Oligonucleotide synthesis
Encyclopedia
Oligonucleotide synthesis is the chemical synthesis of relatively short fragments of nucleic acid
s with defined chemical structure (sequence). The technique is extremely useful in current laboratory practice because it provides a rapid and inexpensive access to custom-made oligonucleotides of the desired sequence. Whereas enzyme
s synthesize DNA
and RNA
in a 5' to 3' direction, chemical oligonucleotide synthesis is carried out in the opposite, 3' to 5' direction. Currently, the process is implemented as solid-phase synthesis
using phosphoramidite method and phosphoramidite building blocks derived from protected 2'-deoxynucleosides
(dA
, dC
, dG
, and T
), ribonucleosides
(A, C
, G
, and U
), or chemically modified nucleosides, e.g. LNA
. To obtain the desired oligonucleotide, the building blocks are sequentially coupled to the growing oligonucleotide chain in the order required by the sequence of the product (see Synthetic cycle below). The process has been fully automated since the late 1970s. Upon the completion of the chain assembly, the product is released from the solid phase to solution, deprotected, and collected. The occurrence of side reactions sets practical limits for the length of synthetic oligonucleotides (up to about 200 nucleotide
residues) because the number of errors accumulates with the length of the oligonucleotide being synthesized. Products are often isolated by HPLC
to obtain the desired oligonucleotides in high purity. Typically, synthetic oligonucleotides are single-stranded DNA or RNA molecules around 15–25 bases in length. They are most commonly used as antisense oligonucleotides, small interfering RNA
, primers
for DNA sequencing
and amplification
, probes
for detecting complementary DNA or RNA via molecular hybridization, tools for the targeted introduction of mutation
s and restriction sites, and for the synthesis of artificial gene
s.
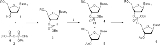
 In the early 1950s, Alexander Todd
In the early 1950s, Alexander Todd
’s group pioneered H-phosphonate and phosphate
triester methods of oligonucleotide synthesis. The reaction of compounds 1 and 2 to form H-phosphonate diester 3 is an H-phosphonate coupling in solution while that of compounds 4 and 5 to give 6 is a phosphotriester coupling (see Phosphotriester synthesis below).
 Thirty years later, this work inspired, independently, two research groups to adopt the H-phosphonate chemistry to the solid-phase synthesis using nucleoside H-phosphonate monoesters 7 as building blocks and pivaloyl chloride, 2,4,6-triisopropylbenzenesulfonyl chloride (TPS-Cl), and other compounds as activators. The practical implementation of H-phosphonate method resulted in a very short and simple synthetic cycle consisting of only two steps, detritylation and coupling (Scheme 2). Oxidation of internucleosidic H-phosphonate diester linkages in 8 to phosphodiester linkages in 9 (X = O) with a solution of iodine
Thirty years later, this work inspired, independently, two research groups to adopt the H-phosphonate chemistry to the solid-phase synthesis using nucleoside H-phosphonate monoesters 7 as building blocks and pivaloyl chloride, 2,4,6-triisopropylbenzenesulfonyl chloride (TPS-Cl), and other compounds as activators. The practical implementation of H-phosphonate method resulted in a very short and simple synthetic cycle consisting of only two steps, detritylation and coupling (Scheme 2). Oxidation of internucleosidic H-phosphonate diester linkages in 8 to phosphodiester linkages in 9 (X = O) with a solution of iodine
in aqueous pyridine
is carried out at the end of the chain assembly rather than as a step in the synthetic cycle. Alternatively, 8 can be converted to phosphorothioate 9 (X = S).
