
Leclanché cell
Encyclopedia
Georges Leclanché
Georges Leclanché was a French electrical engineer chiefly remembered for his invention of the Leclanché cell, one of the first modern electrical batteries and the forerunner of the modern dry cell battery.-Biography:...
invented and patented his battery, the Leclanché cell, in 1866. The battery contained a conducting solution (electrolyte
Electrolyte
In chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
) of ammonium chloride
Ammonium chloride
Ammonium chloride NH4Cl is an inorganic compound with the formula NH4Cl. It is a white crystalline salt that is highly soluble in water. Solutions of ammonium chloride are mildly acidic. Sal ammoniac is a name of natural, mineralogical form of ammonium chloride...
, a cathode
Cathode
A cathode is an electrode through which electric current flows out of a polarized electrical device. Mnemonic: CCD .Cathode polarity is not always negative...
(negative terminal) of carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
, a depolarizer
Depolarizer
A depolarizer or depolariser, in electrochemistry, according to an IUPAC definition, is a synonym of electroactive substance, i.e., a substance which changes its oxidation state, or partakes in a formation or breaking of chemical bonds, in a charge-transfer step of an electrochemical reaction.In...
of manganese dioxide, and an anode
Anode
An anode is an electrode through which electric current flows into a polarized electrical device. Mnemonic: ACID ....
(positive terminal) of zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
. The chemistry of this cell was later successfully adapted to manufacture of dry cell
Dry Cell
-Dry Cell's formation:Part of the band formed in 1998 when guitarist Danny Hartwell and drummer Brandon Brown met at the Ratt Show on the Sunset Strip. They later met up with then-vocalist Judd Gruenbaum. The original name of the band was "Beyond Control"....
s.
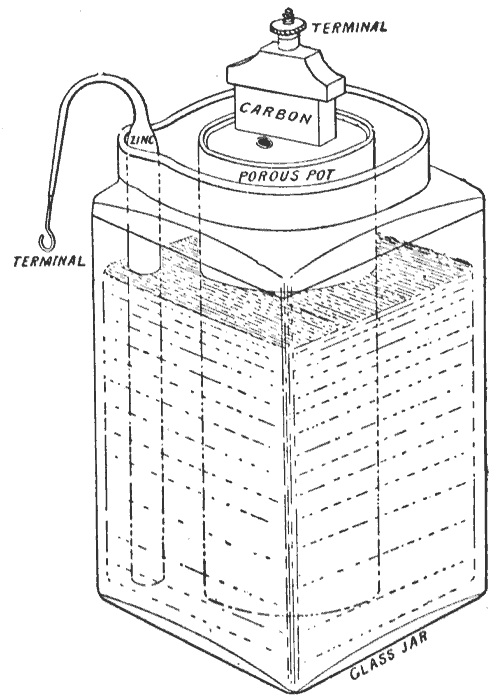
Construction
The original form of the cell used a porous pot. This gave it a relatively high internal resistance and various modifications were made to reduce it. These included the "Agglomerate block cell" and the "Sack cell".Porous pot cell
In Leclanché's original cell the depolarizer, which consisted of crushed manganese dioxide, was packed into a pot, and a carbon rod was inserted to act as the cathode. The anode, which was a zinc rod, was then immersed along with the pot in a solution of ammonium chloride. The liquid solution acted as the electrolyte, permeating through the porous pot to make contact with the cathode.Agglomerate block cell
In 1871 Leclanché dispensed with the porous pot and replaced it with a pair of "agglomerate blocks", attached to the carbon plate by rubber bands. These blocks were made by mixing the manganese dioxide with binding agents and pressing the mixture into moulds.Sack cell
In this cell the porous pot was replaced by a wrapping of canvas or sacking. In addition, the zinc rod was replaced by a zinc cylinder to give a larger surface area. It had a lower internal resistance than either of the above (porous and agglomerate).Chemistry
The chemical process which produces electricity in a Leclanché cell begins when zinc atoms on the surface of the anode oxidize, ie they give up both their electrons to become positively-charged ions. As the zinc ions move away from the anode, leaving their electrons on its surface, the anode becomes more negatively charged than the cathode. When the cell is connected in an external electrical circuit, the excess electrons on the zinc anode flow through the circuit to the carbon rod, the movement of electrons forming an electrical current.When the electrons enter the rod, they combine with manganese dioxide and water, which react with each other to produce manganese oxide and negatively charged hydroxide ions. This is accompanied by a secondary reaction in which the negative hydroxide ions react with positive ammonium ions in the ammonium chloride electrolyte to produce molecules of ammonia and water.
Zn(s) + 2 MnO2(s) + 2 NH4Cl(aq) → ZnCl2 + Mn2O3(s) + 2 NH3(aq) + H2O
Alternately, the reaction proceeds further, the hydroxide ions reacting also with the manganese oxide to form manganese hydroxide.
Zn(s) + 2 MnO2(s) + 2 NH4Cl(aq) + 2H2O(l) → ZnCl2 + 2Mn(OH)3(s) + 2 NH3(aq)
Usage
The electromotive forceElectromotive force
In physics, electromotive force, emf , or electromotance refers to voltage generated by a battery or by the magnetic force according to Faraday's Law, which states that a time varying magnetic field will induce an electric current.It is important to note that the electromotive "force" is not a...
(emf) produced by a Leclanche cell is typically around 1.5 volt
Volt
The volt is the SI derived unit for electric potential, electric potential difference, and electromotive force. The volt is named in honor of the Italian physicist Alessandro Volta , who invented the voltaic pile, possibly the first chemical battery.- Definition :A single volt is defined as the...
s with a resistance
Electrical resistance
The electrical resistance of an electrical element is the opposition to the passage of an electric current through that element; the inverse quantity is electrical conductance, the ease at which an electric current passes. Electrical resistance shares some conceptual parallels with the mechanical...
of several ohm
Ohm
The ohm is the SI unit of electrical resistance, named after German physicist Georg Simon Ohm.- Definition :The ohm is defined as a resistance between two points of a conductor when a constant potential difference of 1 volt, applied to these points, produces in the conductor a current of 1 ampere,...
s where a porous pot is used. It saw extensive usage in telegraphy
Telegraphy
Telegraphy is the long-distance transmission of messages via some form of signalling technology. Telegraphy requires messages to be converted to a code which is known to both sender and receiver...
, signaling, electric bell
Electric bell
An electric bell is a mechanical bell that functions by means of an electromagnet. When an electric current is applied, it produces a repetitive buzzing or clanging sound...
s and similar applications where intermittent current was required and it was desirable that a battery should require little maintenance.
The Leclanché battery (or wet cell as it was referred to) was the forerunner of the modern dry cell
Dry Cell
-Dry Cell's formation:Part of the band formed in 1998 when guitarist Danny Hartwell and drummer Brandon Brown met at the Ratt Show on the Sunset Strip. They later met up with then-vocalist Judd Gruenbaum. The original name of the band was "Beyond Control"....
zinc-carbon battery
Zinc-carbon battery
A zinc–carbon dry cell or battery is packaged in a zinc can that serves as both a container and negative terminal. It was developed from the wet Leclanché cell . The positive terminal is a carbon rod surrounded by a mixture of manganese dioxide and carbon powder. The electrolyte used is a paste of...
.
Sources
- Practical Electricity by W. E. Ayrton and T. Mather, published by Cassell and Company, London, 1911, pp 188-193

