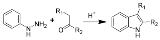
Fischer indole synthesis
Overview
a chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
that produces the aromatic heterocycle
Heterocyclic compound
A heterocyclic compound is a cyclic compound which has atoms of at least two different elements as members of its ring. The counterparts of heterocyclic compounds are homocyclic compounds, the rings of which are made of a single element....
indole
Indole
Indole is an aromatic heterocyclic organic compound. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing pyrrole ring. Indole is a popular component of fragrances and the precursor to many pharmaceuticals. Compounds that contain an...
from a (substituted) phenylhydrazine
Phenylhydrazine
Phenylhydrazine is the chemical compound with the formula C6H5NHNH2. Organic chemists abbreviate the compound as PhNHNH2.- Chemical properties :...
and an aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
or ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
under acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
ic conditions. The reaction was discovered in 1883 by Hermann Emil Fischer
Hermann Emil Fischer
Hermann Emil Fischer, Emil Fischer was a German chemist and 1902 recipient of the Nobel Prize in Chemistry. He discovered the Fischer esterification. He developed the Fischer projection, a symbolic way of drawing asymmetric carbon atoms.-Early years:Fischer was born in Euskirchen, near Cologne,...
. Today antimigraine
Migraine
Migraine is a chronic neurological disorder characterized by moderate to severe headaches, and nausea...
drugs of the triptan
Triptan
Triptans are a family of tryptamine-based drugs used as abortive medication in the treatment of migraines and cluster headaches. They were first introduced in the 1990s...
class are often synthesized by this method.
The choice of acid catalyst is very important. Brønsted acids such as HCl
HCL
HCL or HCl can stand for:* Hairy cell leukemia, an uncommon and slowly progressing B cell leukemia* Hardware compatibility list...
, H2SO4
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
, polyphosphoric acid and p-toluenesulfonic acid
P-Toluenesulfonic acid
p-Toluenesulfonic acid or tosylic acid is an organic compound with the formula CH3C6H4SO3H. It is a white solid that is soluble in water, alcohols, and other polar organic solvents. The 4-CH3C6H4SO2- group is known as the Tosyl group and is often abbreviated as Ts or Tos...
have been used successfully.

