
Battery (electricity)
Encyclopedia
An electrical battery is one or more electrochemical cell
s that convert stored chemical energy
into electrical energy. Since the invention of the first battery (or "voltaic pile
") in 1800 by Alessandro Volta
and especially since the technically improved Daniell cell
in 1836, batteries have become a common power source for many household and industrial applications. According to a 2005 estimate, the worldwide battery industry generates US$
48 billion
in sales each year, with 6% annual growth.
There are two types of batteries: primary batteries (disposable batteries), which are designed to be used once and discarded, and secondary batteries (rechargeable batteries), which are designed to be recharged and used multiple times. Batteries come in many sizes, from miniature cells used to power hearing aid
s and wristwatches to battery banks the size of rooms that provide standby power for telephone exchange
s and computer data center
s.
in 1792, and in 1800 he invented the first battery, a "pile" of many cells in series.
The usage of "battery" to describe electrical devices dates to Benjamin Franklin
, who in 1748 described multiple Leyden jar
s (early electrical capacitor
s) by analogy to a battery of cannons
. Thus Franklin's usage to describe multiple Leyden jars predated Volta's use of multiple galvanic cells. It is speculated, but not established, that several ancient artifacts consisting of copper sheets and iron bars, and known as Baghdad batteries
may have been galvanic cells.
Volta's work was stimulated by the Italian anatomist and physiologist Luigi Galvani
, who in 1780 noticed that dissected frog's legs would twitch when struck by a spark from a Leyden jar
, an external source of electricity. In 1786 he noticed that twitching would occur during lightning storms. After many years Galvani learned how to produce twitching without using any external source of electricity. In 1791 he published a report on "animal electricity." He created an electric circuit consisting of the frog's leg (FL) and two different metals A and B, each metal touching the frog's leg and each other, thus producing the circuit A–FL–B–A–FL–B...etc. In modern terms, the frog's leg served as both the electrolyte
and the sensor
, and the metals served as electrode
s. He noticed that even though the frog was dead, its legs would twitch when he touched them with the metals.
Within a year, Volta realized the frog's moist tissues could be replaced by cardboard soaked in salt water, and the frog's muscular response could be replaced by another form of electrical detection. He already had studied the electrostatic phenomenon of capacitance
, which required measurements of electric charge and of electrical potential ("tension"). Building on this experience, Volta was able to detect electric current through his system, also called a Galvanic cell
. The terminal voltage of a cell that is not discharging is called its electromotive force
(emf), and has the same unit as electrical potential, named (voltage
) and measured in volt
s, in honor of Volta. In 1800, Volta invented the battery by placing many voltaic cells in series, piling them one above the other. This voltaic pile gave a greatly enhanced net emf for the combination, with a voltage of about 50 volts for a 32-cell pile. In many parts of Europe batteries continue to be called piles.
Volta did not appreciate that the voltage was due to chemical reactions. He thought that his cells were an inexhaustible source of energy, and that the associated corrosion effects at the electrodes were a mere nuisance, rather than an unavoidable consequence of their operation, as Michael Faraday
showed in 1834. According to Faraday, cations (positively charged ions) are attracted to the cathode
, and anions (negatively charged ions) are attracted to the anode
.
Although early batteries were of great value for experimental purposes, in practice their voltages fluctuated and they could not provide a large current for a sustained period. Later, starting with the Daniell cell
in 1836, batteries provided more reliable currents and were adopted by industry for use in stationary devices, in particular in telegraph networks where they were the only practical source of electricity, since electrical distribution networks did not exist at the time. These wet cells used liquid electrolytes, which were prone to leakage and spillage if not handled correctly. Many used glass jars to hold their components, which made them fragile. These characteristics made wet cells unsuitable for portable appliances. Near the end of the nineteenth century, the invention of dry cell batteries
, which replaced the liquid electrolyte with a paste, made portable electrical devices practical.
Since then, batteries have gained popularity as they became portable and useful for a variety of purposes.
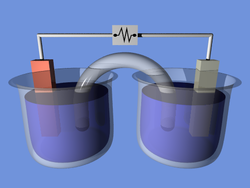 A battery is a device that converts chemical energy directly to electrical energy. It consists of a number of voltaic cells; each voltaic cell consists of two half-cells connected in series by a conductive electrolyte containing anions and cations. One half-cell includes electrolyte and the electrode to which anions (negatively charged ions) migrate, i.e., the anode
A battery is a device that converts chemical energy directly to electrical energy. It consists of a number of voltaic cells; each voltaic cell consists of two half-cells connected in series by a conductive electrolyte containing anions and cations. One half-cell includes electrolyte and the electrode to which anions (negatively charged ions) migrate, i.e., the anode
or negative electrode; the other half-cell includes electrolyte and the electrode to which cations (positively charged ions) migrate, i.e., the cathode
or positive electrode. In the redox
reaction that powers the battery, cations are reduced (electrons are added) at the cathode, while anions are oxidized (electrons are removed) at the anode. The electrodes do not touch each other but are electrically connected by the electrolyte
. Some cells use two half-cells with different electrolytes. A separator between half-cells allows ions to flow, but prevents mixing of the electrolytes.
Each half-cell has an electromotive force (or emf), determined by its ability to drive electric current from the interior to the exterior of the cell. The net emf of the cell is the difference between the emfs of its half-cells, as first recognized by Volta. Therefore, if the electrodes have emfs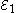 and
and 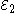 , then the net emf is
, then the net emf is 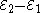 ; in other words, the net emf is the difference between the reduction potential
; in other words, the net emf is the difference between the reduction potential
s of the half-reaction
s.
The electrical driving force or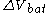 across the terminals of a cell is known as the terminal voltage (difference) and is measured in volt
across the terminals of a cell is known as the terminal voltage (difference) and is measured in volt
s. The terminal voltage of a cell that is neither charging nor discharging is called the open-circuit voltage
and equals the emf of the cell. Because of internal resistance, the terminal voltage of a cell that is discharging is smaller in magnitude than the open-circuit voltage and the terminal voltage of a cell that is charging exceeds the open-circuit voltage. An ideal cell has negligible internal resistance, so it would maintain a constant terminal voltage of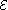 until exhausted, then dropping to zero. If such a cell maintained 1.5 volts and stored a charge of one coulomb then on complete discharge it would perform 1.5 joule
until exhausted, then dropping to zero. If such a cell maintained 1.5 volts and stored a charge of one coulomb then on complete discharge it would perform 1.5 joule
of work. In actual cells, the internal resistance increases under discharge, and the open circuit voltage also decreases under discharge. If the voltage and resistance are plotted against time, the resulting graphs typically are a curve; the shape of the curve varies according to the chemistry and internal arrangement employed.
As stated above, the voltage developed across a cell's terminals depends on the energy release of the chemical reactions of its electrodes and electrolyte. Alkaline and zinc–carbon cells have different chemistries but approximately the same emf of 1.5 volts; likewise NiCd and NiMH cells have different chemistries, but approximately the same emf of 1.2 volts. On the other hand the high electrochemical potential changes in the reactions of lithium
compounds give lithium cells emfs of 3 volts or more.
Some types of primary batteries used, for example, for telegraph
circuits, were restored to operation by replacing the components of the battery consumed by the chemical reaction. Secondary batteries are not indefinitely rechargeable due to dissipation of the active materials, loss of electrolyte and internal corrosion.
Common types of disposable batteries include zinc–carbon batteries and alkaline batteries. In general, these have higher energy densities than rechargeable batteries, but disposable batteries do not fare well under high-drain applications with loads under 75 ohms
(75 Ω).
s that occur during its use. Devices to supply the appropriate current are called chargers or rechargers.
The oldest form of rechargeable battery is the lead–acid battery. This battery is notable in that it contains a liquid in an unsealed container, requiring that the battery be kept upright and the area be well ventilated to ensure safe dispersal of the hydrogen
gas produced by these batteries during overcharging. The lead–acid battery is also very heavy for the amount of electrical energy it can supply. Despite this, its low manufacturing cost and its high surge current levels make its use common where a large capacity (over approximately 10 Ah) is required or where the weight and ease of handling are not concerns.
A common form of the lead–acid battery is the modern car battery
, which can, in general, deliver a peak current of 450 ampere
s. An improved type of liquid electrolyte battery is the sealed valve regulated lead acid (VRLA
) battery, popular in the automotive industry as a replacement for the lead–acid wet cell. The VRLA battery uses an immobilized sulfuric acid electrolyte, reducing the chance of leakage and extending shelf life. VRLA batteries have the electrolyte immobilized, usually by one of two means:
Other portable rechargeable batteries include several "dry cell" types, which are sealed units and are, therefore, useful in appliances such as mobile phone
s and laptop computers
. Cells of this type (in order of increasing power density
and cost) include nickel–cadmium (NiCd), nickel–zinc (NiZn), nickel metal hydride
(NiMH), and lithium-ion (Li-ion) cells. By far, Li-ion has the highest share of the dry cell rechargeable market. Meanwhile, NiMH has replaced NiCd in most applications due to its higher capacity, but NiCd remains in use in power tool
s, two-way radio
s, and medical equipment
. NiZn is a new technology that is not yet well established commercially.
Recent developments include batteries with embedded electronics such as USBCELL, which allows charging an AA cell through a USB connector, and smart battery packs with state-of-charge monitors and battery protection circuits to prevent damage on over-discharge. low self-discharge
(LSD) allows secondary cells to be precharged prior to shipping.
s, electrolytic cell
s, fuel cell
s, flow cells
and voltaic piles.
. Other names are flooded cell, since the liquid covers all internal parts, or vented cell, since gases produced during operation can escape to the air. Wet cells were a precursor to dry cells and are commonly used as a learning tool for electrochemistry
. It is often built with common laboratory supplies, such as beakers
, for demonstrations of how electrochemical cells work. A particular type of wet cell known as a concentration cell
is important in understanding corrosion
. Wet cells may be primary cell
s (non-rechargeable) or secondary cells (rechargeable). Originally, all practical primary batteries such as the Daniell cell
were built as open-topped glass jar wet cells. Other primary wet cells are the Leclanche cell
, Grove cell
, Bunsen cell
, Chromic acid cell
, Clark cell
, and Weston cell
. The Leclanche cell chemistry was adapted to the first dry cells. Wet cells are still used in automobile batteries
and in industry for standby power for switchgear
, telecommunication or large uninterruptible power supplies
, but in many places batteries with gel cells have been used instead. These applications commonly use lead–acid or nickel–cadmium cells.
While a dry cell's electrolyte is not truly completely free of moisture and must contain some moisture to function, it has the advantage of containing no sloshing liquid that might leak or drip out when inverted or handled roughly, making it highly suitable for small portable electric devices. By comparison, the first wet cells were typically fragile glass containers with lead rods hanging from the open top, and needed careful handling to avoid spillage. An inverted wet cell would leak, whereas a dry cell would not. Lead–acid batteries would not achieve the safety and portability of the dry cell until the development of the gel battery.
