in the s-cis conformation
. Butadiene itself normally prefers the s-trans conformation, with the two double bonds as far away from each other as possible. If there are substituents larger than hydrogen then steric hindrance may influence the relative stabilities of the conformations. For simple cases, the barrier to rotation about the central bond is small and rotation to the less favourable but reactive s-cis
conformation is rapid.
Cyclic dienes that are permanently in the s-cis conformation are exceptionally reactive in Diels–Alder reactions (cyclopentadiene
is a classic example), while cyclic dienes that are permanently in the s-trans conformation and cannot adopt the s-cis conformation will not undergo the Diels–Alder reaction at all. An especially reactive diene is Danishefsky’s diene
.
Dendralenes
are a new class of experimental DA dienes.
Unstable dienes, such as o-quinodimethane, can be generated in situ
. Aromatic stabilization in the product of a DA reaction using such a diene is, in some cases, the reason behind the very high reactivity and lack of stability of such diene. The use of such unstable dienes is advantageous, despite the trouble, in that the products will contain newly formed aromatic six-membered rings.
Benzenoid compounds rarely undergo DA reactions and often require very reactive dienophiles. One example of such rare reaction is the Wagner-Jauregg reaction
to the alkene. Though common, this feature is not exclusive of Diels–Alder dienophiles. There must be some extra conjugation, at least a phenyl group or chlorine
atom. The dienophile can be activated by a Lewis acid
such as niobium pentachloride.
Cyclopentadiene
does not react with cyclohexenone
in ethyl acetate
unless the Lewis acid is present. The yield improves when reaction temperature is lowered to −78°C because polymerization
side reactions are prevented. Niobium pentachloride catalysis gives only the endo conformer. The same reaction with aluminium chloride
results in an endo and exo mixture. Many of these Lewis acid
s are not good catalysts for the reaction of α,β-unsaturated carbonyls, this is because the carbonyl oxygen binds too tightly to the metal centre. A far better catalyst for such a system is a combination of silver
perchlorate
and Lawesson's reagent
in cold dichloromethane
.
It is well known that it is possible to use heteroatom
containing dienophiles for Diels–Alder reactions, for instance Lawesson's reagent
(and diferrocenyl dithiadiphosphetane disulfide) can react with 1,3-dienes to form six membered ring adducts. Also selenoketones and thioketones are able to react in the same way with 1,3-dienes. Imines are reactants in the Aza Diels–Alder reaction and carbonyl
groups the reactant in Oxo Diels–Alder reaction
s. These reactions are collectively called hetero Diels–Alder reactions.
Dienophiles can be chosen to contain a "masked functionality". The dienophile undergoes Diels–Alder reaction with a diene introducing such a functionality onto the product molecule. A series of reactions then follow to transform the functionality into a desirable group. The end product cannot not be made in a single DA step because equivalent dienophile is either unreactive or inaccessible. An example of such approach is the use of α-chloroacrylonitrile (CH2=CClCN). When reacted with a diene, this dienophile will introduce alpha-chloronitrile functionality onto the product molecule. This is a "masked functionality" which can be then hydrolyzed to form a ketone. α-Chloroacrylonitrile dienophile is an equivalent of ketene
dienophile (CH2=C=O), which would produce same product in one DA step. The problem is that ketene itself cannot be used in Diels–Alder reactions because it reacts with dienes in unwanted manner (by [2+2] cycloaddition
), and therefore "masked functionality" approach has to be used.
Other such functionalities are phosphonium
substituents (yielding exocyclic double bonds after Wittig reaction
), various sulfoxide
and sulfonyl
functionalities (both are acetylene
equivalents), and nitro groups (ketene equivalents).
groups, for example, can successfully react with dienes to yield pyran
oid rings. Generally, the endo transition state
is favored in this case.
Nitroso compounds (N=O) react to form oxazine-like compounds (cyclic molecules with nitrogen and oxygen present in the six-membered ring). Another group of dienophiles successfully used for Diels–Alder reactions is imine
s. Such reactions are useful for preparation of alkaloid
and other polycyclic compounds.
Chlorosulfonyl isocyanate
can be utilized as a dienophile to prepare Vince Lactam
.
s and stereoisomers (enantiomer
s and diastereomer
s). Identity of major products can usually be predicted, however.
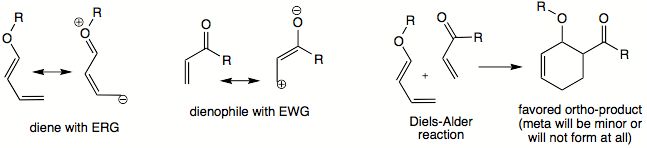
In unsymmetrically substituted diene and dienophile, pseudo-ortho
and para
orientations in products are usually favored over meta
orientation. A particular preference in location of substitutents in the product can, in some cases, be explained in terms of frontier orbital theory. Most commonly, diene
bears an electron-releasing group (ERG) and dienophile bears an electron-withdrawing group (EWG). The strongest interaction takes place between HOMO
of diene and LUMO
of dienophile. Carbons that have the highest coefficients in two frontier orbitals will begin to bond; therefore these carbons will direct the orientation of substituents and thus identity of major product of a DA reaction.
Dealing with the actual frontier orbital coefficients can be avoided since the preferred orientation in product can be described in terms of partial positive and negative charges that exist in diene and dienophile. Carbon with a partial negative charge will interact most readily with carbon bearing a partial positive charge. Therefore those two carbons will start coming together, thus dictating the relative orientation of substituents. The existence of partial positive/negative charge can always be determined by drawing resonance contributors for diene and dienophile, taking their ERG and EWG into consideration.