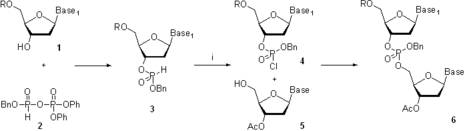
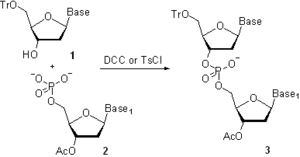 In the 1950s, Khorana and co-workers developed a phosphodiester method where 3’-O-acetylnucleoside-5’-O-phosphate 2 (Scheme 3) was activated with N,N-dicyclohexylcarbodiimide (DCC) or 4-toluenesulfonyl chloride
In the 1950s, Khorana and co-workers developed a phosphodiester method where 3’-O-acetylnucleoside-5’-O-phosphate 2 (Scheme 3) was activated with N,N-dicyclohexylcarbodiimide (DCC) or 4-toluenesulfonyl chloride
(Ts-Cl). The activated species were reacted with a 5’-O-protected nucleoside 1 to give a protected dinucleoside monophosphate 3. Upon the removal of 3’-O-acetyl group using base-catalyzed hydrolysis, further chain elongation was carried out. Following this methodology, sets of tri- and tetradeoxyribonucleotides were synthesized and were enzymatically converted to longer oligonucleotides, which allowed elucidation of the genetic code
. The major limitation of the phosphodiester method consisted in the formation of pyrophosphate oligomers and oligonucleotides branched at the internucleosidic phosphate. The method seems to be a step back from the more selective chemistry described earlier; however, at that time, most phosphate-protecting groups available now had not yet been introduced. The lack of the convenient protection strategy necessitated taking a retreat to a slower and less selective chemistry to achieve the ultimate goal of the study.
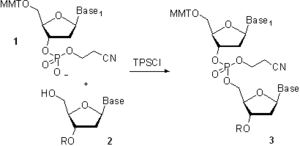 In the 1960s, groups led by R. Letsinger and C. Reese developed a phosphotriester approach. The defining difference from the phosphodiester approach was the protection of the phosphate moiety in the building block 1 (Scheme 4) and in the product 3 with 2-cyanoethyl group. This precluded the formation of oligonucleotides branched at the internucleosidic phosphate. The higher selectivity of the method allowed the use of more efficient coupling agents and catalysts, which dramatically reduced the length of the synthesis. The method, initially developed for the solution-phase synthesis, was also implemented on low-cross-linked “popcorn” polystyrene, which initiated a massive research effort in solid-phase synthesis of oligonucleotides and eventually led to the automation of the oligonucleotide chain assembly.
In the 1960s, groups led by R. Letsinger and C. Reese developed a phosphotriester approach. The defining difference from the phosphodiester approach was the protection of the phosphate moiety in the building block 1 (Scheme 4) and in the product 3 with 2-cyanoethyl group. This precluded the formation of oligonucleotides branched at the internucleosidic phosphate. The higher selectivity of the method allowed the use of more efficient coupling agents and catalysts, which dramatically reduced the length of the synthesis. The method, initially developed for the solution-phase synthesis, was also implemented on low-cross-linked “popcorn” polystyrene, which initiated a massive research effort in solid-phase synthesis of oligonucleotides and eventually led to the automation of the oligonucleotide chain assembly.
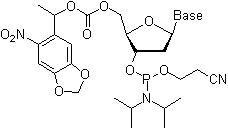
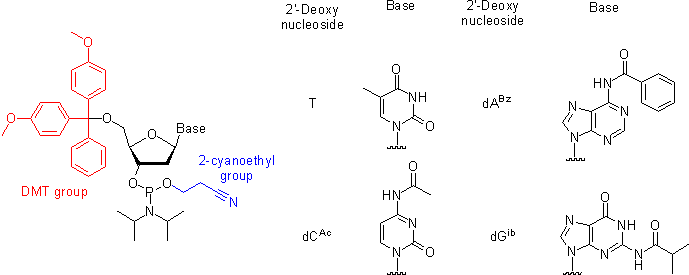 As mentioned above, the naturally occurring nucleotides (nucleoside-3'- or 5'-phosphates) and their phosphodiester analogs are insufficiently reactive to afford an expedite synthetic preparation of oligonucleotides in high yields. The selectivity and the rate of the formation of internucleosidic linkages is dramatically improved by using 3'-O-(N,N-diisopropyl phosphoramidite) derivatives of nucleosides (nucleoside phosphoramidites) that serve as building blocks in phosphite triester methodology. To prevent undesired side reactions, all other functional groups present in nucleosides have to be rendered unreactive (protected) by attaching protecting groups. Upon the completion of the oligonucleotide chain assembly, all the protecting groups are removed to yield the desired oligonucleotides. Below, the protecting groups currently used in commercially available and most common nucleoside phosphoramidite building blocks are briefly reviewed:
As mentioned above, the naturally occurring nucleotides (nucleoside-3'- or 5'-phosphates) and their phosphodiester analogs are insufficiently reactive to afford an expedite synthetic preparation of oligonucleotides in high yields. The selectivity and the rate of the formation of internucleosidic linkages is dramatically improved by using 3'-O-(N,N-diisopropyl phosphoramidite) derivatives of nucleosides (nucleoside phosphoramidites) that serve as building blocks in phosphite triester methodology. To prevent undesired side reactions, all other functional groups present in nucleosides have to be rendered unreactive (protected) by attaching protecting groups. Upon the completion of the oligonucleotide chain assembly, all the protecting groups are removed to yield the desired oligonucleotides. Below, the protecting groups currently used in commercially available and most common nucleoside phosphoramidite building blocks are briefly reviewed:
Non-nucleosidic phosphoramidites are used to introduce desired groups that are not available in natural nucleosides or that can be introduced more readily using simpler chemical designs. A very short selection of commercial phosphoramidite reagents is shown in Scheme for the demonstration of the available structural and functional diversity. These reagents serve for the attachment of 5'-terminal phosphate (1), NH2 (2), SH (3), aldehydo (4), and carboxylic groups (5), CC triple bonds (6), non-radioactive labels and quenchers
(exemplified by 6-FAM amidite
7 for the attachment of fluorescein
and dabcyl amidite 8, respectively), hydrophilic and hydrophobic modifiers (exemplified by hexaethyleneglycol amidite 9 and cholesterol
amidite 10, respectively), and biotin
amidite 11.
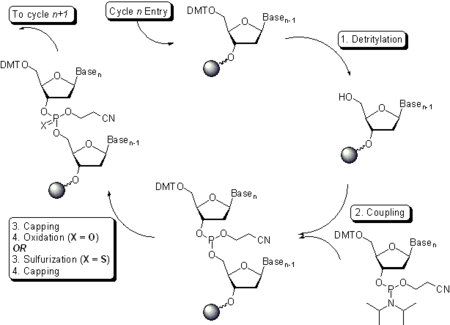 Oligonucleotide synthesis is carried out by a stepwise addition of nucleotide residues to the 5'-terminus of the growing chain until the desired sequence is assembled. Each addition is referred to as a synthetic cycle (Scheme 6) and consists of four chemical reactions:
Oligonucleotide synthesis is carried out by a stepwise addition of nucleotide residues to the 5'-terminus of the growing chain until the desired sequence is assembled. Each addition is referred to as a synthetic cycle (Scheme 6) and consists of four chemical reactions:
or 3% Dichloroacetic acid
(DCA), in an inert solvent (dichloromethane
or toluene
). The orange-colored DMT cation formed is washed out; the step results in the solid support-bound oligonucleotide precursor bearing a free 5'-terminal hydroxyl group.
It is worth remembering that conducting detritylation for an extended time or with stronger than recommended solutions of acids leads to depurination
of solid support-bound oligonucleotide and thus reduces the yield of the desired full-length product.
is activated by a 0.2 - 0.7M solution of an acidic azole
catalyst, 1H-tetrazole
, 2-ethylthiotetrazole, 2-benzylthiotetrazole, 4,5-dicyanoimidazole
, or a number of similar compounds. The mixing is usually very brief and occurs in fluid lines of oligonucleotide synthesizers (see below) while the components are being delivered to the reactors containing solid support. The activated phosphoramidite in 1.5 - 20-fold excess over the support-bound material is then brought in contact with the starting solid support (first coupling) or a support-bound oligonucleotide precursor (following couplings) whose 5'-hydroxy group reacts with the activated phosphoramidite moiety of the incoming nucleoside phosphoramidite to form a phosphite triester linkage. The phosphoramidite coupling is very rapid and requires, on small scale, about 20 s for its completion. The reaction is also highly sensitive to the presence of water, particularly when dilute solutions of phosphoramidites are used, and is commonly carried out in anhydrous acetonitrile. Generally, the larger the scale of the synthesis, the lower the excess and the higher the concentration of the phosphoramidites is used. In contrast, the concentration of the activator is primarily determined by its solubility in acetonitrile and is irrespective of the scale of the synthesis. Upon the completion of the coupling, any unbound reagents and by-products are removed by washing.
or, less often, DMAP
as catalysts and, in the phosphoramidite method, serves two purposes.