A common dry cell battery is the zinc–carbon battery, using a cell sometimes called the dry Leclanché cell
, with a nominal voltage of 1.5 volt
s, the same nominal voltage as the alkaline battery
(since both use the same zinc
–manganese dioxide combination).
The makeup of a standard dry cell is a zinc
anode (negative pole), usually in the form of a cylindrical pot, with a carbon
cathode (positive pole) in the form of a central rod. The electrolyte is ammonium chloride
in the form of a paste next to the zinc anode. The remaining space between the electrolyte and carbon cathode is taken up by a second paste consisting of ammonium chloride and manganese dioxide, the latter acting as a depolariser. In some more modern types of so-called 'high-power' batteries, the ammonium chloride has been replaced by zinc chloride
.
is a primary or secondary battery that uses a molten salt
as its electrolyte. Their energy density
and power density
give them potential for use in electric vehicles, but they must be carefully insulated to retain heat.
can be stored for a long period of time and is activated when its internal parts (usually electrolyte) are assembled. For example, a battery for an electronic fuze
might be activated by the impact of firing a gun, breaking a capsule of electrolyte to activate the battery and power the fuze's circuits. Reserve batteries are usually designed for a short service life (seconds or minutes) after long storage (years). A water-activated battery
for oceanographic instruments or military applications becomes activated on immersion in water.
, and over lifetime due to many factors including internal chemistry, current
drain, and temperature.
it can store. The more electrolyte and electrode material there is in the cell the greater the capacity of the cell. A small cell has less capacity than a larger cell with the same chemistry, and they develop the same open-circuit voltage.
Because of the chemical reactions within the cells, the capacity of a battery depends on the discharge conditions such as the magnitude of the current (which may vary with time), the allowable terminal voltage of the battery, temperature, and other factors. The available capacity of a battery depends upon the rate at which it is discharged. If a battery is discharged at a relatively high rate, the available capacity will be lower than expected.
The battery capacity that battery manufacturers print on a battery is usually the product of 20 hours multiplied by the maximum constant current that a new battery can supply for 20 hours at 68 F° (20 C°), down to a predetermined terminal voltage per cell. A battery rated at 100 A·h will deliver 5 A over a 20 hour period at room temperature
. However, if it is instead discharged at 50 A, it will have a lower apparent capacity.
The relationship between current, discharge time, and capacity for a lead acid battery is approximated (over a certain range of current values) by Peukert's law
: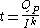
where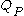 is the capacity when discharged at a rate of 1 amp.
is the capacity when discharged at a rate of 1 amp.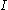 is the current drawn from battery (A).
is the current drawn from battery (A). is the amount of time (in hours) that a battery can sustain.
is the amount of time (in hours) that a battery can sustain.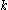 is a constant around 1.3.
is a constant around 1.3.
For low values of I internal self-discharge must be included.
In practical batteries, internal energy losses, and limited rate of diffusion of ions through the electrolyte, cause the efficiency
of a battery to vary at different discharge rates. When discharging at low rate, the battery's energy is delivered more efficiently than at higher discharge rates, but if the rate is too low, it will self-discharge during the long time of operation, again lowering its efficiency.
Installing batteries with different A·h ratings will not affect the operation of a device rated for a specific voltage unless the load limits of the battery are exceeded. High-drain loads like digital camera
s can result in lower actual energy, such as for alkaline batteries. For example, a battery rated at 2000 mA·h would not sustain a current of 1 A for the full two hours, if it had been rated at a 10-hour or 20-hour discharge.
are the fastest charging and discharging, next to supercapacitor
s. The world's largest battery is in Fairbanks, Alaska
, composed of Ni–Cd cells. Sodium–sulfur batteries are being used to store wind power
. Lithium–sulfur batteries have been used on the longest and highest solar powered flight. The speed of recharging for lithium-ion batteries may be increased by manipulation.
Discharging performance of all batteries drops at low temperature.
Old chemistry rechargeable batteries self-discharge more rapidly than disposable alkaline batteries, especially nickel
-based batteries; a freshly charged nickel cadmium (NiCd) battery loses 10% of its charge in the first 24 hours, and thereafter discharges at a rate of about 10% a month. However, newer low self-discharge nickel metal hydride (NiMH) batteries
and modern lithium designs have reduced the self-discharge rate to a relatively low level (but still poorer than for primary batteries). Most nickel-based batteries are partially discharged when purchased, and must be charged before first use. Newer NiMH batteries are ready to be used when purchased, and have only 15% discharge in a year.
Although rechargeable batteries have their energy content restored by charging, some deterioration occurs on each charge–discharge cycle. Low-capacity NiMH batteries (1700–2000 mA·h) can be charged for about 1000 cycles, whereas high-capacity NiMH batteries (above 2500 mA·h) can be charged for about 500 cycles. NiCd batteries tend to be rated for 1000 cycles before their internal resistance permanently increases beyond usable values. Under normal circumstances, a fast charge, rather than a slow overnight charge, will shorten battery lifespan. However, if the overnight charger is not "smart" and cannot detect when the battery is fully charged, then overcharging is likely, which also damages the battery. Degradation usually occurs because electrolyte migrates away from the electrodes or because active material falls off the electrodes. NiCd batteries suffer the drawback that they should be fully discharged before recharge. Without full discharge, crystals may build up on the electrodes, thus decreasing the active surface area and increasing internal resistance. This decreases battery capacity and causes the "memory effect
". These electrode crystals can also penetrate the electrolyte separator, thereby causing shorts. NiMH, although similar in chemistry, does not suffer from memory effect to quite this extent. When a battery reaches the end of its lifetime, it will not suddenly lose all of its capacity; rather, its capacity will gradually decrease.
Automotive lead–acid rechargeable batteries have a much harder life. Because of vibration, shock, heat, cold, and sulfation of their lead plates, few automotive batteries last beyond six years of regular use. Automotive starting batteries have many thin plates to provide as much current as possible in a reasonably small package. In general, the thicker the plates, the longer the life of the battery. They are typically drained only a small amount before recharge. Care should be taken to avoid deep discharging a starting battery, since each charge and discharge cycle causes active material to be shed from the plates.
"Deep-cycle" lead–acid batteries such as those used in electric golf carts have much thicker plates to aid their longevity. The main benefit of the lead–acid battery is its low cost; the main drawbacks are its large size and weight for a given capacity and voltage. Lead–acid batteries should never be discharged to below 20% of their full capacity, because internal resistance will cause heat and damage when they are recharged. Deep-cycle lead–acid systems often use a low-charge warning light or a low-charge power cut-off switch to prevent the type of damage that will shorten the battery's life.
or freezer, which slows the chemical reactions in the battery. Such storage can extend the life of alkaline batteries by about 5%, while the charge of rechargeable batteries can be extended from a few days up to several months. To reach their maximum voltage, batteries must be returned to room temperature; discharging an alkaline battery at 250 mA at 0°C is only half as efficient as it is at 20°C. As a result, alkaline battery manufacturers like Duracell
do not recommend refrigerating or freezing batteries.
Cell balancing principle
Battery pack cells are balanced when all the cells in the battery pack meet two conditions:
Cell balancing electronics
Cell balancing is defined as the application of differential currents to individual cells (or combinations of cells) in a series string. Under normal circumstances, of course, cells in a series string receive identical currents. A battery pack requires additional components and circuitry to achieve cell balancing. However, the use of a fully integrated analog front end for cell balancing reduces the required external components to just balancing resistors.
It is important to recognize that the cell mismatch results more from limitations in process control and inspection than from variations inherent in the lithium ion chemistry. The use of a fully integrated analog front end for cell balancing can improve the performance of series connected Li-ion Cells by addressing both SOC and C/E issues. SOC mismatch can be remedied by balancing the cell during an initial conditioning period and subsequently only during the charge phase. C/E mismatch remedies are more difficult to implement and harder to measure and require balancing during both charge and discharge periods.
This solution eliminates the quantity of external components, as for discrete capacitors, diodes, and most other resistors to achieve balance.
s used for electric watches, to the No. 6 cell used for signal circuits or other long duration applications. Secondary cells are made in very large sizes, from car batteries to systems used for uninterruptible power supply
in large computer data centers. Large secondary batteries have been connected to the electrical grid to stabilize the system and help level out peak loads.
ing a battery. With car batteries, explosions are most likely to occur when a short circuit generates very large currents. In addition, car batteries liberate hydrogen
when they are overcharged (because of electrolysis
of the water in the electrolyte). The amount of overcharging is usually very small, as is the amount of explosive gas developed, and the gas dissipates quickly. However, when "jumping" a car battery, the high current can cause the rapid release of large volumes of hydrogen, which can be ignited by a nearby spark (for example, when removing the jumper cables).
When a battery is recharged at an excessive rate, an explosive gas mixture of hydrogen and oxygen may be produced faster than it can escape from within the walls of the battery, leading to pressure build-up and the possibility of bursting of the battery case. In extreme cases, the battery acid may spray violently from the casing of the battery and cause injury. Overcharging—that is, attempting to charge a battery beyond its electrical capacity—can also lead to a battery explosion, leakage, or irreversible damage to the battery. It may also cause damage to the charger or device in which the overcharged battery is later used. In addition, disposing of a battery in fire may cause an explosion as steam builds up within the sealed case of the battery.
For example, disposable batteries often use a zinc "can" both as a reactant and as the container to hold the other reagents. If this kind of battery is run all the way down, or if it is recharged after running down too far, the reagents can emerge through the cardboard and plastic that form the remainder of the container. The active chemical leakage can then damage the equipment that the batteries were inserted into. For this reason, many electronic device manufacturers recommend removing the batteries from devices that will not be used for extended periods of time.
, such as toxic metal pollution. Battery manufacture consumes resources and often involves hazardous chemicals. Used batteries also contribute to electronic waste
. Some areas now have battery recycling
services available to recover some of the materials from used batteries. Batteries may be harmful or fatal if swallowed
. Recycling or proper disposal prevents dangerous elements (such as lead
, mercury
, and cadmium
) found in some types of batteries from entering the environment. In the United States, Americans purchase nearly three billion batteries annually, and about 179,000 tons of those end up in landfills across the country.
In the United States, the Mercury-Containing and Rechargeable Battery Management Act
of 1996 banned the sale of mercury-containing batteries, enacted uniform labeling requirements for rechargeable batteries, and required that rechargeable batteries be easily removable. California, and New York City prohibit the disposal of rechargeable batteries in solid waste, and along with Maine require recycling of cell phones. The rechargeable battery industry has nationwide recycling programs in the United States and Canada, with dropoff points at local retailers.
The Battery Directive
of the European Union has similar requirements, in addition to requiring increased recycling of batteries, and promoting research on improved battery recycling methods.