The initial potential of the reaction was utilized in the form of insecticides, which ultimately led to the endo rule. Otto Diels’ and Kurt Alder’s reaction allowed for the production of weapons against agricultural pests to be fully realized in the 1930s. Most of these chemicals contained chlorine, of which two are called Dieldrin
and Aldrin
after the appropriate individuals. These chemicals have been long-since discontinued because of their toxicity to not only invertebrates, but to higher orders of organisms as well. Fortunately, insecticide use may continue unabated with the recent introduction of various safe treatments. Though insecticides like Dieldrin and Aldrin cause a slew of cardiac and respiratory illnesses (as well as reproductive failure), they served as an important step towards understanding why the endo product was the major yield. The study of the insecticide’s fused norcamphane rings became a highly popular topic in the 1930s and 1940s. Oxidative degradation revealed high specificity of the stereochemistry; after much research by Kurt Alder and H.F. Rickert, it became clear that steric hindrance is not as important in Diels–Alder reactions as it is in other reactions. This led to the secondary orbital explanation as well as a satisfactory hypothesis that elucidated polymerization of certain Diels–Alder adducts.
of substituents in the starting material is retained in the product. This means that if a cis-dienophile is reacted, both of the cis-substituent
s will end up on same side (face) of the product ring. Trans-dienophile will yield a product where both of trans-substituents (that came originally from the dienophile) will be on different sides of the product ring. The same principle applies to diene
s. Trans, trans or cis, cis 1,4-substituents will end up on same side of the ring, whereas trans, cis 1,4-substituents will be oriented towards different faces of the ring.
Besides the ortho/meta/para-forming orientations, the diene
and dienophile may arrange themselves in different ways to yield exo and endo
transition state
s which result in different products. To determine which is the endo and which is the exo transition state, the two molecules are oriented parallel
to each other, such that diene's single bond (one which connects two double bonds) is parallel to dienophile's double or triple bond
. It makes no difference whether the dienophile is positioned above or below the diene. The single substituent (or cis-substituents on the dienophile) is oriented to point in the direction of diene's pi-system. This is the endo transition state (pictured below). If these substituents are pointed away from the diene
, this would be the exo transition state.
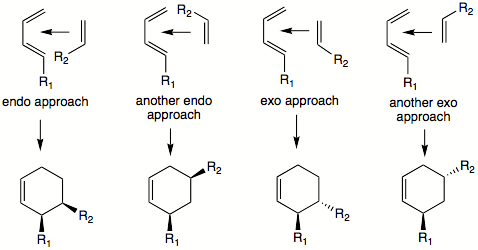 Using the 'cis principle' it is understood that cis-substituents on dienophile, for example, will end up on same side of the molecule. It is not obvious where the substituent
Using the 'cis principle' it is understood that cis-substituents on dienophile, for example, will end up on same side of the molecule. It is not obvious where the substituent
s on both diene
and dienophile will end up relative to each other. To predict the cis or trans orientation of substituents that are coming from different molecules we have to examine possible transition state
s. The most stable transition state will lead to the major product. Transition states will also dictate the relative orientation of the diene's and dienophile's substituents on the product ring. In some cases another rule can be applied: the endo addition rule. According to this rule, the most stable transition state
results when there is a 'maximum accumulation of double bonds'. This rule is not always followed. It most often applies when dealing with cyclic diene
s and dienophiles. For example, the DA reaction of cyclopentadiene
and maleic anhydride
yields over 95% of the endo
product.
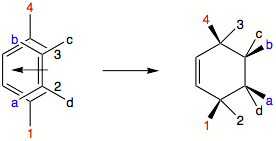
It is important to note that labels "exo" and "endo" relate to the orientation of substituent
s in the transition state
and not to a specific orientation of substituents in the product molecule. In each individual case, the transition state has to be examined to see the most favored relative orientation of substituents. It is not true for the endo transition state that the substituents on dienophile and 1,4-substituents on diene will always point towards the same side of the newly formed ring. "Endo" and "exo" define specific transition states, not orientation of substituents. In the picture below, it just happens that the endo transition state will yield substituents on same side of the ring. This is not always so. In the case of maleic anhydride
and cyclopentadiene
the endo product will have the R groups of the diene
and dienophile oriented toward the opposite sides of the newly formed ring.
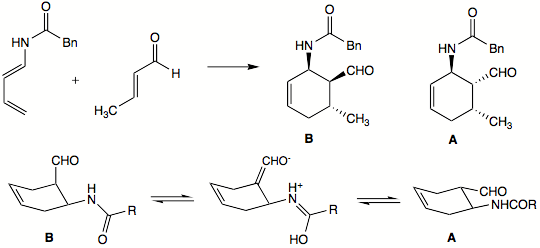
 The exo product can predominate, however, for some reactions. This can happen when the resulting endo product can easily dissociate back into the starting material. In such reactions, the exo product predominates with extended reaction times because the exo product is thermodynamically favored
The exo product can predominate, however, for some reactions. This can happen when the resulting endo product can easily dissociate back into the starting material. In such reactions, the exo product predominates with extended reaction times because the exo product is thermodynamically favored
. In other cases, the endo product can convert to what would be the exo product of the reaction. In the example below, endo product B was the only one isolated after the Diels–Alder reaction. However, letting the reaction go for prolonged periods of time also yielded substantial amounts of exo product A. The authors speculated that endo product B can epimer
ize to exo product A in the following way:
 In summary, diastereoselectivity is based on the postulation of the transition state
In summary, diastereoselectivity is based on the postulation of the transition state
. For any given DA reaction, one can imagine one possible transition state being favored over the other due to steric, stereoelectronic, and complexing factors. Thus, predictions can be made on the identity of major product of a particular DA reaction by looking at the starting material available.