) oxidizes the phosphite triester into a tetracoordinated phosphate triester, a protected precursor of the naturally occurring phosphate diester internucleosidic linkage. This step is substituted with a sulfurization step to obtain oligonucleotide phosphorothioates. In the latter case, the sulfurization step is best carried out prior to capping.
bound, via its 3'-terminal hydroxy group, to a solid support material and remains attached to it over the entire course of the chain assembly. The solid support is contained in columns whose dimensions depend on the scale of synthesis and may vary between 0.05 mL and several litres. The overwhelming majority of oligonucleotides are synthesized on small scale ranging from 40 nmol
to 1 μmol. More recently, high-throughput oligonucleotide synthesis where the solid support is contained in the wells of multi-well plates (most often, 96 or 384 wells per plate) became a method of choice for parallel synthesis of oligonucleotides on small scale. At the end of the chain assembly, the oligonucleotide is released from the solid support and eluted from the column or the well.
, the synthesis of oligonucleotides proceeds best on non-swellable or low-swellable solid supports. The two most often used solid-phase materials are Controlled Pore Glass
(CPG) and macroporous polystyrene
(MPPS).
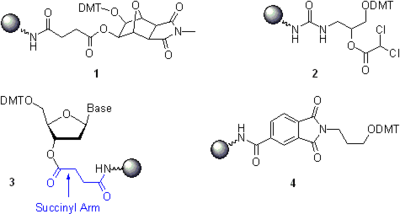
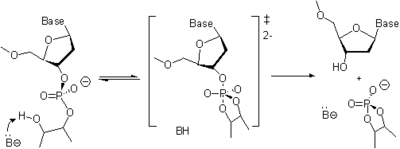 To make the solid support material suitable for oligonucleotide synthesis, non-nucleosidic linkers or nucleoside succinates are covalently attached to the reactive amino groups in Aminopropyl CPG, LCAA CPG, or Aminomethyl MPPS. The remaining unreacted amino groups are capped with acetic anhydride
To make the solid support material suitable for oligonucleotide synthesis, non-nucleosidic linkers or nucleoside succinates are covalently attached to the reactive amino groups in Aminopropyl CPG, LCAA CPG, or Aminomethyl MPPS. The remaining unreacted amino groups are capped with acetic anhydride
. Typically, three conceptually different groups of solid supports are used.
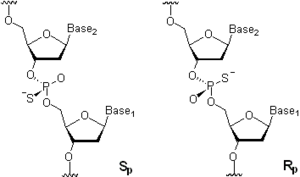 Oligonucleotide phosphorothioates (OPS) are modified oligonucleotides where one of the oxygen atoms in the phosphate moiety is replaced by sulfur. Only the phosphorothioates having sulfur at a non-bridging position as shown in figure are widely used and are available commercially. The replacement of the non-bridging oxygen with sulfur creates a new center of chirality
Oligonucleotide phosphorothioates (OPS) are modified oligonucleotides where one of the oxygen atoms in the phosphate moiety is replaced by sulfur. Only the phosphorothioates having sulfur at a non-bridging position as shown in figure are widely used and are available commercially. The replacement of the non-bridging oxygen with sulfur creates a new center of chirality
at phosphorus
. In a simple case of a dinucleotide, this results in the formation of a diastereomeric pair of Sp- and Rp-dinucleoside monophosphorothioates whose structures are shown in Figure. In a n-mer oligonucleotide where all (n – 1) internucleosidic linkages are phosphorothioate linkages, the number of diastereomers m is calculated as m = 2(n – 1). Being non-natural analogs of nucleic acids, OPS are substantially more stable towards hydrolysis
by nucleases, the class of enzymes that destoy nucleic acids by breaking the bridging P-O bond of the phosphodiester moiety. This property determines the use of OPS as antisense oligonucleotides in in vitro
and in vivo
applications where the extensive exposure to nucleases is inevitable. Similarly, to improve the stability of siRNA
, at least one phosphorothioate linkage is often introduced at the 3'-terminus of both sense
and antisense strands. In chirally pure OPS, all-Sp diastereomers are more stable to enzymatic degradation than their all-Rp analogs. However, the preparation of chirally pure OPS remains a synthetic challenge. In laboratory practice, mixtures of diastereomers of OPS are commonly used.