In accordance with this directive all batteries to be sold within the EU must be marked with the "collection symbol" (A crossed out wheeled bin). This must cover at least 3% of the surface of prismatic batteries and 1.5% of the surface of cylindrical batteries. All packaging must be marked likewise.
, potato
, etc. and generate small amounts of electricity. "Two-potato clocks" are also widely available in hobby and toy stores; they consist of a pair of cells, each consisting of a potato (lemon, et cetera) with two electrodes inserted into it, wired in series to form a battery with enough voltage to power a digital clock. Homemade cells of this kind are of no real practical use, because they produce far less current—and cost far more per unit of energy generated—than commercial cells, due to the need for frequent replacement of the fruit or vegetable. In addition, one can make a voltaic pile from two coins (such as a nickel
and a penny
) and a piece of paper towel
dipped in salt water
. Such a pile would make very little voltage itself, but, when many of them are stacked together in series, they can replace normal batteries for a short amount of time.
Sony has developed a biological battery that generates electricity from sugar in a way that is similar to the processes observed in living organisms. The battery generates electricity through the use of enzymes that break down carbohydrates, which are, in essence, sugar. A similarly designed sugar drink powers a phone using enzymes to generate electricity from carbohydrates that covers the phone’s electrical needs. It needs only a pack of sugary drink and it generates water and oxygen while the battery dies out.
Lead acid cells can easily be manufactured at home, but a tedious charge/discharge cycle is needed to 'form' the plates. This is a process in which lead sulfate forms on the plates, and during charge is converted to lead dioxide (positive plate) and pure lead (negative plate). Repeating this process results in a microscopically rough surface, with far greater surface area being exposed. This increases the current the cell can deliver. For an example, see http://windpower.org.za/batteries/batteries.html.
Daniell cell
s are also easy to make at home. Aluminum–air batteries can also be produced with high-purity aluminum. Aluminum foil batteries will produce some electricity, but they are not very efficient, in part because a significant amount of hydrogen
gas is produced.
Electrochemical cell
An electrochemical cell is a device capable of either deriving electrical energy from chemical reactions, or facilitating chemical reactions through the introduction of electrical energy. A common example of an electrochemical cell is a standard 1.5-volt "battery"...
s that convert stored chemical energy
Energy
In physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
into electrical energy. Since the invention of the first battery (or "voltaic pile
Voltaic pile
A voltaic pile is a set of individual Galvanic cells placed in series. The voltaic pile, invented by Alessandro Volta in 1800, was the first electric battery...
") in 1800 by Alessandro Volta
Alessandro Volta
Count Alessandro Giuseppe Antonio Anastasio Gerolamo Umberto Volta was a Lombard physicist known especially for the invention of the battery in 1800.-Early life and works:...
and especially since the technically improved Daniell cell
Daniell cell
The Daniell cell was invented in 1836 by John Frederic Daniell, a British chemist and meteorologist, and consisted of a copper pot filled with a copper sulfate solution, in which was immersed an unglazed earthenware container filled with sulfuric acid and a zinc electrode...
in 1836, batteries have become a common power source for many household and industrial applications. According to a 2005 estimate, the worldwide battery industry generates US$
United States dollar
The United States dollar , also referred to as the American dollar, is the official currency of the United States of America. It is divided into 100 smaller units called cents or pennies....
48 billion
1000000000 (number)
1,000,000,000 is the natural number following 999,999,999 and preceding 1,000,000,001.In scientific notation, it is written as 109....
in sales each year, with 6% annual growth.
There are two types of batteries: primary batteries (disposable batteries), which are designed to be used once and discarded, and secondary batteries (rechargeable batteries), which are designed to be recharged and used multiple times. Batteries come in many sizes, from miniature cells used to power hearing aid
Hearing aid
A hearing aid is an electroacoustic device which typically fits in or behind the wearer's ear, and is designed to amplify and modulate sound for the wearer. Earlier devices, known as "ear trumpets" or "ear horns", were passive funnel-like amplification cones designed to gather sound energy and...
s and wristwatches to battery banks the size of rooms that provide standby power for telephone exchange
Telephone exchange
In the field of telecommunications, a telephone exchange or telephone switch is a system of electronic components that connects telephone calls...
s and computer data center
Data center
A data center is a facility used to house computer systems and associated components, such as telecommunications and storage systems...
s.
History
In strict terms, a battery is a collection of multiple electrochemical cells, but in popular usage battery often refers to a single cell. For example, a 1.5-volt AAA battery is a single 1.5-volt cell, and a 9-volt battery has six 1.5-volt cells in series. The first electrochemical cell was developed by the Italian physicist Alessandro VoltaAlessandro Volta
Count Alessandro Giuseppe Antonio Anastasio Gerolamo Umberto Volta was a Lombard physicist known especially for the invention of the battery in 1800.-Early life and works:...
in 1792, and in 1800 he invented the first battery, a "pile" of many cells in series.
The usage of "battery" to describe electrical devices dates to Benjamin Franklin
Benjamin Franklin
Dr. Benjamin Franklin was one of the Founding Fathers of the United States. A noted polymath, Franklin was a leading author, printer, political theorist, politician, postmaster, scientist, musician, inventor, satirist, civic activist, statesman, and diplomat...
, who in 1748 described multiple Leyden jar
Leyden jar
A Leyden jar, or Leiden jar, is a device that "stores" static electricity between two electrodes on the inside and outside of a jar. It was invented independently by German cleric Ewald Georg von Kleist on 11 October 1745 and by Dutch scientist Pieter van Musschenbroek of Leiden in 1745–1746. The...
s (early electrical capacitor
Capacitor
A capacitor is a passive two-terminal electrical component used to store energy in an electric field. The forms of practical capacitors vary widely, but all contain at least two electrical conductors separated by a dielectric ; for example, one common construction consists of metal foils separated...
s) by analogy to a battery of cannons
Artillery battery
In military organizations, an artillery battery is a unit of guns, mortars, rockets or missiles so grouped in order to facilitate better battlefield communication and command and control, as well as to provide dispersion for its constituent gunnery crews and their systems...
. Thus Franklin's usage to describe multiple Leyden jars predated Volta's use of multiple galvanic cells. It is speculated, but not established, that several ancient artifacts consisting of copper sheets and iron bars, and known as Baghdad batteries
Baghdad Battery
The Baghdad Battery, sometimes referred to as the Parthian Battery, is the common name for a number of artifacts created in Mesopotamia, during the dynasties of Parthian or Sassanid period , and probably discovered in 1936 in the village of Khuyut Rabbou'a, near Baghdad, Iraq...
may have been galvanic cells.
Volta's work was stimulated by the Italian anatomist and physiologist Luigi Galvani
Luigi Galvani
Luigi Aloisio Galvani was an Italian physician and physicist who lived and died in Bologna. In 1791, he discovered that the muscles of dead frogs legs twitched when struck by a spark...
, who in 1780 noticed that dissected frog's legs would twitch when struck by a spark from a Leyden jar
Leyden jar
A Leyden jar, or Leiden jar, is a device that "stores" static electricity between two electrodes on the inside and outside of a jar. It was invented independently by German cleric Ewald Georg von Kleist on 11 October 1745 and by Dutch scientist Pieter van Musschenbroek of Leiden in 1745–1746. The...
, an external source of electricity. In 1786 he noticed that twitching would occur during lightning storms. After many years Galvani learned how to produce twitching without using any external source of electricity. In 1791 he published a report on "animal electricity." He created an electric circuit consisting of the frog's leg (FL) and two different metals A and B, each metal touching the frog's leg and each other, thus producing the circuit A–FL–B–A–FL–B...etc. In modern terms, the frog's leg served as both the electrolyte
Electrolyte
In chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
and the sensor
Sensor
A sensor is a device that measures a physical quantity and converts it into a signal which can be read by an observer or by an instrument. For example, a mercury-in-glass thermometer converts the measured temperature into expansion and contraction of a liquid which can be read on a calibrated...
, and the metals served as electrode
Electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit...
s. He noticed that even though the frog was dead, its legs would twitch when he touched them with the metals.
Within a year, Volta realized the frog's moist tissues could be replaced by cardboard soaked in salt water, and the frog's muscular response could be replaced by another form of electrical detection. He already had studied the electrostatic phenomenon of capacitance
Capacitance
In electromagnetism and electronics, capacitance is the ability of a capacitor to store energy in an electric field. Capacitance is also a measure of the amount of electric potential energy stored for a given electric potential. A common form of energy storage device is a parallel-plate capacitor...
, which required measurements of electric charge and of electrical potential ("tension"). Building on this experience, Volta was able to detect electric current through his system, also called a Galvanic cell
Galvanic cell
A Galvanic cell, or Voltaic cell, named after Luigi Galvani, or Alessandro Volta respectively, is an electrochemical cell that derives electrical energy from spontaneous redox reaction taking place within the cell...
. The terminal voltage of a cell that is not discharging is called its electromotive force
Electromotive force
In physics, electromotive force, emf , or electromotance refers to voltage generated by a battery or by the magnetic force according to Faraday's Law, which states that a time varying magnetic field will induce an electric current.It is important to note that the electromotive "force" is not a...
(emf), and has the same unit as electrical potential, named (voltage
Voltage
Voltage, otherwise known as electrical potential difference or electric tension is the difference in electric potential between two points — or the difference in electric potential energy per unit charge between two points...
) and measured in volt
Volt
The volt is the SI derived unit for electric potential, electric potential difference, and electromotive force. The volt is named in honor of the Italian physicist Alessandro Volta , who invented the voltaic pile, possibly the first chemical battery.- Definition :A single volt is defined as the...
s, in honor of Volta. In 1800, Volta invented the battery by placing many voltaic cells in series, piling them one above the other. This voltaic pile gave a greatly enhanced net emf for the combination, with a voltage of about 50 volts for a 32-cell pile. In many parts of Europe batteries continue to be called piles.
Volta did not appreciate that the voltage was due to chemical reactions. He thought that his cells were an inexhaustible source of energy, and that the associated corrosion effects at the electrodes were a mere nuisance, rather than an unavoidable consequence of their operation, as Michael Faraday
Michael Faraday
Michael Faraday, FRS was an English chemist and physicist who contributed to the fields of electromagnetism and electrochemistry....
showed in 1834. According to Faraday, cations (positively charged ions) are attracted to the cathode
Cathode
A cathode is an electrode through which electric current flows out of a polarized electrical device. Mnemonic: CCD .Cathode polarity is not always negative...
, and anions (negatively charged ions) are attracted to the anode
Anode
An anode is an electrode through which electric current flows into a polarized electrical device. Mnemonic: ACID ....
.