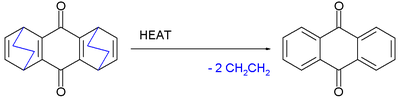
and 1,3-cyclohexadiene
at elevated temperatures eliminates ethylene
to form anthraquinone
.
between an electron-rich dienophile and an electron-poor diene
.
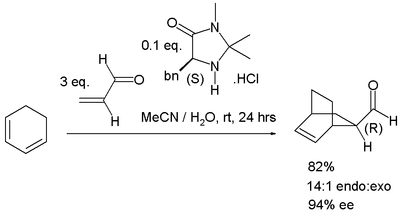
is possible with catalysts based on an imidazoline
skeleton (the MacMillan catalyst) for instance in the reaction of cyclohexadiene
with acrolein
:
 Diels–Alder reactions also lend themselves to chiral synthesis
Diels–Alder reactions also lend themselves to chiral synthesis
with chiral auxiliaries
. In one research effort, the auxiliary is derived from L-asparagine
. The telescopic synthesis with cyclopentadiene
and acrylic acid
yields the DA adduct with three stereocenter
s as predominantly the endo conformer and with 54% ee
.
Lewis acid
s (AlCl3
, ZnCl2
, and others) act as catalysts by coordinating to the dienophile. The complexed dienophile becomes more electrophilic and more reactive toward the diene. This increases the rate and often the stereoselectivity of a DA reaction.
Cycloaddition
A cycloaddition is a pericyclic chemical reaction, in which "two or more unsaturated molecules combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity." The resulting reaction is a cyclization reaction.Cycloadditions are usually described by the...
) between a conjugated diene
Diene
In organic chemistry a diene or diolefin is a hydrocarbon that contains two carbon double bonds.Conjugated dienes are functional groups, with a general formula of CnH2n-2. Dienes and alkynes are functional isomers...
and a substituted alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
, commonly termed the dienophile, to form a substituted cyclohexene
Cyclohexene
Cyclohexene is a hydrocarbon with the formula C6H10. This cycloalkene is a colorless liquid with a sharp smell. It is an intermediate in various industrial processes...
system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
. Some of the Diels–Alder reactions are reversible; the decomposition reaction of the cyclic system is then called the retro-Diels–Alder. For example, retro-Diels–Alder compounds are commonly observed when a Diels–Alder product is analyzed via mass spectrometry
Mass spectrometry
Mass spectrometry is an analytical technique that measures the mass-to-charge ratio of charged particles.It is used for determining masses of particles, for determining the elemental composition of a sample or molecule, and for elucidating the chemical structures of molecules, such as peptides and...
.
Otto Paul Hermann Diels
Otto Diels
Otto Paul Hermann Diels was a German chemist. He was the son of a professor of philology at the University of Berlin, where he himself earned his doctorate in chemistry, in the group of Emil Fischer....
and Kurt Alder
Kurt Alder
Kurt Alder was a German chemist and Nobel laureate.-Biography:Alder was born in the industrial area of Königshütte, Silesia , where he received his early schooling...
first documented the novel reaction in 1928 for which they were awarded the Nobel Prize in Chemistry
Nobel Prize in Chemistry
The Nobel Prize in Chemistry is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for outstanding contributions in chemistry, physics, literature,...
in 1950 for their work on the eponymous reaction.
The Diels–Alder reaction is generally considered the "Mona Lisa" of reactions in organic chemistry since it requires very little energy to create a cyclohexene
Cyclohexene
Cyclohexene is a hydrocarbon with the formula C6H10. This cycloalkene is a colorless liquid with a sharp smell. It is an intermediate in various industrial processes...
ring, which is useful in many other organic reactions.
Reaction mechanism
The reaction occurs via a single transition stateTransition state
The transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest energy along this reaction coordinate. At this point, assuming a perfectly irreversible reaction, colliding reactant molecules will always...
, which has a smaller volume than either the starting materials or the product. It is an associative type of reaction, and it is sped up by very high pressures. Diels–Alder is an example of a pericyclic reaction.
Some free-radical versions of this reaction have been observed, though these are not Diels–Alder reactions since the stereochemistry
Stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms within molecules. An important branch of stereochemistry is the study of chiral molecules....
at the carbons is scrambled. These are step-wise reactions of the free radicals which form the new bonds in at least two steps. An example of this type of reaction is the reaction of selenobenzophenone with a 1,3-diene (See: thioketones).
The diene
The dieneDiene
In organic chemistry a diene or diolefin is a hydrocarbon that contains two carbon double bonds.Conjugated dienes are functional groups, with a general formula of CnH2n-2. Dienes and alkynes are functional isomers...
component in the Diels–Alder reaction can be open-chain or cyclic and it can have many different kinds of substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
s. There is only one limitation: it must be able to exist in the s-cis conformation
Geometric isomerism
In organic chemistry, cis/trans isomerism or geometric isomerism or configuration isomerism or E/Z isomerism is a form of stereoisomerism describing the orientation of functional groups within a molecule...
. Butadiene itself normally prefers the s-trans conformation, with the two double bonds as far away from each other as possible. If there are substituents larger than hydrogen then steric hindrance may influence the relative stabilities of the conformations. For simple cases, the barrier to rotation about the central bond is small and rotation to the less favourable but reactive s-cis
conformation is rapid.
Cyclic dienes that are permanently in the s-cis conformation are exceptionally reactive in Diels–Alder reactions (cyclopentadiene
Cyclopentadiene
Cyclopentadiene is an organic compound with the formula C5H6. This colorless liquid has a strong and unpleasant odor. At room temperature, this cyclic diene dimerizes over the course of hours to give dicyclopentadiene via a Diels–Alder reaction...
is a classic example), while cyclic dienes that are permanently in the s-trans conformation and cannot adopt the s-cis conformation will not undergo the Diels–Alder reaction at all. An especially reactive diene is Danishefsky’s diene
Danishefsky’s diene
Danishefsky’s diene is an organosilicon compound and a diene with the formal name trans-1-methoxy-3-trimethylsilyloxy-1,3-butadiene named after Samuel J. Danishefsky. Because the diene is very electron-rich it is a very reactive reagent in Diels-Alder reactions...
