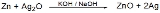
Silver-oxide battery
Encyclopedia
A silver oxide battery (IEC code: S), not to be confused with a similar but different silver–zinc battery, which is a secondary cell, is a primary cell
with relatively very high energy/weight ratio. They are costly due to the high price of silver. They are available in either very small sizes as button cell
s where the amount of silver used is small and not a significant contributor to the overall product costs, or in large custom design batteries where the superior performance characteristics of the silver oxide chemistry
outweigh cost considerations. The large cells found some applications with the military, for example in Mark 37 torpedo
es or on Alfa class submarine
s. Spent batteries may be processed for their silver content.
Silver-oxide primary batteries account for 21% of all primary battery sales in Japan (14% of all portable batteries) but by contrast only 0.05% of all battery sales in the UK including secondary types.
There exists a related device, usually called the silver–zinc battery, which uses a variation of silver–zinc chemistry to create a rechargeable power source. It shares most of the pros and cons of a silver-oxide battery, in addition to being able to withstand the largest loads of all known secondary power sources. Long used in specialist applications, they are now geared to enter the mainstream markets, for example as laptop
batteries.
battery uses silver oxide as the positive electrode
(cathode
), zinc
as the negative electrode (anode
) plus an alkaline electrolyte, usually sodium hydroxide (NaOH) or potassium hydroxide
(KOH). The silver
is reduced at the cathode from Ag(I) to Ag and the zinc
is oxidized from Zn to Zn(II). The chemical reaction that takes place inside the battery is the following:
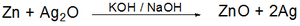 Zinc is the activator in the negative electrode and corrodes in alkaline solution. When this happens, it becomes difficult to maintain the capacity of the unused battery. The zinc corrosion causes electrolysis
Zinc is the activator in the negative electrode and corrodes in alkaline solution. When this happens, it becomes difficult to maintain the capacity of the unused battery. The zinc corrosion causes electrolysis
in the electrolyte
, resulting in the production of hydrogen
gas, a rise of inner pressure and expansion of the cell. Mercury
has been used in the past to suppress the corrosion, despite its harmful effects on the environment
.
The silver–zinc battery, on the other hand, uses the opposite electrode composition, the cathode
being made of pure silver, while the anode
is made from a mixture of zinc oxide
and pure zinc
powders. The electrolyte used is the pure caustic potash
solution without any added NaOH. Chemical processes during the discharge are similar to the silver oxide cell, while the different starting electrode composition makes it possible to recharge such a cell.
During the charge process, silver is first oxidized to silver(I) oxide
: 2Ag + 2OH– → Ag2O + H2O + 2e– and then to silver(II) oxide
: Ag + 2OH– → 2AgO + H2O + 2e–, while the zinc oxide is reduced to metallic zinc: 2Zn(OH)2 + 4e– = 2Zn + 4OH–. This process continues until the cell potential reaches the level where the electrolysis
of the hydroxide ion is possible, about 1.55 V. This is usually taken as the sign of the end of the charge, as at this point no other charge is taken, and the oxygen
generated poses a mechanical and fire hazard for the cell.
, and a flatter discharge curve than a standard alkaline battery
.
It provides up to 40 percent more run time than lithium-ion batteries and also feature a water-based chemistry that is free from the thermal runaway
and flammability problems that have plagued the lithium-ion alternatives.
prior to lithium technologies. Primarily developed for aircraft, they have long been used in space launchers and crewed spacecraft where their short cycle life is not a drawback. Non-rechargeable silver–zinc batteries powered the Saturn
launch vehicles, the Apollo Lunar Module
, lunar rover
and life support backpack
.
The primary power sources for the command module
were the hydrogen/oxygen fuel cells in the service module. They provided greater energy densities than any conventional battery, but peak power limitations required supplementation by silver–zinc batteries in the CM that also became its sole power supply during re-entry after separation of the service module. Only these batteries were recharged in flight.