Although early batteries were of great value for experimental purposes, in practice their voltages fluctuated and they could not provide a large current for a sustained period. Later, starting with the Daniell cell
Daniell cell
The Daniell cell was invented in 1836 by John Frederic Daniell, a British chemist and meteorologist, and consisted of a copper pot filled with a copper sulfate solution, in which was immersed an unglazed earthenware container filled with sulfuric acid and a zinc electrode...
in 1836, batteries provided more reliable currents and were adopted by industry for use in stationary devices, in particular in telegraph networks where they were the only practical source of electricity, since electrical distribution networks did not exist at the time. These wet cells used liquid electrolytes, which were prone to leakage and spillage if not handled correctly. Many used glass jars to hold their components, which made them fragile. These characteristics made wet cells unsuitable for portable appliances. Near the end of the nineteenth century, the invention of dry cell batteries
Dry Cell
-Dry Cell's formation:Part of the band formed in 1998 when guitarist Danny Hartwell and drummer Brandon Brown met at the Ratt Show on the Sunset Strip. They later met up with then-vocalist Judd Gruenbaum. The original name of the band was "Beyond Control"....
, which replaced the liquid electrolyte with a paste, made portable electrical devices practical.
Since then, batteries have gained popularity as they became portable and useful for a variety of purposes.
Principle of operation

Anode
An anode is an electrode through which electric current flows into a polarized electrical device. Mnemonic: ACID ....
or negative electrode; the other half-cell includes electrolyte and the electrode to which cations (positively charged ions) migrate, i.e., the cathode
Cathode
A cathode is an electrode through which electric current flows out of a polarized electrical device. Mnemonic: CCD .Cathode polarity is not always negative...
or positive electrode. In the redox
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
reaction that powers the battery, cations are reduced (electrons are added) at the cathode, while anions are oxidized (electrons are removed) at the anode. The electrodes do not touch each other but are electrically connected by the electrolyte
Electrolyte
In chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
. Some cells use two half-cells with different electrolytes. A separator between half-cells allows ions to flow, but prevents mixing of the electrolytes.
Each half-cell has an electromotive force (or emf), determined by its ability to drive electric current from the interior to the exterior of the cell. The net emf of the cell is the difference between the emfs of its half-cells, as first recognized by Volta. Therefore, if the electrodes have emfs
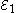 and
and 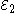 , then the net emf is
, then the net emf is 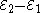 ; in other words, the net emf is the difference between the reduction potential
; in other words, the net emf is the difference between the reduction potentialReduction potential
Reduction potential is a measure of the tendency of a chemical species to acquire electrons and thereby be reduced. Reduction potential is measured in volts , or millivolts...
s of the half-reaction
Half-reaction
A half reaction is either the oxidation or reduction reaction component of a redox reaction. A half reaction is obtained by considering the change in oxidation states of individual substances involved in the redox reaction.-Example:...
s.
The electrical driving force or
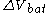 across the terminals of a cell is known as the terminal voltage (difference) and is measured in volt
across the terminals of a cell is known as the terminal voltage (difference) and is measured in voltVolt
The volt is the SI derived unit for electric potential, electric potential difference, and electromotive force. The volt is named in honor of the Italian physicist Alessandro Volta , who invented the voltaic pile, possibly the first chemical battery.- Definition :A single volt is defined as the...
s. The terminal voltage of a cell that is neither charging nor discharging is called the open-circuit voltage
Open-circuit voltage
Open-circuit voltage is the difference of electrical potential between two terminals of a device when there is no external load connected, i.e. the circuit is broken or open. Under these conditions there is no external electric current between the terminals, even though there may be current...
and equals the emf of the cell. Because of internal resistance, the terminal voltage of a cell that is discharging is smaller in magnitude than the open-circuit voltage and the terminal voltage of a cell that is charging exceeds the open-circuit voltage. An ideal cell has negligible internal resistance, so it would maintain a constant terminal voltage of
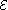 until exhausted, then dropping to zero. If such a cell maintained 1.5 volts and stored a charge of one coulomb then on complete discharge it would perform 1.5 joule
until exhausted, then dropping to zero. If such a cell maintained 1.5 volts and stored a charge of one coulomb then on complete discharge it would perform 1.5 jouleJoule
The joule ; symbol J) is a derived unit of energy or work in the International System of Units. It is equal to the energy expended in applying a force of one newton through a distance of one metre , or in passing an electric current of one ampere through a resistance of one ohm for one second...
of work. In actual cells, the internal resistance increases under discharge, and the open circuit voltage also decreases under discharge. If the voltage and resistance are plotted against time, the resulting graphs typically are a curve; the shape of the curve varies according to the chemistry and internal arrangement employed.
As stated above, the voltage developed across a cell's terminals depends on the energy release of the chemical reactions of its electrodes and electrolyte. Alkaline and zinc–carbon cells have different chemistries but approximately the same emf of 1.5 volts; likewise NiCd and NiMH cells have different chemistries, but approximately the same emf of 1.2 volts. On the other hand the high electrochemical potential changes in the reactions of lithium
Lithium
Lithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly...
compounds give lithium cells emfs of 3 volts or more.
Categories and types of batteries
Batteries are classified into two broad categories, each type with advantages and disadvantages.- Primary batteries irreversibly (within limits of practicality) transform chemical energy to electrical energy. When the initial supply of reactants is exhausted, energy cannot be readily restored to the battery by electrical means.
- Secondary batteries can be recharged; that is, they can have their chemical reactions reversed by supplying electrical energy to the cell, restoring their original composition.
Some types of primary batteries used, for example, for telegraph
Telegraphy
Telegraphy is the long-distance transmission of messages via some form of signalling technology. Telegraphy requires messages to be converted to a code which is known to both sender and receiver...
circuits, were restored to operation by replacing the components of the battery consumed by the chemical reaction. Secondary batteries are not indefinitely rechargeable due to dissipation of the active materials, loss of electrolyte and internal corrosion.
Primary batteries
Primary batteries can produce current immediately on assembly. Disposable batteries are intended to be used once and discarded. These are most commonly used in portable devices that have low current drain, are used only intermittently, or are used well away from an alternative power source, such as in alarm and communication circuits where other electric power is only intermittently available. Disposable primary cells cannot be reliably recharged, since the chemical reactions are not easily reversible and active materials may not return to their original forms. Battery manufacturers recommend against attempting to recharge primary cells.Common types of disposable batteries include zinc–carbon batteries and alkaline batteries. In general, these have higher energy densities than rechargeable batteries, but disposable batteries do not fare well under high-drain applications with loads under 75 ohms
Ohms
OHMS may refer to:* The plural of ohm, a unit of resistance, named after Georg Ohm* Ohm's Law of electric currents, first proposed by Georg Ohm* O.H.M.S., On His/Her Majesty's Service...
(75 Ω).
Secondary batteries
Secondary batteries must be charged before use; they are usually assembled with active materials in the discharged state. Rechargeable batteries or secondary cells can be recharged by applying electric current, which reverses the chemical reactionChemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
s that occur during its use. Devices to supply the appropriate current are called chargers or rechargers.
The oldest form of rechargeable battery is the lead–acid battery. This battery is notable in that it contains a liquid in an unsealed container, requiring that the battery be kept upright and the area be well ventilated to ensure safe dispersal of the hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
gas produced by these batteries during overcharging. The lead–acid battery is also very heavy for the amount of electrical energy it can supply. Despite this, its low manufacturing cost and its high surge current levels make its use common where a large capacity (over approximately 10 Ah) is required or where the weight and ease of handling are not concerns.
A common form of the lead–acid battery is the modern car battery
Car battery
An automotive battery is a type of rechargeable battery that supplies electric energy to an automobile. Usually this refers to an SLI battery to power the starter motor, the lights, and the ignition system of a vehicle’s engine...
, which can, in general, deliver a peak current of 450 ampere
Ampere
The ampere , often shortened to amp, is the SI unit of electric current and is one of the seven SI base units. It is named after André-Marie Ampère , French mathematician and physicist, considered the father of electrodynamics...
s. An improved type of liquid electrolyte battery is the sealed valve regulated lead acid (VRLA
VRLA
A VRLA battery is a type of low-maintenance lead–acid rechargeable battery. Because of their construction, VRLA batteries do not require regular addition of water to the cells....
) battery, popular in the automotive industry as a replacement for the lead–acid wet cell. The VRLA battery uses an immobilized sulfuric acid electrolyte, reducing the chance of leakage and extending shelf life. VRLA batteries have the electrolyte immobilized, usually by one of two means:
- Gel batteries (or "gel cell") contain a semi-solid electrolyte to prevent spillage.
- Absorbed Glass Mat (AGM) batteries absorb the electrolyte in a special fiberglass matting.
Other portable rechargeable batteries include several "dry cell" types, which are sealed units and are, therefore, useful in appliances such as mobile phone
Mobile phone
A mobile phone is a device which can make and receive telephone calls over a radio link whilst moving around a wide geographic area. It does so by connecting to a cellular network provided by a mobile network operator...
s and laptop computers
Laptop
A laptop, also called a notebook, is a personal computer for mobile use. A laptop integrates most of the typical components of a desktop computer, including a display, a keyboard, a pointing device and speakers into a single unit...
. Cells of this type (in order of increasing power density
Power density
Power density is the amount of power per unit volume....
and cost) include nickel–cadmium (NiCd), nickel–zinc (NiZn), nickel metal hydride
Nickel metal hydride battery
A nickel–metal hydride cell, abbreviated NiMH, is a type of rechargeable battery similar to the nickel–cadmium cell. The NiMH battery uses a hydrogen-absorbing alloy for the negative electrode instead of cadmium. As in NiCd cells, the positive electrode is nickel oxyhydroxide...
(NiMH), and lithium-ion (Li-ion) cells. By far, Li-ion has the highest share of the dry cell rechargeable market. Meanwhile, NiMH has replaced NiCd in most applications due to its higher capacity, but NiCd remains in use in power tool
Power tool
A power tool is a tool that is actuated by an additional power source and mechanism other than the solely manual labour used with hand tools. The most common types of power tools use electric motors. Internal combustion engines and compressed air are also commonly used...
s, two-way radio
Two-way radio
A two-way radio is a radio that can both transmit and receive , unlike a broadcast receiver which only receives content. The term refers to a personal radio transceiver that allows the operator to have a two-way conversation with other similar radios operating on the same radio frequency...
s, and medical equipment
Medical equipment
Medical equipment is designed to aid in the diagnosis, monitoring or treatment of medical conditions.-Types:There are several basic types:* Diagnostic equipment includes medical imaging machines, used to aid in diagnosis...
. NiZn is a new technology that is not yet well established commercially.
Recent developments include batteries with embedded electronics such as USBCELL, which allows charging an AA cell through a USB connector, and smart battery packs with state-of-charge monitors and battery protection circuits to prevent damage on over-discharge. low self-discharge
Low self-discharge NiMH battery
The low self-discharge nickel-metal hydride battery was introduced in November 2005. These batteries were developed by Sanyo, who called them "eneloop". Subsequently, other manufacturers also offered LSD NiMH....