.
Dendralenes
Dendralenes
A dendralene is a discrete acyclic cross-conjugated polyene. The simplest dendralene is buta-1,3-diene or [2]dendralene followed by [3]dendralene , [4]dendralene and [5]dendralene and so forth. [2]dendralene is the only one not cross-conjugated.The name dendralene is pulled together from the...
are a new class of experimental DA dienes.
Unstable dienes, such as o-quinodimethane, can be generated in situ
In situ
In situ is a Latin phrase which translated literally as 'In position'. It is used in many different contexts.-Aerospace:In the aerospace industry, equipment on board aircraft must be tested in situ, or in place, to confirm everything functions properly as a system. Individually, each piece may...
. Aromatic stabilization in the product of a DA reaction using such a diene is, in some cases, the reason behind the very high reactivity and lack of stability of such diene. The use of such unstable dienes is advantageous, despite the trouble, in that the products will contain newly formed aromatic six-membered rings.
Benzenoid compounds rarely undergo DA reactions and often require very reactive dienophiles. One example of such rare reaction is the Wagner-Jauregg reaction
Wagner-Jauregg reaction
The Wagner-Jauregg reaction is a classic organic reaction in organic chemistry, named after Theodor Wagner-Jauregg, describing the double Diels–Alder reaction of 2 equivalents of maleic anhydride with a 1,1-diarylethylene...
The dienophile
In a typical Diels–Alder reaction, the dienophile has an electron-withdrawing group conjugatedConjugated system
In chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds, which in general may lower the overall energy of the molecule and increase stability. Lone pairs, radicals or carbenium ions may be part of the...
to the alkene. Though common, this feature is not exclusive of Diels–Alder dienophiles. There must be some extra conjugation, at least a phenyl group or chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
atom. The dienophile can be activated by a Lewis acid
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
such as niobium pentachloride.
Cyclopentadiene
Cyclopentadiene
Cyclopentadiene is an organic compound with the formula C5H6. This colorless liquid has a strong and unpleasant odor. At room temperature, this cyclic diene dimerizes over the course of hours to give dicyclopentadiene via a Diels–Alder reaction...
does not react with cyclohexenone
Cyclohexenone
Cyclohexenone is an organic compound which is a versatile intermediate used in the synthesis of a variety of chemical products such as pharmaceuticals and fragrances...
in ethyl acetate
Ethyl acetate
Ethyl acetate is the organic compound with the formula CH3COOCH2CH3. This colorless liquid has a characteristic sweet smell and is used in glues, nail polish removers, and cigarettes...
unless the Lewis acid is present. The yield improves when reaction temperature is lowered to −78°C because polymerization
Polymerization
In polymer chemistry, polymerization is a process of reacting monomer molecules together in a chemical reaction to form three-dimensional networks or polymer chains...
side reactions are prevented. Niobium pentachloride catalysis gives only the endo conformer. The same reaction with aluminium chloride
Aluminium chloride
Aluminium chloride is the main compound of aluminium and chlorine. It is white, but samples are often contaminated with iron trichloride, giving it a yellow colour. The solid has a low melting and boiling point. It is mainly produced and consumed in the production of aluminium metal, but large...
results in an endo and exo mixture. Many of these Lewis acid
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
s are not good catalysts for the reaction of α,β-unsaturated carbonyls, this is because the carbonyl oxygen binds too tightly to the metal centre. A far better catalyst for such a system is a combination of silver
Silver
Silver is a metallic chemical element with the chemical symbol Ag and atomic number 47. A soft, white, lustrous transition metal, it has the highest electrical conductivity of any element and the highest thermal conductivity of any metal...
perchlorate
Perchlorate
Perchlorates are the salts derived from perchloric acid . They occur both naturally and through manufacturing. They have been used as a medicine for more than 50 years to treat thyroid gland disorders. They are used extensively within the pyrotechnics industry, and ammonium perchlorate is also a...
and Lawesson's reagent
Lawesson's reagent
Lawesson's reagent, or LR, is a chemical compound used in organic synthesis as a thiation agent. Lawesson's reagent was first made popular by Sven-Olov Lawesson, who did not, however, invent it. Lawesson's reagent was first made in 1956 during a systematic study of the reactions of arenes with...
in cold dichloromethane
Dichloromethane
Dichloromethane is an organic compound with the formula CH2Cl2. This colorless, volatile liquid with a moderately sweet aroma is widely used as a solvent. Although it is not miscible with water, it is miscible with many organic solvents...
.
It is well known that it is possible to use heteroatom
Heteroatom
In organic chemistry, a heteroatom is any atom that is not carbon or hydrogen. Usually, the term is used to indicate that non-carbon atoms have replaced carbon in the backbone of the molecular structure...
containing dienophiles for Diels–Alder reactions, for instance Lawesson's reagent
Lawesson's reagent
Lawesson's reagent, or LR, is a chemical compound used in organic synthesis as a thiation agent. Lawesson's reagent was first made popular by Sven-Olov Lawesson, who did not, however, invent it. Lawesson's reagent was first made in 1956 during a systematic study of the reactions of arenes with...
