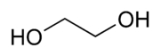
Ethylene glycol
Overview
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
widely used as an automotive
Automobile
An automobile, autocar, motor car or car is a wheeled motor vehicle used for transporting passengers, which also carries its own engine or motor...
antifreeze
Antifreeze
Antifreeze is a freeze preventive used in internal combustion engines and other heat transfer applications, such as HVAC chillers and solar water heaters....
and a precursor to polymers. In its pure form, it is an odorless, colorless, syrupy, sweet-tasting liquid. Ethylene glycol is toxic, and ingestion can result in death.
Ethylene glycol is not to be confused with diethylene glycol
Diethylene glycol
Diethylene glycol is an organic compound with the formula 2O. It is a colorless, practically odorless, poisonous, and hygroscopic liquid with a sweetish taste. It is miscible in water, alcohol, ether, acetone, and ethylene glycol. DEG is a widely used solvent...
, a heavier ether diol
Diol
A diol or glycol is a chemical compound containing two hydroxyl groups A geminal diol has two hydroxyl groups bonded to the same atom...
, or with polyethylene glycol
Polyethylene glycol
Polyethylene glycol is a polyether compound with many applications from industrial manufacturing to medicine. It has also been known as polyethylene oxide or polyoxyethylene , depending on its molecular weight, and under the tradename Carbowax.-Available forms:PEG, PEO, or POE refers to an...
, a nontoxic polyether polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
.
Ethylene glycol was first prepared in 1859 by the French
France
The French Republic , The French Republic , The French Republic , (commonly known as France , is a unitary semi-presidential republic in Western Europe with several overseas territories and islands located on other continents and in the Indian, Pacific, and Atlantic oceans. Metropolitan France...
chemist Charles-Adolphe Wurtz
Charles-Adolphe Wurtz
Adolphe Wurtz was an Alsatian French chemist. He is best remembered for his decades-long advocacy for the atomic theory and for ideas about the structures of chemical compounds, against the skeptical opinions of chemists such as Marcellin Berthelot and Etienne Henri Sainte-Claire Deville...
from ethylene glycol diacetate via saponification
Saponification
Saponification is a process that produces soap, usually from fats and lye. In technical terms, saponification involves base hydrolysis of triglycerides, which are esters of fatty acids, to form the sodium salt of a carboxylate. In addition to soap, such traditional saponification processes...
with potassium hydroxide
Potassium hydroxide
Potassium hydroxide is an inorganic compound with the formula KOH, commonly called caustic potash.Along with sodium hydroxide , this colorless solid is a prototypical strong base. It has many industrial and niche applications. Most applications exploit its reactivity toward acids and its corrosive...
and, in 1860, from the hydration
Hydration reaction
In organic chemistry, a hydration reaction is a chemical reaction in which a hydroxyl group and a hydrogen cation are added to the two carbon atoms bonded together in the carbon-carbon double bond which makes up an alkene functional group. The reaction usually runs in a strong acidic, aqueous...
of ethylene oxide
Ethylene oxide
Ethylene oxide, also called oxirane, is the organic compound with the formula . It is a cyclic ether. This means that it is composed of two alkyl groups attached to an oxygen atom in a cyclic shape . This colorless flammable gas with a faintly sweet odor is the simplest epoxide, a three-membered...
.
Unanswered Questions