(LSD) allows secondary cells to be precharged prior to shipping.
Battery cell types
There are many general types of electrochemical cells, according to chemical processes applied and design chosen. The variation includes galvanic cellGalvanic cell
A Galvanic cell, or Voltaic cell, named after Luigi Galvani, or Alessandro Volta respectively, is an electrochemical cell that derives electrical energy from spontaneous redox reaction taking place within the cell...
s, electrolytic cell
Electrolytic cell
An electrolytic cell decomposes chemical compounds by means of electrical energy, in a process called electrolysis; the Greek word lysis means to break up. The result is that the chemical energy is increased...
s, fuel cell
Fuel cell
A fuel cell is a device that converts the chemical energy from a fuel into electricity through a chemical reaction with oxygen or another oxidizing agent. Hydrogen is the most common fuel, but hydrocarbons such as natural gas and alcohols like methanol are sometimes used...
s, flow cells
Flow battery
A flow battery is a form of rechargeable battery in which electrolyte containing one or more dissolved electroactive species flows through an electrochemical cell that converts chemical energy directly to electricity...
and voltaic piles.
Wet cell
A wet cell battery has a liquid electrolyteElectrolyte
In chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
. Other names are flooded cell, since the liquid covers all internal parts, or vented cell, since gases produced during operation can escape to the air. Wet cells were a precursor to dry cells and are commonly used as a learning tool for electrochemistry
Electrochemistry
Electrochemistry is a branch of chemistry that studies chemical reactions which take place in a solution at the interface of an electron conductor and an ionic conductor , and which involve electron transfer between the electrode and the electrolyte or species in solution.If a chemical reaction is...
. It is often built with common laboratory supplies, such as beakers
Beaker (glassware)
A beaker is a simple container for stirring, mixing and heating liquids commonly used in many laboratories. Beakers are generally cylindrical in shape, with a flat bottom. Most also have a small spout to aid pouring as shown in the picture...
, for demonstrations of how electrochemical cells work. A particular type of wet cell known as a concentration cell
Concentration cell
A concentration cell is a limited form of a galvanic cell that has two equivalent half-cells of the same material differing only in concentrations. One can calculate the potential developed by such a cell using the Nernst Equation. A concentration cell produces a voltage as it attempts to reach...
is important in understanding corrosion
Corrosion
Corrosion is the disintegration of an engineered material into its constituent atoms due to chemical reactions with its surroundings. In the most common use of the word, this means electrochemical oxidation of metals in reaction with an oxidant such as oxygen...
. Wet cells may be primary cell
Primary cell
A primary cell is any kind of battery in which the electrochemical reaction is not reversible, rendering the cell non-rechargeable. A common example of a primary cell is the disposable battery. Unlike a secondary cell, the reaction cannot be reversed by running a current into the cell; the chemical...
s (non-rechargeable) or secondary cells (rechargeable). Originally, all practical primary batteries such as the Daniell cell
Daniell cell
The Daniell cell was invented in 1836 by John Frederic Daniell, a British chemist and meteorologist, and consisted of a copper pot filled with a copper sulfate solution, in which was immersed an unglazed earthenware container filled with sulfuric acid and a zinc electrode...
were built as open-topped glass jar wet cells. Other primary wet cells are the Leclanche cell
Leclanché cell
Georges Leclanché invented and patented his battery, the Leclanché cell, in 1866. The battery contained a conducting solution of ammonium chloride, a cathode of carbon, a depolarizer of manganese dioxide, and an anode of zinc...
, Grove cell
Grove cell
The Grove cell was an early electric primary cell named after its inventor, British chemist William Robert Grove, and consisted of a zinc anode in dilute sulfuric acid and a platinum cathode in concentrated nitric acid, the two separated by a porous ceramic pot.-Cell details:The Grove cell voltage...
, Bunsen cell
Bunsen cell
The Bunsen cell is a zinc-carbon primary cell composed of a zinc anode in dilute sulfuric acid separated by a porous pot from a carbon cathode in nitric or chromic acid.- Cell details :...
, Chromic acid cell
Chromic acid cell
The Chromic acid cell was a type of primary cell which used chromic acid as a depolarizer. The chromic acid was usually made by acidifying a solution of potassium dichromate. The old name for potassium dichromate was potassium bichromate and the cell was often called a Bichromate cell...
, Clark cell
Clark cell
The Clark cell, invented by English engineer Josiah Latimer Clark in 1873, is a wet-chemical cell that produces a highly stable voltage usable as a laboratory standard.-Chemistry:...
, and Weston cell
Weston cell
The Weston cell, invented by Edward Weston in 1893, is a wet-chemical cell that produces a highly stable voltage suitable as a laboratory standard for calibration of voltmeters...
. The Leclanche cell chemistry was adapted to the first dry cells. Wet cells are still used in automobile batteries
Car battery
An automotive battery is a type of rechargeable battery that supplies electric energy to an automobile. Usually this refers to an SLI battery to power the starter motor, the lights, and the ignition system of a vehicle’s engine...
and in industry for standby power for switchgear
Switchgear
The term switchgear, used in association with the electric power system, or grid, refers to the combination of electrical disconnects, fuses and/or circuit breakers used to isolate electrical equipment. Switchgear is used both to de-energize equipment to allow work to be done and to clear faults...
, telecommunication or large uninterruptible power supplies
Uninterruptible power supply
An uninterruptible power supply, also uninterruptible power source, UPS or battery/flywheel backup, is an electrical apparatus that provides emergency power to a load when the input power source, typically mains power, fails...
, but in many places batteries with gel cells have been used instead. These applications commonly use lead–acid or nickel–cadmium cells.
Dry cell
A dry cell has the electrolyte immobilized as a paste, with only enough moisture in the paste to allow current to flow. As opposed to a wet cell, the battery can be operated in any random position, and will not spill its electrolyte if inverted.While a dry cell's electrolyte is not truly completely free of moisture and must contain some moisture to function, it has the advantage of containing no sloshing liquid that might leak or drip out when inverted or handled roughly, making it highly suitable for small portable electric devices. By comparison, the first wet cells were typically fragile glass containers with lead rods hanging from the open top, and needed careful handling to avoid spillage. An inverted wet cell would leak, whereas a dry cell would not. Lead–acid batteries would not achieve the safety and portability of the dry cell until the development of the gel battery.
A common dry cell battery is the zinc–carbon battery, using a cell sometimes called the dry Leclanché cell
Leclanché cell
Georges Leclanché invented and patented his battery, the Leclanché cell, in 1866. The battery contained a conducting solution of ammonium chloride, a cathode of carbon, a depolarizer of manganese dioxide, and an anode of zinc...
, with a nominal voltage of 1.5 volt
Volt
The volt is the SI derived unit for electric potential, electric potential difference, and electromotive force. The volt is named in honor of the Italian physicist Alessandro Volta , who invented the voltaic pile, possibly the first chemical battery.- Definition :A single volt is defined as the...
s, the same nominal voltage as the alkaline battery
Alkaline battery
Alkaline batteries are a type of primary batteries dependent upon the reaction between zinc and manganese dioxide . A rechargeable alkaline battery allows reuse of specially designed cells....
(since both use the same zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
–manganese dioxide combination).
The makeup of a standard dry cell is a zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
anode (negative pole), usually in the form of a cylindrical pot, with a carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
cathode (positive pole) in the form of a central rod. The electrolyte is ammonium chloride
Ammonium chloride
Ammonium chloride NH4Cl is an inorganic compound with the formula NH4Cl. It is a white crystalline salt that is highly soluble in water. Solutions of ammonium chloride are mildly acidic. Sal ammoniac is a name of natural, mineralogical form of ammonium chloride...
in the form of a paste next to the zinc anode. The remaining space between the electrolyte and carbon cathode is taken up by a second paste consisting of ammonium chloride and manganese dioxide, the latter acting as a depolariser. In some more modern types of so-called 'high-power' batteries, the ammonium chloride has been replaced by zinc chloride
Zinc chloride
Zinc chloride is the name of chemical compound with the formula ZnCl2 and its hydrates. Zinc chlorides, of which nine crystalline forms are known, are colorless or white, and are highly soluble in water. ZnCl2 itself is hygroscopic and even deliquescent. Samples should therefore be protected from...
.
Molten salt
A molten salt batteryMolten salt battery
Molten salt batteries or liquid sodium battery are a class of primary cell and secondary cell high-temperature electric battery that use molten salts as an electrolyte. They offer both a higher energy density through the proper selection of reactant pairs as well as a higher power density by means...
is a primary or secondary battery that uses a molten salt
Salt
In chemistry, salts are ionic compounds that result from the neutralization reaction of an acid and a base. They are composed of cations and anions so that the product is electrically neutral...
as its electrolyte. Their energy density
Energy density
Energy density is a term used for the amount of energy stored in a given system or region of space per unit volume. Often only the useful or extractable energy is quantified, which is to say that chemically inaccessible energy such as rest mass energy is ignored...
and power density
Power density
Power density is the amount of power per unit volume....
give them potential for use in electric vehicles, but they must be carefully insulated to retain heat.
Reserve
A reserve batteryReserve battery
A reserve battery, also called stand-by battery, is a primary battery where part is isolated until the battery needs to be used. When long storage is required, reserve batteries are often used, since the active chemicals of the cell are segregated until needed, thus reducing self-discharge...
can be stored for a long period of time and is activated when its internal parts (usually electrolyte) are assembled. For example, a battery for an electronic fuze
Fuze
Fuze Beverage, commercially referred to as just Fuze , is a manufacturer of teas and non-carbonated fruit drinks enriched with vitamins. Currently the brand consists of five vitamin-infused lines: Slenderize, Refresh, Tea, Defensify, and Vitalize...
might be activated by the impact of firing a gun, breaking a capsule of electrolyte to activate the battery and power the fuze's circuits. Reserve batteries are usually designed for a short service life (seconds or minutes) after long storage (years). A water-activated battery
Water-activated battery
A water-activated battery is a disposable reserve battery that does not contain an electrolyte and hence produces no voltage until it is soaked in water for several minutes.-Description:...
for oceanographic instruments or military applications becomes activated on immersion in water.
Battery cell performance
A battery's characteristics may vary over load cycle, over charge cycleCharge cycle
A charge cycle is the process of charging a rechargeable battery and discharging it as required into a load. The term is typically used to specify a battery's expected life, as the number of charge cycles affects life more than the mere passage of time...
, and over lifetime due to many factors including internal chemistry, current
Electric current
Electric current is a flow of electric charge through a medium.This charge is typically carried by moving electrons in a conductor such as wire...
drain, and temperature.