(and diferrocenyl dithiadiphosphetane disulfide) can react with 1,3-dienes to form six membered ring adducts. Also selenoketones and thioketones are able to react in the same way with 1,3-dienes. Imines are reactants in the Aza Diels–Alder reaction and carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
groups the reactant in Oxo Diels–Alder reaction
Oxo Diels–Alder reaction
An Oxo Diels–Alder reaction is an organic reaction and a variation of the Diels-Alder reaction in which a suitable diene reacts with an aldehyde to form a dihydropyran ring...
s. These reactions are collectively called hetero Diels–Alder reactions.
Dienophiles can be chosen to contain a "masked functionality". The dienophile undergoes Diels–Alder reaction with a diene introducing such a functionality onto the product molecule. A series of reactions then follow to transform the functionality into a desirable group. The end product cannot not be made in a single DA step because equivalent dienophile is either unreactive or inaccessible. An example of such approach is the use of α-chloroacrylonitrile (CH2=CClCN). When reacted with a diene, this dienophile will introduce alpha-chloronitrile functionality onto the product molecule. This is a "masked functionality" which can be then hydrolyzed to form a ketone. α-Chloroacrylonitrile dienophile is an equivalent of ketene
Ketene
A ketene is an organic compound of the form R'RC=C=O. The term is also used specifically to mean ethenone, the simplest ketene, where R' and R are hydrogen atoms.Ketenes were first studied as a class by Hermann Staudinger.-Formation:...
dienophile (CH2=C=O), which would produce same product in one DA step. The problem is that ketene itself cannot be used in Diels–Alder reactions because it reacts with dienes in unwanted manner (by [2+2] cycloaddition
Cycloaddition
A cycloaddition is a pericyclic chemical reaction, in which "two or more unsaturated molecules combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity." The resulting reaction is a cyclization reaction.Cycloadditions are usually described by the...
), and therefore "masked functionality" approach has to be used.
Other such functionalities are phosphonium
Phosphonium
The phosphonium cation describes positively charged polyatomic cations with the chemical formula . Salts of the parent PH4+ are rarely encountered, but this ion is an intermediate in the preparation of the industrially useful tetrakisphosphonium chloride:Organic phosphonium salts are common...
substituents (yielding exocyclic double bonds after Wittig reaction
Wittig reaction
The Wittig reaction is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide to give an alkene and triphenylphosphine oxide....
), various sulfoxide
Sulfoxide
A sulfoxide is a chemical compound containing a sulfinyl functional group attached to two carbon atoms. Sulfoxides can be considered as oxidized sulfides...
and sulfonyl
Sulfonyl
A sulfonyl group can refer either to a functional group found primarily in sulfones or to a substituent obtained from a sulfonic acid by the removal of the hydroxyl group similarly to acyl groups...
functionalities (both are acetylene
Acetylene
Acetylene is the chemical compound with the formula C2H2. It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in pure form and thus is usually handled as a solution.As an alkyne, acetylene is unsaturated because...
equivalents), and nitro groups (ketene equivalents).
Heterodienophiles
No major loss in reactivity of dienophile is seen when one, or both, of the carbons are substituted for another variety of atom. CarbonylCarbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
groups, for example, can successfully react with dienes to yield pyran
Pyran
In chemistry, a pyran, or oxine, is a six-membered heterocyclic, non-aromatic ring, consisting of five carbon atoms and one oxygen atom and containing two double bonds. The molecular formula is C5H6O. There are two isomers of pyran that differ by the location of the double bonds...
oid rings. Generally, the endo transition state
Transition state
The transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest energy along this reaction coordinate. At this point, assuming a perfectly irreversible reaction, colliding reactant molecules will always...
is favored in this case.
Nitroso compounds (N=O) react to form oxazine-like compounds (cyclic molecules with nitrogen and oxygen present in the six-membered ring). Another group of dienophiles successfully used for Diels–Alder reactions is imine
Imine
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
s. Such reactions are useful for preparation of alkaloid
Alkaloid
Alkaloids are a group of naturally occurring chemical compounds that contain mostly basic nitrogen atoms. This group also includes some related compounds with neutral and even weakly acidic properties. Also some synthetic compounds of similar structure are attributed to alkaloids...
and other polycyclic compounds.
Chlorosulfonyl isocyanate
Chlorosulfonyl isocyanate
Chlorosulfonyl isocyanate is the chemical compound ClSO2NCO, known as CSI. This compound is a versatile reagent in organic synthesis.-Preparation, structure, handling:...
can be utilized as a dienophile to prepare Vince Lactam
Vince lactam
Vince lactam is the commercial name given to the bicyclic molecule γ-lactam 2-azabicyclo[2.2.1]hept-5-en-3-one. This lactam is a versatile chemical intermediate used in organic and medicinal chemistry. It is used as a synthetic precursor for three drugs...
.
Stereoselectivity in DA reactions
Diels–Alder reactions can lead to formation of a variety of structural isomerIsomer
In chemistry, isomers are compounds with the same molecular formula but different structural formulas. Isomers do not necessarily share similar properties, unless they also have the same functional groups. There are many different classes of isomers, like stereoisomers, enantiomers, geometrical...
s and stereoisomers (enantiomer
Enantiomer
In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable , much as one's left and right hands are the same except for opposite orientation. It can be clearly understood if you try to place your hands one over the other without...
s and diastereomer
Diastereomer
Diastereomers are stereoisomers that are not enantiomers.Diastereomerism occurs when two or more stereoisomers of a compound have different configurations at one or more of the equivalent stereocenters and are not mirror images of each other.When two diastereoisomers differ from each other at...
s). Identity of major products can usually be predicted, however.