After the Apollo 13
near-disaster, an auxiliary silver–zinc battery was added to the service module as a backup to the fuel cells. The Apollo service modules used as crew ferries to the Skylab
space station were powered by three silver–zinc batteries between undocking and SM jettison as the hydrogen and oxygen tanks could not store fuel cell reactants through the long stays at the station.
. The mercury was incorporated into the zinc anode to inhibit corrosion in the alkaline environment. Sony started producing the first silver oxide batteries without added mercury in 2004.
Primary cell
A primary cell is any kind of battery in which the electrochemical reaction is not reversible, rendering the cell non-rechargeable. A common example of a primary cell is the disposable battery. Unlike a secondary cell, the reaction cannot be reversed by running a current into the cell; the chemical...
with relatively very high energy/weight ratio. They are costly due to the high price of silver. They are available in either very small sizes as button cell
Button cell
A watch battery or button cell is a small single cell battery shaped as a squat cylinder typically 5 to 12 mm in diameter and 1 to 6 mm high—like a button on a garment, hence the name. Button cells are used to power small portable electronics devices such as wrist watches, pocket...
s where the amount of silver used is small and not a significant contributor to the overall product costs, or in large custom design batteries where the superior performance characteristics of the silver oxide chemistry
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
outweigh cost considerations. The large cells found some applications with the military, for example in Mark 37 torpedo
Mark 37 torpedo
The Mark 37 torpedo is a torpedo with electrical propulsion, developed for the US Navy after World War II. It entered service with the US Navy in the early 1950s, with over 3,300 produced. It was phased out of service with the US Navy during the 1970s, and the stockpiles were sold to foreign...
es or on Alfa class submarine
Alfa class submarine
The Soviet Union/Russian Navy Project 705 was a class of hunter/killer nuclear powered submarines. The class is also known by the NATO reporting name of Alfa...
s. Spent batteries may be processed for their silver content.
Silver-oxide primary batteries account for 21% of all primary battery sales in Japan (14% of all portable batteries) but by contrast only 0.05% of all battery sales in the UK including secondary types.
There exists a related device, usually called the silver–zinc battery, which uses a variation of silver–zinc chemistry to create a rechargeable power source. It shares most of the pros and cons of a silver-oxide battery, in addition to being able to withstand the largest loads of all known secondary power sources. Long used in specialist applications, they are now geared to enter the mainstream markets, for example as laptop
Laptop
A laptop, also called a notebook, is a personal computer for mobile use. A laptop integrates most of the typical components of a desktop computer, including a display, a keyboard, a pointing device and speakers into a single unit...
batteries.
Chemistry
A silver oxideSilver(I) oxide
Silver oxide is the chemical compound with the formula Ag2O. It is a fine black or dark brown powder that is used to prepare other silver compounds.-Preparation:...
battery uses silver oxide as the positive electrode
Electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit...
(cathode
Cathode
A cathode is an electrode through which electric current flows out of a polarized electrical device. Mnemonic: CCD .Cathode polarity is not always negative...
), zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
as the negative electrode (anode
Anode
An anode is an electrode through which electric current flows into a polarized electrical device. Mnemonic: ACID ....
) plus an alkaline electrolyte, usually sodium hydroxide (NaOH) or potassium hydroxide
Potassium hydroxide
Potassium hydroxide is an inorganic compound with the formula KOH, commonly called caustic potash.Along with sodium hydroxide , this colorless solid is a prototypical strong base. It has many industrial and niche applications. Most applications exploit its reactivity toward acids and its corrosive...
(KOH). The silver
Silver
Silver is a metallic chemical element with the chemical symbol Ag and atomic number 47. A soft, white, lustrous transition metal, it has the highest electrical conductivity of any element and the highest thermal conductivity of any metal...
is reduced at the cathode from Ag(I) to Ag and the zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
is oxidized from Zn to Zn(II). The chemical reaction that takes place inside the battery is the following:
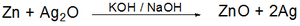
Electrolysis
In chemistry and manufacturing, electrolysis is a method of using a direct electric current to drive an otherwise non-spontaneous chemical reaction...
in the electrolyte
Electrolyte
In chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
, resulting in the production of hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
gas, a rise of inner pressure and expansion of the cell. Mercury
Mercury (element)
Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum...
has been used in the past to suppress the corrosion, despite its harmful effects on the environment
Natural environment
The natural environment encompasses all living and non-living things occurring naturally on Earth or some region thereof. It is an environment that encompasses the interaction of all living species....