Battery capacity and discharging
A battery's capacity is the amount of electric chargeElectric charge
Electric charge is a physical property of matter that causes it to experience a force when near other electrically charged matter. Electric charge comes in two types, called positive and negative. Two positively charged substances, or objects, experience a mutual repulsive force, as do two...
it can store. The more electrolyte and electrode material there is in the cell the greater the capacity of the cell. A small cell has less capacity than a larger cell with the same chemistry, and they develop the same open-circuit voltage.
Because of the chemical reactions within the cells, the capacity of a battery depends on the discharge conditions such as the magnitude of the current (which may vary with time), the allowable terminal voltage of the battery, temperature, and other factors. The available capacity of a battery depends upon the rate at which it is discharged. If a battery is discharged at a relatively high rate, the available capacity will be lower than expected.
The battery capacity that battery manufacturers print on a battery is usually the product of 20 hours multiplied by the maximum constant current that a new battery can supply for 20 hours at 68 F° (20 C°), down to a predetermined terminal voltage per cell. A battery rated at 100 A·h will deliver 5 A over a 20 hour period at room temperature
Room temperature
-Comfort levels:The American Society of Heating, Refrigerating and Air-Conditioning Engineers has listings for suggested temperatures and air flow rates in different types of buildings and different environmental circumstances. For example, a single office in a building has an occupancy ratio per...
. However, if it is instead discharged at 50 A, it will have a lower apparent capacity.
The relationship between current, discharge time, and capacity for a lead acid battery is approximated (over a certain range of current values) by Peukert's law
Peukert's law
Peukert's law, presented by the German scientist W. Peukert in 1897, expresses the capacity of a lead–acid battery in terms of the rate at which it is discharged. As the rate increases, the battery's available capacity decreases....
:
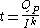
where
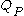 is the capacity when discharged at a rate of 1 amp.
is the capacity when discharged at a rate of 1 amp.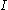 is the current drawn from battery (A).
is the current drawn from battery (A). is the amount of time (in hours) that a battery can sustain.
is the amount of time (in hours) that a battery can sustain.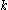 is a constant around 1.3.
is a constant around 1.3.For low values of I internal self-discharge must be included.
In practical batteries, internal energy losses, and limited rate of diffusion of ions through the electrolyte, cause the efficiency
Efficient energy use
Efficient energy use, sometimes simply called energy efficiency, is the goal of efforts to reduce the amount of energy required to provide products and services. For example, insulating a home allows a building to use less heating and cooling energy to achieve and maintain a comfortable temperature...
of a battery to vary at different discharge rates. When discharging at low rate, the battery's energy is delivered more efficiently than at higher discharge rates, but if the rate is too low, it will self-discharge during the long time of operation, again lowering its efficiency.
Installing batteries with different A·h ratings will not affect the operation of a device rated for a specific voltage unless the load limits of the battery are exceeded. High-drain loads like digital camera
Digital camera
A digital camera is a camera that takes video or still photographs, or both, digitally by recording images via an electronic image sensor. It is the main device used in the field of digital photography...
s can result in lower actual energy, such as for alkaline batteries. For example, a battery rated at 2000 mA·h would not sustain a current of 1 A for the full two hours, if it had been rated at a 10-hour or 20-hour discharge.
Fastest charging, largest, and lightest batteries
Lithium iron phosphate (LiFePO4) batteriesLithium iron phosphate
Lithium iron phosphate , also known as LFP, is a compound used in lithium iron phosphate batteries . It is targeted for use in power tools and electric vehicles...
are the fastest charging and discharging, next to supercapacitor
Supercapacitor
An electric double-layer capacitor , also known as supercapacitor, supercondenser, electrochemical double layer capacitor, or ultracapacitor, is an electrochemical capacitor with relatively high energy density. Their energy density is typically hundreds of times greater than conventional...
s. The world's largest battery is in Fairbanks, Alaska
Fairbanks, Alaska
Fairbanks is a home rule city in and the borough seat of the Fairbanks North Star Borough in the U.S. state of Alaska.Fairbanks is the largest city in the Interior region of Alaska, and second largest in the state behind Anchorage...
, composed of Ni–Cd cells. Sodium–sulfur batteries are being used to store wind power
Wind power
Wind power is the conversion of wind energy into a useful form of energy, such as using wind turbines to make electricity, windmills for mechanical power, windpumps for water pumping or drainage, or sails to propel ships....
. Lithium–sulfur batteries have been used on the longest and highest solar powered flight. The speed of recharging for lithium-ion batteries may be increased by manipulation.
Primary batteries
Even if never taken out of the original package, disposable (or "primary") batteries can lose 8 to 20 percent of their original charge every year at a temperature of about 20°–30°C. This is known as the "self discharge" rate and is due to non-current-producing "side" chemical reactions, which occur within the cell even if no load is applied to it. The rate of the side reactions is reduced if the batteries are stored at low temperature, although some batteries can be damaged by freezing. High or low temperatures may reduce battery performance. This will affect the initial voltage of the battery. For an AA alkaline battery, this initial voltage is approximately normally distributed around 1.6 volts.Discharging performance of all batteries drops at low temperature.
Secondary batteries
Storage life of secondary batteries is limited by chemical reactions that occur between the battery parts and the electrolyte; these are called "side reactions". Internal parts may corrode and fail, or the active materials may be slowly converted to inactive forms. Since the active material on the battery plates changes chemical composition on each charge and discharge cycle, active material may be lost due to physical changes of volume; this may limit the cycle life of the battery.Old chemistry rechargeable batteries self-discharge more rapidly than disposable alkaline batteries, especially nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
-based batteries; a freshly charged nickel cadmium (NiCd) battery loses 10% of its charge in the first 24 hours, and thereafter discharges at a rate of about 10% a month. However, newer low self-discharge nickel metal hydride (NiMH) batteries
Low self-discharge NiMH battery
The low self-discharge nickel-metal hydride battery was introduced in November 2005. These batteries were developed by Sanyo, who called them "eneloop". Subsequently, other manufacturers also offered LSD NiMH....
and modern lithium designs have reduced the self-discharge rate to a relatively low level (but still poorer than for primary batteries). Most nickel-based batteries are partially discharged when purchased, and must be charged before first use. Newer NiMH batteries are ready to be used when purchased, and have only 15% discharge in a year.
Although rechargeable batteries have their energy content restored by charging, some deterioration occurs on each charge–discharge cycle. Low-capacity NiMH batteries (1700–2000 mA·h) can be charged for about 1000 cycles, whereas high-capacity NiMH batteries (above 2500 mA·h) can be charged for about 500 cycles. NiCd batteries tend to be rated for 1000 cycles before their internal resistance permanently increases beyond usable values. Under normal circumstances, a fast charge, rather than a slow overnight charge, will shorten battery lifespan. However, if the overnight charger is not "smart" and cannot detect when the battery is fully charged, then overcharging is likely, which also damages the battery. Degradation usually occurs because electrolyte migrates away from the electrodes or because active material falls off the electrodes. NiCd batteries suffer the drawback that they should be fully discharged before recharge. Without full discharge, crystals may build up on the electrodes, thus decreasing the active surface area and increasing internal resistance. This decreases battery capacity and causes the "memory effect
Memory effect
Memory effect, also known as battery effect, lazy battery effect or battery memory, is an alleged effect observed in nickel cadmium rechargeable batteries that causes them to hold less charge...
". These electrode crystals can also penetrate the electrolyte separator, thereby causing shorts. NiMH, although similar in chemistry, does not suffer from memory effect to quite this extent. When a battery reaches the end of its lifetime, it will not suddenly lose all of its capacity; rather, its capacity will gradually decrease.
Automotive lead–acid rechargeable batteries have a much harder life. Because of vibration, shock, heat, cold, and sulfation of their lead plates, few automotive batteries last beyond six years of regular use. Automotive starting batteries have many thin plates to provide as much current as possible in a reasonably small package. In general, the thicker the plates, the longer the life of the battery. They are typically drained only a small amount before recharge. Care should be taken to avoid deep discharging a starting battery, since each charge and discharge cycle causes active material to be shed from the plates.
"Deep-cycle" lead–acid batteries such as those used in electric golf carts have much thicker plates to aid their longevity. The main benefit of the lead–acid battery is its low cost; the main drawbacks are its large size and weight for a given capacity and voltage. Lead–acid batteries should never be discharged to below 20% of their full capacity, because internal resistance will cause heat and damage when they are recharged. Deep-cycle lead–acid systems often use a low-charge warning light or a low-charge power cut-off switch to prevent the type of damage that will shorten the battery's life.
Extending battery life
Battery life can be extended by storing the batteries at a low temperature, as in a refrigeratorRefrigerator
A refrigerator is a common household appliance that consists of a thermally insulated compartment and a heat pump that transfers heat from the inside of the fridge to its external environment so that the inside of the fridge is cooled to a temperature below the ambient temperature of the room...
or freezer, which slows the chemical reactions in the battery. Such storage can extend the life of alkaline batteries by about 5%, while the charge of rechargeable batteries can be extended from a few days up to several months. To reach their maximum voltage, batteries must be returned to room temperature; discharging an alkaline battery at 250 mA at 0°C is only half as efficient as it is at 20°C. As a result, alkaline battery manufacturers like Duracell
Duracell
Duracell is a brand of batteries manufactured by Procter & Gamble.Additionally, Duracell owns the Procell professional-use brand.-Products:Duracell manufactures alkaline batteries in many common sizes, such as AAA, AA, C, D, and 9V...
do not recommend refrigerating or freezing batteries.
Prolonging life in multiple cells through cell balancing
Analog front ends that balance cells and eliminate mismatches of cells in series or parallel combination significantly improve battery efficiency and increase the overall pack capacity. As the number of cells and load currents increase, the potential for mismatch also increases. There are two kinds of mismatch in the pack: state-of-charge (SOC) mismatch and capacity/energy (C/E) mismatch. Though the SOC mismatch is more common, each problem limits the pack capacity (mAh) to the capacity of the weakest cell.Cell balancing principle
Battery pack cells are balanced when all the cells in the battery pack meet two conditions:
- If all cells have the same capacity, then they are balanced when they have the same State of Charge (SOC.) In this case, the Open Circuit Voltage (OCV) is a good measure of the SOC. If, in an out of balance pack, all cells can be differentially charged to full capacity (balanced), then they will subsequently cycle normally without any additional adjustments. This is mostly a one-shot fix.
- If the cells have different capacities, they are also considered balanced when the SOC is the same. But, since SOC is a relative measure, the absolute amount of capacity for each cell is different. To keep the cells with different capacities at the same SOC, cell balancing must provide differential amounts of current to cells in the series string during both charge and discharge on every cycle.
