Diene
Encyclopedia
In organic chemistry
a diene (icon ) or diolefin (d ) is a hydrocarbon
that contains two carbon double bonds.
Conjugated
dienes are functional group
s, with a general formula of CnH2n-2. Dienes and alkynes are functional isomers. Dienes occur occasionally in nature but are widely used in the polymer
industry.
Compounds that contain more than two double bonds are called polyene
s. Polyenes and dienes, share many of their properties.
In the laboratory, more directed and more delicate processes are employed such as dehydrohalogenation
s and condensations. Myriad methods
have been developed, such as the Whiting reaction
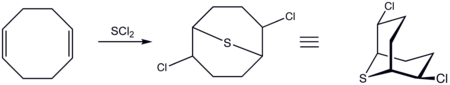
. Families of nonconjugated dienes are derived from the oligomerization and dimerization of conjugated dienes. For example, 1,5-cyclooctadiene and vinylcyclohexene are produced by dimerization of 1,3-butadiene.
Diene-containing fatty acid
s are biosynthesized
from acetyl CoA.
is a related but synthetic monomer.
. Many specialized dienes have been developed to exploit this reactivity for the synthesis of natural product
s, e.g. Danishefsky’s diene
.
reactions. These reactions require metal catalyst:
.
s in organometallic chemistry
. In some cases they serve as placeholder ligands, being removed during a catalytic cycle. For example, the cyclooctadiene ("cod") ligands in bis(cyclooctadiene)nickel(0)
are labile. In some cases, dienes are spectator ligands, remaining coordinated throughout a catalytic cycle and influencing the product distributions. Chiral dienes have also been described.
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
a diene (icon ) or diolefin (d ) is a hydrocarbon
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
that contains two carbon double bonds.
Conjugated
Conjugated system
In chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds, which in general may lower the overall energy of the molecule and increase stability. Lone pairs, radicals or carbenium ions may be part of the...
dienes are functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
s, with a general formula of CnH2n-2. Dienes and alkynes are functional isomers. Dienes occur occasionally in nature but are widely used in the polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
industry.
Classes
Dienes can be divided into three classes, depending on the relative location of the double bonds:- Cumulated dienes have the double bonds sharing a common atom as in a group of compounds called alleneAlleneAn allene is a compound in which one carbon atom has double bonds with each of its two adjacent carbon centres. Allenes are classified as polyenes with cumulated dienes. The parent compound of allene is propadiene. Compounds with an allene-type structure but with more than three carbon atoms are...
s. - Conjugated dienes have conjugatedConjugated systemIn chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds, which in general may lower the overall energy of the molecule and increase stability. Lone pairs, radicals or carbenium ions may be part of the...
double bonds separated by one single bond. - Unconjugated dienes have the double bonds separated by two or more single bonds. They are usually less stable than isomeric conjugated dienes. This can also be known as an isolated diene.
Compounds that contain more than two double bonds are called polyene
Polyene
Polyenes are poly-unsaturated organic compounds that contain one or more sequences of alternating double and single carbon-carbon bonds. These double carbon-carbon bonds interact in a process known as conjugation, which results in an overall lower energy state of the molecule.Organic compounds with...
s. Polyenes and dienes, share many of their properties.
Synthesis of dienes
On an industrial scale, butadiene is prepared by thermal cracking of butanes. In a similarly non-selective process, dicyclopentadiene is obtained from coal tars.In the laboratory, more directed and more delicate processes are employed such as dehydrohalogenation
Dehydrohalogenation
Dehydrohalogenation is an organic reaction from which an alkene is obtained from an alkyl halide . It is also called a β-Elimination reaction and is a type of elimination reaction....
s and condensations. Myriad methods
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
have been developed, such as the Whiting reaction
Whiting reaction
The Whiting reaction is an organic reaction converting a propargyl diol into a diene using lithium aluminium hydride.This organic reduction has been applied in the synthesis of fecapentaene....
. Families of nonconjugated dienes are derived from the oligomerization and dimerization of conjugated dienes. For example, 1,5-cyclooctadiene and vinylcyclohexene are produced by dimerization of 1,3-butadiene.
Diene-containing fatty acid
Fatty acid
In chemistry, especially biochemistry, a fatty acid is a carboxylic acid with a long unbranched aliphatic tail , which is either saturated or unsaturated. Most naturally occurring fatty acids have a chain of an even number of carbon atoms, from 4 to 28. Fatty acids are usually derived from...
s are biosynthesized
Biosynthesis
Biosynthesis is an enzyme-catalyzed process in cells of living organisms by which substrates are converted to more complex products. The biosynthesis process often consists of several enzymatic steps in which the product of one step is used as substrate in the following step...
from acetyl CoA.
Polymerization
The most heavily practiced reaction of alkenes, dienes included, is polymerization. Butadiene is a precursor to rubber used in tires, and isoprene is the precursor to natural rubber. ChloropreneChloroprene
Chloroprene is the common name for the organic compound 2-chlorobuta-1,3-diene, which has the formula CH2=CCl-CH=CH2. This colorless liquid is the monomer for the production of the polymer polychloroprene, a type of synthetic rubber...
is a related but synthetic monomer.
Cycloadditions
An important reaction for conjugated dienes is the Diels-Alder reactionDiels-Alder reaction
The Diels–Alder reaction is an organic chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon...
. Many specialized dienes have been developed to exploit this reactivity for the synthesis of natural product
Natural product
A natural product is a chemical compound or substance produced by a living organism - found in nature that usually has a pharmacological or biological activity for use in pharmaceutical drug discovery and drug design...
s, e.g. Danishefsky’s diene
Danishefsky’s diene
Danishefsky’s diene is an organosilicon compound and a diene with the formal name trans-1-methoxy-3-trimethylsilyloxy-1,3-butadiene named after Samuel J. Danishefsky. Because the diene is very electron-rich it is a very reactive reagent in Diels-Alder reactions...
.
Other addition reactions
Conjugated dienes add reagents such as bromine and hydrogen by both 1,2-addition and 1,4-addition pathways. Addition of polar reagents can generate complex architectures:Metathesis reactions
Nonconjugated dienes are substrates for ring-closing metathesisRing-closing metathesis
Ring-closing metathesis or RCM is a variation on olefin metathesis that allows the closing of previously hard to make rings...
reactions. These reactions require metal catalyst:
Acidity
Conjugated and 1,4-dienes generally are somewhat acidic since deprotonation of both classes gives pentadienyl anions. The acidifying effect of the diene is very pronounced in cyclopentadieneCyclopentadiene
Cyclopentadiene is an organic compound with the formula C5H6. This colorless liquid has a strong and unpleasant odor. At room temperature, this cyclic diene dimerizes over the course of hours to give dicyclopentadiene via a Diels–Alder reaction...
.
As ligands
Dienes are widely used chelating ligandLigand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
s in organometallic chemistry
Organometallic chemistry
Organometallic chemistry is the study of chemical compounds containing bonds between carbon and a metal. Since many compounds without such bonds are chemically similar, an alternative may be compounds containing metal-element bonds of a largely covalent character...
. In some cases they serve as placeholder ligands, being removed during a catalytic cycle. For example, the cyclooctadiene ("cod") ligands in bis(cyclooctadiene)nickel(0)
Bis(cyclooctadiene)nickel(0)
Bisnickel is the organometallic compound with the formula Ni2. This air-sensitive yellow solid is a common source of Ni in chemical synthesis....
are labile. In some cases, dienes are spectator ligands, remaining coordinated throughout a catalytic cycle and influencing the product distributions. Chiral dienes have also been described.