.
The silver–zinc battery, on the other hand, uses the opposite electrode composition, the cathode
Cathode
A cathode is an electrode through which electric current flows out of a polarized electrical device. Mnemonic: CCD .Cathode polarity is not always negative...
being made of pure silver, while the anode
Anode
An anode is an electrode through which electric current flows into a polarized electrical device. Mnemonic: ACID ....
is made from a mixture of zinc oxide
Zinc oxide
Zinc oxide is an inorganic compound with the formula ZnO. It is a white powder that is insoluble in water. The powder is widely used as an additive into numerous materials and products including plastics, ceramics, glass, cement, rubber , lubricants, paints, ointments, adhesives, sealants,...
and pure zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
powders. The electrolyte used is the pure caustic potash
Potassium hydroxide
Potassium hydroxide is an inorganic compound with the formula KOH, commonly called caustic potash.Along with sodium hydroxide , this colorless solid is a prototypical strong base. It has many industrial and niche applications. Most applications exploit its reactivity toward acids and its corrosive...
solution without any added NaOH. Chemical processes during the discharge are similar to the silver oxide cell, while the different starting electrode composition makes it possible to recharge such a cell.
During the charge process, silver is first oxidized to silver(I) oxide
Silver(I) oxide
Silver oxide is the chemical compound with the formula Ag2O. It is a fine black or dark brown powder that is used to prepare other silver compounds.-Preparation:...
: 2Ag + 2OH– → Ag2O + H2O + 2e– and then to silver(II) oxide
Silver(II) oxide
Silver oxide is the inorganic compound with the formula AgO. It is a component of silver oxide-zinc alkaline batteries. It can be prepared by the slow addition of a silver salt to a persulfate solution e.g. AgNO3 to a Na2S2O8 solution...
: Ag + 2OH– → 2AgO + H2O + 2e–, while the zinc oxide is reduced to metallic zinc: 2Zn(OH)2 + 4e– = 2Zn + 4OH–. This process continues until the cell potential reaches the level where the electrolysis
Electrolysis
In chemistry and manufacturing, electrolysis is a method of using a direct electric current to drive an otherwise non-spontaneous chemical reaction...
of the hydroxide ion is possible, about 1.55 V. This is usually taken as the sign of the end of the charge, as at this point no other charge is taken, and the oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
generated poses a mechanical and fire hazard for the cell.
Characteristics
Compared to other batteries, a silver oxide battery has a higher open circuit potential than a mercury batteryMercury battery
A mercury battery is a non-rechargeable electrochemical battery, a primary cell. Due to the content of mercury, and the resulting environmental concerns, the sale of mercury batteries is banned in many countries. Both ANSI and IEC have withdrawn standards for mercury batteries...
, and a flatter discharge curve than a standard alkaline battery
Alkaline battery
Alkaline batteries are a type of primary batteries dependent upon the reaction between zinc and manganese dioxide . A rechargeable alkaline battery allows reuse of specially designed cells....
.
It provides up to 40 percent more run time than lithium-ion batteries and also feature a water-based chemistry that is free from the thermal runaway
Thermal runaway
Thermal runaway refers to a situation where an increase in temperature changes the conditions in a way that causes a further increase in temperature, often leading to a destructive result...
and flammability problems that have plagued the lithium-ion alternatives.