Cell balancing electronics
Cell balancing is defined as the application of differential currents to individual cells (or combinations of cells) in a series string. Under normal circumstances, of course, cells in a series string receive identical currents. A battery pack requires additional components and circuitry to achieve cell balancing. However, the use of a fully integrated analog front end for cell balancing reduces the required external components to just balancing resistors.
It is important to recognize that the cell mismatch results more from limitations in process control and inspection than from variations inherent in the lithium ion chemistry. The use of a fully integrated analog front end for cell balancing can improve the performance of series connected Li-ion Cells by addressing both SOC and C/E issues. SOC mismatch can be remedied by balancing the cell during an initial conditioning period and subsequently only during the charge phase. C/E mismatch remedies are more difficult to implement and harder to measure and require balancing during both charge and discharge periods.
This solution eliminates the quantity of external components, as for discrete capacitors, diodes, and most other resistors to achieve balance.
Battery sizes
Primary batteries readily available to consumers range from tiny button cellButton cell
A watch battery or button cell is a small single cell battery shaped as a squat cylinder typically 5 to 12 mm in diameter and 1 to 6 mm high—like a button on a garment, hence the name. Button cells are used to power small portable electronics devices such as wrist watches, pocket...
s used for electric watches, to the No. 6 cell used for signal circuits or other long duration applications. Secondary cells are made in very large sizes, from car batteries to systems used for uninterruptible power supply
Uninterruptible power supply
An uninterruptible power supply, also uninterruptible power source, UPS or battery/flywheel backup, is an electrical apparatus that provides emergency power to a load when the input power source, typically mains power, fails...
in large computer data centers. Large secondary batteries have been connected to the electrical grid to stabilize the system and help level out peak loads.
Explosion
A battery explosion is caused by the misuse or malfunction of a battery, such as attempting to recharge a primary (non-rechargeable) battery, or short circuitShort circuit
A short circuit in an electrical circuit that allows a current to travel along an unintended path, often where essentially no electrical impedance is encountered....
ing a battery. With car batteries, explosions are most likely to occur when a short circuit generates very large currents. In addition, car batteries liberate hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
when they are overcharged (because of electrolysis
Electrolysis
In chemistry and manufacturing, electrolysis is a method of using a direct electric current to drive an otherwise non-spontaneous chemical reaction...
of the water in the electrolyte). The amount of overcharging is usually very small, as is the amount of explosive gas developed, and the gas dissipates quickly. However, when "jumping" a car battery, the high current can cause the rapid release of large volumes of hydrogen, which can be ignited by a nearby spark (for example, when removing the jumper cables).
When a battery is recharged at an excessive rate, an explosive gas mixture of hydrogen and oxygen may be produced faster than it can escape from within the walls of the battery, leading to pressure build-up and the possibility of bursting of the battery case. In extreme cases, the battery acid may spray violently from the casing of the battery and cause injury. Overcharging—that is, attempting to charge a battery beyond its electrical capacity—can also lead to a battery explosion, leakage, or irreversible damage to the battery. It may also cause damage to the charger or device in which the overcharged battery is later used. In addition, disposing of a battery in fire may cause an explosion as steam builds up within the sealed case of the battery.
Leakage
Many battery chemicals are corrosive, poisonous, or both. If leakage occurs, either spontaneously or through accident, the chemicals released may be dangerous.For example, disposable batteries often use a zinc "can" both as a reactant and as the container to hold the other reagents. If this kind of battery is run all the way down, or if it is recharged after running down too far, the reagents can emerge through the cardboard and plastic that form the remainder of the container. The active chemical leakage can then damage the equipment that the batteries were inserted into. For this reason, many electronic device manufacturers recommend removing the batteries from devices that will not be used for extended periods of time.
Environmental concerns
The widespread use of batteries has created many environmental concernsElectronic waste
Electronic waste, e-waste, e-scrap, or Waste Electrical and Electronic Equipment describes discarded electrical or electronic devices. There is a lack of consensus as to whether the term should apply to resale, reuse, and refurbishing industries, or only to product that cannot be used for its...
, such as toxic metal pollution. Battery manufacture consumes resources and often involves hazardous chemicals. Used batteries also contribute to electronic waste
Electronic waste
Electronic waste, e-waste, e-scrap, or Waste Electrical and Electronic Equipment describes discarded electrical or electronic devices. There is a lack of consensus as to whether the term should apply to resale, reuse, and refurbishing industries, or only to product that cannot be used for its...
. Some areas now have battery recycling
Recycling
Recycling is processing used materials into new products to prevent waste of potentially useful materials, reduce the consumption of fresh raw materials, reduce energy usage, reduce air pollution and water pollution by reducing the need for "conventional" waste disposal, and lower greenhouse...
services available to recover some of the materials from used batteries. Batteries may be harmful or fatal if swallowed
Swallowing
Swallowing, known scientifically as deglutition, is the process in the human or animal body that makes something pass from the mouth, to the pharynx, and into the esophagus, while shutting the epiglottis. If this fails and the object goes through the trachea, then choking or pulmonary aspiration...
. Recycling or proper disposal prevents dangerous elements (such as lead
Lead
Lead is a main-group element in the carbon group with the symbol Pb and atomic number 82. Lead is a soft, malleable poor metal. It is also counted as one of the heavy metals. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed...
, mercury
Mercury (element)
Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum...
, and cadmium
Cadmium
Cadmium is a chemical element with the symbol Cd and atomic number 48. This soft, bluish-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Similar to zinc, it prefers oxidation state +2 in most of its compounds and similar to mercury it shows a low...
) found in some types of batteries from entering the environment. In the United States, Americans purchase nearly three billion batteries annually, and about 179,000 tons of those end up in landfills across the country.
In the United States, the Mercury-Containing and Rechargeable Battery Management Act
Mercury-containing and Rechargeable Battery Management Act
In the United States, the Mercury-Containing and Rechargeable Battery Management Act was signed into law on May 13, 1996...
of 1996 banned the sale of mercury-containing batteries, enacted uniform labeling requirements for rechargeable batteries, and required that rechargeable batteries be easily removable. California, and New York City prohibit the disposal of rechargeable batteries in solid waste, and along with Maine require recycling of cell phones. The rechargeable battery industry has nationwide recycling programs in the United States and Canada, with dropoff points at local retailers.
The Battery Directive
Battery Directive
Directive 2006/66/EC of the European Parliament and of the Council of 6 September 2006 on batteries and accumulators and waste batteries and accumulators and repealing Directive 91/157/EEC, commonly known as the Battery Directive, regulates the manufacture and disposal of batteries in the European...
of the European Union has similar requirements, in addition to requiring increased recycling of batteries, and promoting research on improved battery recycling methods.
In accordance with this directive all batteries to be sold within the EU must be marked with the "collection symbol" (A crossed out wheeled bin). This must cover at least 3% of the surface of prismatic batteries and 1.5% of the surface of cylindrical batteries. All packaging must be marked likewise.
Ingestion
Small button/disk batteries can be swallowed by young children. While in the digestive tract the battery's electrical discharge may lead to tissue damage; such damage is occasionally serious and very rarely even leads to death. Disk batteries do not usually cause problems unless they become lodged in the gastrointestinal (GI) tract. The most common place disk batteries become lodged, resulting in clinical sequelae, is the esophagus. Batteries that successfully traverse the esophagus are unlikely to lodge at any other location. The likelihood that a disk battery will lodge in the esophagus is a function of the patient's age and the size of the battery. Disk batteries of 16 mm have become lodged in the esophagi of 2 children younger than 1 year. Older children do not have problems with batteries smaller than 21–23 mm. Liquefaction necrosis may occur because sodium hydroxide is generated by the current produced by the battery (usually at the anode). Perforation has occurred as rapidly as 6 hours after ingestion.Primary battery chemistries
| Chemistry | Nominal Cell Voltage | Specific Energy [MJ/kg] | Elaboration |
|---|---|---|---|
| Zinc–carbon | 1.5 | 0.13 | Inexpensive. |
| Zinc–chloride | 1.5 | Also known as "heavy duty", inexpensive. | |
| Alkaline Alkaline battery Alkaline batteries are a type of primary batteries dependent upon the reaction between zinc and manganese dioxide . A rechargeable alkaline battery allows reuse of specially designed cells.... (zinc–manganese dioxide) |
1.5 | 0.4-0.59 | Moderate energy density. Good for high and low drain uses. |
| Nickel oxyhydroxide Nickel oxyhydroxide battery Nickel oxyhydroxide battery is a type of primary cell . NiOx batteries excel in high-drain applications such as digital cameras, and in this role, they can provide up to twice the life of an alkaline battery.... (zinc–manganese dioxide/nickel oxyhydroxide) |
1.7 | Moderate energy density. Good for high drain uses |
|
| Lithium Lithium battery Lithium batteries are disposable batteries that have lithium metal or lithium compounds as an anode. Depending on the design and chemical compounds used, lithium cells can produce voltages from 1.5 V to about 3.7 V, over twice the voltage of an ordinary zinc–carbon battery or alkaline battery... (lithium–copper oxide) Li–CuO |
1.7 | No longer manufactured. Replaced by silver oxide (IEC International Electrotechnical Commission The International Electrotechnical Commission is a non-profit, non-governmental international standards organization that prepares and publishes International Standards for all electrical, electronic and related technologies – collectively known as "electrotechnology"... -type "SR") batteries. |
|
| Lithium Lithium battery Lithium batteries are disposable batteries that have lithium metal or lithium compounds as an anode. Depending on the design and chemical compounds used, lithium cells can produce voltages from 1.5 V to about 3.7 V, over twice the voltage of an ordinary zinc–carbon battery or alkaline battery... (lithium–iron disulfide) LiFeS2 |
1.5 | Expensive. Used in 'plus' or 'extra' batteries. |
|
| Lithium Lithium battery Lithium batteries are disposable batteries that have lithium metal or lithium compounds as an anode. Depending on the design and chemical compounds used, lithium cells can produce voltages from 1.5 V to about 3.7 V, over twice the voltage of an ordinary zinc–carbon battery or alkaline battery... (lithium–manganese dioxide) LiMnO2 |
3.0 | 0.83-1.01 | Expensive. Only used in high-drain devices or for long shelf life due to very low rate of self discharge. 'Lithium' alone usually refers to this type of chemistry. |
| Mercury oxide Mercury battery A mercury battery is a non-rechargeable electrochemical battery, a primary cell. Due to the content of mercury, and the resulting environmental concerns, the sale of mercury batteries is banned in many countries. Both ANSI and IEC have withdrawn standards for mercury batteries... |
1.35 | High drain and constant voltage. Banned in most countries because of health concerns. |
|
| Zinc–air | 1.35–1.65 | 1.59 | Mostly used in hearing aids. |
| Silver-oxide Silver-oxide battery A silver oxide battery , not to be confused with a similar but different silver–zinc battery, which is a secondary cell, is a primary cell with relatively very high energy/weight ratio. They are costly due to the high price of silver... (silver–zinc) |
1.55 | 0.47 | Very expensive. Only used commercially in 'button' cells. |
Rechargeable battery chemistries
| Chemistry | Cell Voltage | Specific Energy [MJ/kg] | Comments |
|---|---|---|---|
| NiCd | 1.2 | 0.14 | Inexpensive. High/low drain, moderate energy density. Can withstand very high discharge rates with virtually no loss of capacity. Moderate rate of self discharge. Reputed to suffer from memory effect Memory effect Memory effect, also known as battery effect, lazy battery effect or battery memory, is an alleged effect observed in nickel cadmium rechargeable batteries that causes them to hold less charge... (which is alleged to cause early failure). Environmental hazard due to Cadmium – use now virtually prohibited in Europe. |
| Lead–acid | 2.1 | 0.14 | Moderately expensive. Moderate energy density. Moderate rate of self discharge. Higher discharge rates result in considerable loss of capacity. Does not suffer from memory effect Memory effect Memory effect, also known as battery effect, lazy battery effect or battery memory, is an alleged effect observed in nickel cadmium rechargeable batteries that causes them to hold less charge... . Environmental hazard due to Lead. Common use – Automobile batteries |
| NiMH | 1.2 | 0.36 | Inexpensive. Performs better than alkaline batteries in higher drain devices. Traditional chemistry has high energy density, but also a high rate of self-discharge. Newer chemistry has low self-discharge rate Low self-discharge NiMH battery The low self-discharge nickel-metal hydride battery was introduced in November 2005. These batteries were developed by Sanyo, who called them "eneloop". Subsequently, other manufacturers also offered LSD NiMH.... , but also a ~25% lower energy density. Very heavy. Used in some cars. |
| NiZn | 1.6 | 0.36 | Moderately inexpensive. High drain device suitable. Low self-discharge rate. Voltage closer to alkaline primary cells than other secondary cells. No toxic components. Newly introduced to the market (2009). Has not yet established a track record. Limited size availability. |
| Lithium ion | 3.6 | 0.46 | Very expensive. Very high energy density. Not usually available in "common" battery sizes. Very common in laptop computers, moderate to high-end digital cameras and camcorders, and cellphones. Very low rate of self discharge. Volatile: Chance of explosion if short circuited, allowed to overheat, or not manufactured with rigorous quality standards. |
Homemade cells
Almost any liquid or moist object that has enough ions to be electrically conductive can serve as the electrolyte for a cell. As a novelty or science demonstration, it is possible to insert two electrodes made of different metals into a lemonLemon battery
A lemon battery is a device used in experiments proposed in many science textbooks around the world. It is made by inserting two different metallic objects, for example a galvanized nail and a copper coin, into a lemon. The copper coin serves as the positive electrode or cathode and the galvanized...