In unsymmetrically substituted diene and dienophile, pseudo-ortho
Arene substitution patterns
Arene substitution patterns are part of organic chemistry IUPAC nomenclature and pinpoint the position of substituents other than hydrogen in relation to each other on an aromatic hydrocarbon.- Ortho, meta, and para substitution :...
and para
Arene substitution patterns
Arene substitution patterns are part of organic chemistry IUPAC nomenclature and pinpoint the position of substituents other than hydrogen in relation to each other on an aromatic hydrocarbon.- Ortho, meta, and para substitution :...
orientations in products are usually favored over meta
Arene substitution patterns
Arene substitution patterns are part of organic chemistry IUPAC nomenclature and pinpoint the position of substituents other than hydrogen in relation to each other on an aromatic hydrocarbon.- Ortho, meta, and para substitution :...
orientation. A particular preference in location of substitutents in the product can, in some cases, be explained in terms of frontier orbital theory. Most commonly, diene
Diene
In organic chemistry a diene or diolefin is a hydrocarbon that contains two carbon double bonds.Conjugated dienes are functional groups, with a general formula of CnH2n-2. Dienes and alkynes are functional isomers...
bears an electron-releasing group (ERG) and dienophile bears an electron-withdrawing group (EWG). The strongest interaction takes place between HOMO
Homo
Homo may refer to:*the Greek prefix ὅμο-, meaning "the same"*the Latin for man, human being*Homo, the taxonomical genus including modern humans...
of diene and LUMO
Lumo
Lumo is a 2007 documentary film about twenty-year-old Lumo Sinai, a woman who fell victim to "Africa's First World War." While returning home one day, Lumo and another woman were gang-raped by a group of soldiers fighting for control of the Democratic Republic of the Congo during the 1994 Rwandan...
of dienophile. Carbons that have the highest coefficients in two frontier orbitals will begin to bond; therefore these carbons will direct the orientation of substituents and thus identity of major product of a DA reaction.
Dealing with the actual frontier orbital coefficients can be avoided since the preferred orientation in product can be described in terms of partial positive and negative charges that exist in diene and dienophile. Carbon with a partial negative charge will interact most readily with carbon bearing a partial positive charge. Therefore those two carbons will start coming together, thus dictating the relative orientation of substituents. The existence of partial positive/negative charge can always be determined by drawing resonance contributors for diene and dienophile, taking their ERG and EWG into consideration.
The initial potential of the reaction was utilized in the form of insecticides, which ultimately led to the endo rule. Otto Diels’ and Kurt Alder’s reaction allowed for the production of weapons against agricultural pests to be fully realized in the 1930s. Most of these chemicals contained chlorine, of which two are called Dieldrin
Dieldrin
Dieldrin is a chlorinated hydrocarbon originally produced in 1948 by J. Hyman & Co, Denver, as an insecticide. Dieldrin is closely related to aldrin, which reacts further to form dieldrin. Aldrin is not toxic to insects; it is oxidized in the insect to form dieldrin which is the active compound...
and Aldrin
Aldrin
Aldrin is an organochlorine insecticide that was widely used until the 1970s, when it was banned in most countries. It is a colourless solid. Before the ban, it was heavily used as a pesticide to treat seed and soil...
after the appropriate individuals. These chemicals have been long-since discontinued because of their toxicity to not only invertebrates, but to higher orders of organisms as well. Fortunately, insecticide use may continue unabated with the recent introduction of various safe treatments. Though insecticides like Dieldrin and Aldrin cause a slew of cardiac and respiratory illnesses (as well as reproductive failure), they served as an important step towards understanding why the endo product was the major yield. The study of the insecticide’s fused norcamphane rings became a highly popular topic in the 1930s and 1940s. Oxidative degradation revealed high specificity of the stereochemistry; after much research by Kurt Alder and H.F. Rickert, it became clear that steric hindrance is not as important in Diels–Alder reactions as it is in other reactions. This led to the secondary orbital explanation as well as a satisfactory hypothesis that elucidated polymerization of certain Diels–Alder adducts.

Cis principle
According to the cis principle or the Alder–Stein rules formulated by Alder and Stein in 1937, the stereochemistryStereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms within molecules. An important branch of stereochemistry is the study of chiral molecules....
of substituents in the starting material is retained in the product. This means that if a cis-dienophile is reacted, both of the cis-substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
s will end up on same side (face) of the product ring. Trans-dienophile will yield a product where both of trans-substituents (that came originally from the dienophile) will be on different sides of the product ring. The same principle applies to diene
Diene
In organic chemistry a diene or diolefin is a hydrocarbon that contains two carbon double bonds.Conjugated dienes are functional groups, with a general formula of CnH2n-2. Dienes and alkynes are functional isomers...
s. Trans, trans or cis, cis 1,4-substituents will end up on same side of the ring, whereas trans, cis 1,4-substituents will be oriented towards different faces of the ring.
Besides the ortho/meta/para-forming orientations, the diene
Diene
In organic chemistry a diene or diolefin is a hydrocarbon that contains two carbon double bonds.Conjugated dienes are functional groups, with a general formula of CnH2n-2. Dienes and alkynes are functional isomers...
and dienophile may arrange themselves in different ways to yield exo and endo
Endo-exo isomerism
Endo-exo isomerism is a special type of isomerism found in organic compounds with a substituent on a bridged ring system. The prefix endo is reserved for the isomer with the substituent located closest, or "syn," to the longest bridge. The prefix exo is reserved for the isomer with the substituent...
transition state
Transition state
The transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest energy along this reaction coordinate. At this point, assuming a perfectly irreversible reaction, colliding reactant molecules will always...
s which result in different products. To determine which is the endo and which is the exo transition state, the two molecules are oriented parallel
Parallel (geometry)
Parallelism is a term in geometry and in everyday life that refers to a property in Euclidean space of two or more lines or planes, or a combination of these. The assumed existence and properties of parallel lines are the basis of Euclid's parallel postulate. Two lines in a plane that do not...
to each other, such that diene's single bond (one which connects two double bonds) is parallel to dienophile's double or triple bond
Triple bond
A triple bond in chemistry is a chemical bond between two chemical elements involving six bonding electrons instead of the usual two in a covalent single bond. The most common triple bond, that between two carbon atoms, can be found in alkynes. Other functional groups containing a triple bond are...