History
This technology had the highest energy densityEnergy density
Energy density is a term used for the amount of energy stored in a given system or region of space per unit volume. Often only the useful or extractable energy is quantified, which is to say that chemically inaccessible energy such as rest mass energy is ignored...
prior to lithium technologies. Primarily developed for aircraft, they have long been used in space launchers and crewed spacecraft where their short cycle life is not a drawback. Non-rechargeable silver–zinc batteries powered the Saturn
Saturn (rocket family)
The Saturn family of American rocket boosters was developed by a team of mostly German rocket scientists led by Wernher von Braun to launch heavy payloads to Earth orbit and beyond. Originally proposed as a military satellite launcher, they were adopted as the launch vehicles for the Apollo moon...
launch vehicles, the Apollo Lunar Module
Apollo Lunar Module
The Apollo Lunar Module was the lander portion of the Apollo spacecraft built for the US Apollo program by Grumman to carry a crew of two from lunar orbit to the surface and back...
, lunar rover
Lunar rover
The Lunar Roving Vehicle or lunar rover was a battery-powered four-wheeled rover used on the Moon in the last three missions of the American Apollo program during 1971 and 1972...
and life support backpack
Primary Life Support System
A Primary Life Support System , is a device connected to an astronaut's or cosmonaut's spacesuit, which allows extra-vehicular activity with maximum freedom, independent of a spacecraft's life support system. The PLSS is generally worn like a backpack...
.
The primary power sources for the command module
Apollo Command/Service Module
The Command/Service Module was one of two spacecraft, along with the Lunar Module, used for the United States Apollo program which landed astronauts on the Moon. It was built for NASA by North American Aviation...
were the hydrogen/oxygen fuel cells in the service module. They provided greater energy densities than any conventional battery, but peak power limitations required supplementation by silver–zinc batteries in the CM that also became its sole power supply during re-entry after separation of the service module. Only these batteries were recharged in flight.
After the Apollo 13
Apollo 13
Apollo 13 was the seventh manned mission in the American Apollo space program and the third intended to land on the Moon. The craft was launched on April 11, 1970, at 13:13 CST. The landing was aborted after an oxygen tank exploded two days later, crippling the service module upon which the Command...
near-disaster, an auxiliary silver–zinc battery was added to the service module as a backup to the fuel cells. The Apollo service modules used as crew ferries to the Skylab
Skylab
Skylab was a space station launched and operated by NASA, the space agency of the United States. Skylab orbited the Earth from 1973 to 1979, and included a workshop, a solar observatory, and other systems. It was launched unmanned by a modified Saturn V rocket, with a mass of...
space station were powered by three silver–zinc batteries between undocking and SM jettison as the hydrogen and oxygen tanks could not store fuel cell reactants through the long stays at the station.
Mercury content
Silver oxide batteries become hazardous on the onset of leakage; this generally takes five years from the time they are put into use (which coincides with their normal shelf life). Until recently, all silver oxide batteries contained up to 0.2% mercuryMercury (element)
Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum...
. The mercury was incorporated into the zinc anode to inhibit corrosion in the alkaline environment. Sony started producing the first silver oxide batteries without added mercury in 2004.
See also
- History of the batteryHistory of the batteryThe history of the development of electrochemical cells is crucial to the scientific study and industrial applications of electricity, for prior to the rise of electrical grids around the end of the 19th century, they were the main source of electricity...
- (Rechargeable) Secondary cell
- Fuel cellFuel cellA fuel cell is a device that converts the chemical energy from a fuel into electricity through a chemical reaction with oxygen or another oxidizing agent. Hydrogen is the most common fuel, but hydrocarbons such as natural gas and alcohols like methanol are sometimes used...
- Battery recyclingBattery recyclingBattery recycling is a recycling activity that aims to reduce the number of batteries being disposed as municipal solid waste. Batteries contain a number of heavy metals and toxic chemicals, their dumping has raised concern over risks of soil contamination and water pollution.-Battery recycling by...
- List of battery types
- Battery nomenclatureBattery nomenclatureStandard battery nomenclature describes portable dry cell batteries that are interchangeable in physical dimensions and electrical characteristics between manufacturers. The long history of disposable dry cells means that many different manufacturer-specific and national standards were used to...