, potato
Potato
The potato is a starchy, tuberous crop from the perennial Solanum tuberosum of the Solanaceae family . The word potato may refer to the plant itself as well as the edible tuber. In the region of the Andes, there are some other closely related cultivated potato species...
, etc. and generate small amounts of electricity. "Two-potato clocks" are also widely available in hobby and toy stores; they consist of a pair of cells, each consisting of a potato (lemon, et cetera) with two electrodes inserted into it, wired in series to form a battery with enough voltage to power a digital clock. Homemade cells of this kind are of no real practical use, because they produce far less current—and cost far more per unit of energy generated—than commercial cells, due to the need for frequent replacement of the fruit or vegetable. In addition, one can make a voltaic pile from two coins (such as a nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
and a penny
Penny
A penny is a coin or a type of currency used in several English-speaking countries. It is often the smallest denomination within a currency system.-Etymology:...
) and a piece of paper towel
Paper towel
A paper towel is an absorbent textile made from paper instead of cloth. Unlike cloth towels, paper towels are disposable and intended to be used only once. Paper towels soak up water because they are loosely woven which enables water to travel between them, even against gravity...
dipped in salt water
Saline water
Saline water is a general term for water that contains a significant concentration of dissolved salts . The concentration is usually expressed in parts per million of salt....
. Such a pile would make very little voltage itself, but, when many of them are stacked together in series, they can replace normal batteries for a short amount of time.
Sony has developed a biological battery that generates electricity from sugar in a way that is similar to the processes observed in living organisms. The battery generates electricity through the use of enzymes that break down carbohydrates, which are, in essence, sugar. A similarly designed sugar drink powers a phone using enzymes to generate electricity from carbohydrates that covers the phone’s electrical needs. It needs only a pack of sugary drink and it generates water and oxygen while the battery dies out.
Lead acid cells can easily be manufactured at home, but a tedious charge/discharge cycle is needed to 'form' the plates. This is a process in which lead sulfate forms on the plates, and during charge is converted to lead dioxide (positive plate) and pure lead (negative plate). Repeating this process results in a microscopically rough surface, with far greater surface area being exposed. This increases the current the cell can deliver. For an example, see http://windpower.org.za/batteries/batteries.html.
Daniell cell
Daniell cell
The Daniell cell was invented in 1836 by John Frederic Daniell, a British chemist and meteorologist, and consisted of a copper pot filled with a copper sulfate solution, in which was immersed an unglazed earthenware container filled with sulfuric acid and a zinc electrode...
s are also easy to make at home. Aluminum–air batteries can also be produced with high-purity aluminum. Aluminum foil batteries will produce some electricity, but they are not very efficient, in part because a significant amount of hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
gas is produced.
See also
- Automotive battery
- Battery electric vehicleBattery electric vehicleA battery electric vehicle, or BEV, is a type of electric vehicle that uses chemical energy stored in rechargeable battery packs. BEVs use electric motors and motor controllers instead of, or in addition to, internal combustion engines for propulsion.A battery-only electric vehicle or...
- Battery (vacuum tube)
- Battery DirectiveBattery DirectiveDirective 2006/66/EC of the European Parliament and of the Council of 6 September 2006 on batteries and accumulators and waste batteries and accumulators and repealing Directive 91/157/EEC, commonly known as the Battery Directive, regulates the manufacture and disposal of batteries in the European...
- Battery holderBattery holderA battery holder is one or more compartments or chambers for holding a battery. For dry cells, the holder must also make electrical contact with the battery terminals...
- Battery isolatorBattery isolatorA battery isolator is an electrical device that divides direct current into multiple branches and only allows current in one direction in each branch. The primary benefit of such an arrangement is the ability to simultaneously charge more than one battery from a single power source without...
- Battery management systemBattery Management SystemA battery management system is any electronic system that manages a rechargeable battery , such as by monitoring its state, calculating secondary data, reporting that data, protecting the battery, controlling its environment, and / or balancing it.-Monitor:A BMS may monitor the state of the...
- Battery nomenclatureBattery nomenclatureStandard battery nomenclature describes portable dry cell batteries that are interchangeable in physical dimensions and electrical characteristics between manufacturers. The long history of disposable dry cells means that many different manufacturer-specific and national standards were used to...
- Battery packBattery packA battery pack is a set of any number of identical batteries or individual battery cells. They may be configured in a series, parallel or a mixture of both to deliver the desired voltage, capacity, or power density...
- Battery recyclingBattery recyclingBattery recycling is a recycling activity that aims to reduce the number of batteries being disposed as municipal solid waste. Batteries contain a number of heavy metals and toxic chemicals, their dumping has raised concern over risks of soil contamination and water pollution.-Battery recycling by...
- Battery terminalsBattery terminalsBattery terminals are the electrical contacts used to connect a load and/or charger to a single cell or multiple-cell battery. These terminals have a wide variety of designs, sizes, and features that are often not well documented....
- Depth of dischargeDepth of dischargeDepth of discharge is an alternate method to indicate a battery's state of charge . The DOD is the inverse of SOC: as one increases, the other decreases. While the SOC units are percent points , the units for DOD can be Ah or percent points...
- Electrochemical cellElectrochemical cellAn electrochemical cell is a device capable of either deriving electrical energy from chemical reactions, or facilitating chemical reactions through the introduction of electrical energy. A common example of an electrochemical cell is a standard 1.5-volt "battery"...
- Energy densityEnergy densityEnergy density is a term used for the amount of energy stored in a given system or region of space per unit volume. Often only the useful or extractable energy is quantified, which is to say that chemically inaccessible energy such as rest mass energy is ignored...
- Energy storageEnergy storageEnergy storage is accomplished by devices or physical media that store some form of energy to perform some useful operation at a later time. A device that stores energy is sometimes called an accumulator....
- Flexible battery
- Galvanic cellGalvanic cellA Galvanic cell, or Voltaic cell, named after Luigi Galvani, or Alessandro Volta respectively, is an electrochemical cell that derives electrical energy from spontaneous redox reaction taking place within the cell...
- List of battery sizes
- List of battery types
- Nano titanateTitanateIn chemistry, titanate usually refers to inorganic compounds composed of titanium oxides. In some cases, the term is used more generally for any titanium-containing anion, e.g. [TiCl6]2- and [Ti7]2-. This article focuses on the oxides....
- Nanowire batteryNanowire batteryA nanowire battery is a lithium-ion battery invented by a team led by Dr. Yi Cui at Stanford University in 2007. The team's invention consists of a stainless steel anode covered in silicon nanowires, to replace the traditional graphite anode...
- Printed battery
- Rechargeable batteryRechargeable batteryA rechargeable battery or storage battery is a group of one or more electrochemical cells. They are known as secondary cells because their electrochemical reactions are electrically reversible. Rechargeable batteries come in many different shapes and sizes, ranging anything from a button cell to...
- State of chargeState of chargeState of charge is the equivalent of a fuel gauge for the battery pack in a battery electric vehicle , hybrid vehicle , or plug-in hybrid electric vehicle...
- State of healthState Of HealthState of health is a figure of merit of the condition of a battery , compared to its ideal conditions. The units of SOH are percent points ....
- Thermal runawayThermal runawayThermal runaway refers to a situation where an increase in temperature changes the conditions in a way that causes a further increase in temperature, often leading to a destructive result...
- Trickle chargingTrickle chargingTrickle charging, or float charging, means charging a battery at a similar rate as its self-discharging rate, thus maintaining a full capacity battery. Most rechargeable batteries, particularly nickel-cadmium batteries or nickel metal hydride batteries, have a moderate rate of self-discharge,...
Further reading
Ch. 21 (pp. 662–695) is on electrochemistry. Chs. 28-31 (pp. 879–995) contain information on electric potential. Chs. 8-9 (pp. 336–418) have more information on batteries.External links
- Battery University
- The Electropaedia on Battery Chemistries and How They Work
- Non-rechargeable batteries
- HowStuffWorks: How batteries work
- Comprehensive knowledge base about battery technology, battery applications, chargers and ancillary equipment
- DoITPoMS Teaching and Learning Package- "Batteries"