. It makes no difference whether the dienophile is positioned above or below the diene. The single substituent (or cis-substituents on the dienophile) is oriented to point in the direction of diene's pi-system. This is the endo transition state (pictured below). If these substituents are pointed away from the diene
Diene
In organic chemistry a diene or diolefin is a hydrocarbon that contains two carbon double bonds.Conjugated dienes are functional groups, with a general formula of CnH2n-2. Dienes and alkynes are functional isomers...
, this would be the exo transition state.
Endo addition rule

Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
s on both diene
Diene
In organic chemistry a diene or diolefin is a hydrocarbon that contains two carbon double bonds.Conjugated dienes are functional groups, with a general formula of CnH2n-2. Dienes and alkynes are functional isomers...
and dienophile will end up relative to each other. To predict the cis or trans orientation of substituents that are coming from different molecules we have to examine possible transition state
Transition state
The transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest energy along this reaction coordinate. At this point, assuming a perfectly irreversible reaction, colliding reactant molecules will always...
s. The most stable transition state will lead to the major product. Transition states will also dictate the relative orientation of the diene's and dienophile's substituents on the product ring. In some cases another rule can be applied: the endo addition rule. According to this rule, the most stable transition state
Transition state
The transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest energy along this reaction coordinate. At this point, assuming a perfectly irreversible reaction, colliding reactant molecules will always...
results when there is a 'maximum accumulation of double bonds'. This rule is not always followed. It most often applies when dealing with cyclic diene
Diene
In organic chemistry a diene or diolefin is a hydrocarbon that contains two carbon double bonds.Conjugated dienes are functional groups, with a general formula of CnH2n-2. Dienes and alkynes are functional isomers...
s and dienophiles. For example, the DA reaction of cyclopentadiene
Cyclopentadiene
Cyclopentadiene is an organic compound with the formula C5H6. This colorless liquid has a strong and unpleasant odor. At room temperature, this cyclic diene dimerizes over the course of hours to give dicyclopentadiene via a Diels–Alder reaction...
and maleic anhydride
Maleic anhydride
Maleic anhydride is an organic compound with the formula C2H22O. It is the acid anhydride of maleic acid and in its pure state it is a colourless or white solid with an acrid odour....
yields over 95% of the endo
Endo-exo isomerism
Endo-exo isomerism is a special type of isomerism found in organic compounds with a substituent on a bridged ring system. The prefix endo is reserved for the isomer with the substituent located closest, or "syn," to the longest bridge. The prefix exo is reserved for the isomer with the substituent...
product.
It is important to note that labels "exo" and "endo" relate to the orientation of substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
s in the transition state
Transition state
The transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest energy along this reaction coordinate. At this point, assuming a perfectly irreversible reaction, colliding reactant molecules will always...
and not to a specific orientation of substituents in the product molecule. In each individual case, the transition state has to be examined to see the most favored relative orientation of substituents. It is not true for the endo transition state that the substituents on dienophile and 1,4-substituents on diene will always point towards the same side of the newly formed ring. "Endo" and "exo" define specific transition states, not orientation of substituents. In the picture below, it just happens that the endo transition state will yield substituents on same side of the ring. This is not always so. In the case of maleic anhydride
Maleic anhydride
Maleic anhydride is an organic compound with the formula C2H22O. It is the acid anhydride of maleic acid and in its pure state it is a colourless or white solid with an acrid odour....
and cyclopentadiene
Cyclopentadiene
Cyclopentadiene is an organic compound with the formula C5H6. This colorless liquid has a strong and unpleasant odor. At room temperature, this cyclic diene dimerizes over the course of hours to give dicyclopentadiene via a Diels–Alder reaction...
the endo product will have the R groups of the diene
Diene
In organic chemistry a diene or diolefin is a hydrocarbon that contains two carbon double bonds.Conjugated dienes are functional groups, with a general formula of CnH2n-2. Dienes and alkynes are functional isomers...
and dienophile oriented toward the opposite sides of the newly formed ring.

Thermodynamic reaction control
Thermodynamic reaction control or kinetic reaction control in a chemical reaction can decide the composition in a reaction product mixture when competing pathways lead to different products and the reaction conditions influence the selectivity...
. In other cases, the endo product can convert to what would be the exo product of the reaction. In the example below, endo product B was the only one isolated after the Diels–Alder reaction. However, letting the reaction go for prolonged periods of time also yielded substantial amounts of exo product A. The authors speculated that endo product B can epimer
Epimer
In chemistry, epimers are diastereomers that differ in configuration of only one stereogenic center. Diastereomers are a class of stereoisomers that are non-superposable, non-mirror images of one another....
ize to exo product A in the following way:

Transition state
The transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest energy along this reaction coordinate. At this point, assuming a perfectly irreversible reaction, colliding reactant molecules will always...
. For any given DA reaction, one can imagine one possible transition state being favored over the other due to steric, stereoelectronic, and complexing factors. Thus, predictions can be made on the identity of major product of a particular DA reaction by looking at the starting material available.
Retro-Diels–Alder reactions
DA reactions are reversible and in a retro-Diels–Alder reaction the diene and alkene are reformed. One representative reaction is the Rickert–Alder reaction in which, thanks to favorable rearomatization, the oxidized cycloadduct of quinoneQuinone
A quinone is a class of organic compounds that are formally "derived from aromatic compounds [such as benzene or naphthalene] by conversion of an even number of –CH= groups into –C– groups with any necessary rearrangement of double bonds," resulting in "a fully conjugated cyclic dione structure."...
and 1,3-cyclohexadiene
1,3-Cyclohexadiene
1,3-Cyclohexadiene is a highly flammable cycloalkene that occurs as a colorless clear liquid. Its refractive index is 1.475 .It can be used as a hydrogen donor in transfer hydrogenation, since its conversion to benzene + hydrogen is in fact exothermic .Despite this apparent instability with respect...
at elevated temperatures eliminates ethylene
Ethylene
Ethylene is a gaseous organic compound with the formula . It is the simplest alkene . Because it contains a carbon-carbon double bond, ethylene is classified as an unsaturated hydrocarbon. Ethylene is widely used in industry and is also a plant hormone...
to form anthraquinone
Anthraquinone
Anthraquinone, also called anthracenedione or dioxoanthracene is an aromatic organic compound with formula . Several isomers are possible, each of which can be viewed as a quinone derivative...
.

Inverse electron demand Diels-Alder reaction
Unlike in the Diels-Alder reaction, the Inverse electron demand Diels-Alder reaction is a cycloadditionCycloaddition
A cycloaddition is a pericyclic chemical reaction, in which "two or more unsaturated molecules combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity." The resulting reaction is a cyclization reaction.Cycloadditions are usually described by the...
between an electron-rich dienophile and an electron-poor diene
Diene
In organic chemistry a diene or diolefin is a hydrocarbon that contains two carbon double bonds.Conjugated dienes are functional groups, with a general formula of CnH2n-2. Dienes and alkynes are functional isomers...
.
Asymmetric DA reactions
In asymmetric Diels–Alder reactions only one of two possible enantiomers is formed. Asymmetric catalysis by organocatalysisOrganocatalysis
In organic chemistry, the term Organocatalysis refers to a form of catalysis, whereby the rate of a chemical reaction is increased by an organic catalyst referred to as an "organocatalyst" consisting of carbon, hydrogen, sulfur and other nonmetal elements found in organic compounds...
is possible with catalysts based on an imidazoline
Imidazoline
Imidazoline is a nitrogen-containing heterocycle with formula C3H6N2, derived from imidazole. The ring contains an imine bond, and the carbons at the 4 and 5 positions are singly bonded, rather than doubly bonded for the case of imidazole...
skeleton (the MacMillan catalyst) for instance in the reaction of cyclohexadiene
1,3-Cyclohexadiene
1,3-Cyclohexadiene is a highly flammable cycloalkene that occurs as a colorless clear liquid. Its refractive index is 1.475 .It can be used as a hydrogen donor in transfer hydrogenation, since its conversion to benzene + hydrogen is in fact exothermic .Despite this apparent instability with respect...
with acrolein
Acrolein
Acrolein is the simplest unsaturated aldehyde. It is produced widely but is most often immediately reacted with other products due to its instability and toxicity...
:

Chiral synthesis
Enantioselective synthesis, also called chiral synthesis, asymmetric synthesis or stereoselective synthesis, is organic synthesis that introduces one or more new and desired elements of chirality...
with chiral auxiliaries
Chiral auxiliary
A chiral auxiliary is a chemical compound or unit that is temporarily incorporated into an organic synthesis so that it can be carried out asymmetrically with the selective formation of one of two enantiomers...
. In one research effort, the auxiliary is derived from L-asparagine
Asparagine
Asparagine is one of the 20 most common natural amino acids on Earth. It has carboxamide as the side-chain's functional group. It is not an essential amino acid...
. The telescopic synthesis with cyclopentadiene
Cyclopentadiene
Cyclopentadiene is an organic compound with the formula C5H6. This colorless liquid has a strong and unpleasant odor. At room temperature, this cyclic diene dimerizes over the course of hours to give dicyclopentadiene via a Diels–Alder reaction...
and acrylic acid
Acrylic acid
Acrylic acid is an organic compound with the formula CH2=CHCO2H. It is the simplest unsaturated carboxylic acid, consisting of a vinyl group connected directly to a carboxylic acid terminus. This colorless liquid has a characteristic acrid or tart smell. It is miscible with water, alcohols,...
yields the DA adduct with three stereocenter
Stereocenter
A stereocenter or stereogenic center is an atom, bearing groups such that an interchanging of any two groups leads to a stereoisomer.A chirality center is a stereocenter consisting of an atom holding a set of ligands in a spatial arrangement which is not superposable on its mirror image...
s as predominantly the endo conformer and with 54% ee
Enantiomeric excess
The enantiomeric excess of a substance is a measure of how pure it is. In this case, the impurity is the undesired enantiomer .-Definition:...
.
Lewis acid
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
s (AlCl3
Aluminium chloride
Aluminium chloride is the main compound of aluminium and chlorine. It is white, but samples are often contaminated with iron trichloride, giving it a yellow colour. The solid has a low melting and boiling point. It is mainly produced and consumed in the production of aluminium metal, but large...
, ZnCl2
Zinc chloride
Zinc chloride is the name of chemical compound with the formula ZnCl2 and its hydrates. Zinc chlorides, of which nine crystalline forms are known, are colorless or white, and are highly soluble in water. ZnCl2 itself is hygroscopic and even deliquescent. Samples should therefore be protected from...
, and others) act as catalysts by coordinating to the dienophile. The complexed dienophile becomes more electrophilic and more reactive toward the diene. This increases the rate and often the stereoselectivity of a DA reaction.
External links
- Asymmetric Hetero-Diels–Alder Reactions
- Semi-empirical calculations of the Diels–Alder reaction.
- Endo Addition Rule