Synthesis of OPS is very similar to that of natural oligonucleotides. The difference is that the oxidation step is replaced by sulfur transfer reaction (sulfurization) and that the capping step is performed after the sulfurization. Of many reported reagents capable of the efficient sulfur transfer, only three are commercially available:
Currently, solid-phase oligonucleotide synthesis is carried out automatically using computer-controlled instruments (oligonucleotide synthesizers) and is technically implemented in column, multi-well plate, and array formats. The column format is best suited for research and large scale applications where a high-throughput is not required. Multi-well plate format is designed specifically for high-throughput synthesis on small scale to satisfy the growing demand of industry and academia for synthetic oligonucleotides. A number of oligonucleotide synthesizers for small scale synthesis and medium to large scale synthesis are available commercially.
To furnish a functional oligonucleotide, all the protecting groups have to be removed. The N-acyl base protection and the 2-cyanoethyl phosphate protection may be, and is often removed simultaneously by treatment with inorganic bases or amines. However, the applicability of this method is limited by the fact that the cleavage of 2-cyanoethyl phosphate protection gives rise to acrylonitrile
as a side product. Under the strong basic conditions required for the removal of N-acyl protection, acrylonitrile is capable of alkylation of nucleic bases, primarily, at the N3-position of thymine and uracil residues to give the respective N3-(2-cyanoethyl) adducts via Michael reaction
. The formation of these side products may be avoided by treating the solid support-boud oligonucleotides with solutions of bases in an organic solvent, for instance, with 50% triethylamine
in acetonitrile
or 10% diethylamine
in acetonitrile. This treatment is strongly recommended for medium- and large scale preparations and is optional for syntheses on small scale where the concentration of acrylonitrile generated in the deprotection mixture is low.
Regardless of whether the phosphate protecting groups were removed first, the solid support-bound oligonucleotides are deprotected using one of the two general approaches.
The fully deprotected product is used as is, or the desired oligonucleotide can be purified by a number of methods. Most commonly, the crude product is desalted using ethanol precipitation
, size exclusion chromatography
, or reverse-phase HPLC. To eliminate unwanted truncation products, the oligonucleotides can be purified via polyacrylamide gel electrophoresis
or anion-exchange HPLC followed by desalting.
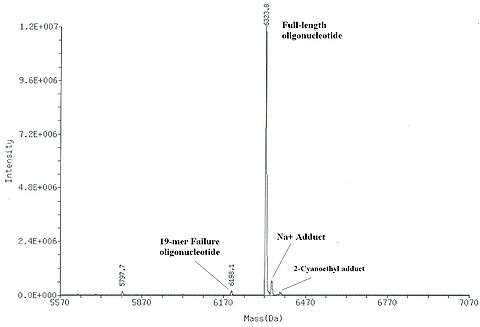 As with any other organic compound, it is prudent to characterize synthetic oligonucleotides upon their preparation. In more complex cases (research and large scale syntheses) oligonucleotides are characterized after their deprotection and after purification. Although the ultimate approach to the characterization is sequencing
As with any other organic compound, it is prudent to characterize synthetic oligonucleotides upon their preparation. In more complex cases (research and large scale syntheses) oligonucleotides are characterized after their deprotection and after purification. Although the ultimate approach to the characterization is sequencing
, a relatively inexpensive and routine procedure, the considerations of the cost reduction preclude its use in routine manufacturing of oligonucleotides. In day-by-day practice, it is sufficient to obtain the molecular mass
of an oligonucleotide by recording its mass spectrum
. Two methods are currently widely used for characterization of oligonucleotides: electrospray mass spectrometry (ES MS) and matrix-assisted laser desorption/ionization
time-of-flight mass spectrometry
(MALDI-TOF). Prior to submitting a sample to the analysis by either of the methods, it is very important to exchange all metal ions that might be present in the sample for ammonium or trialkylammonium (e.c. triethylammonium) ions.
Nucleic acid
Nucleic acids are biological molecules essential for life, and include DNA and RNA . Together with proteins, nucleic acids make up the most important macromolecules; each is found in abundance in all living things, where they function in encoding, transmitting and expressing genetic information...
s with defined chemical structure (sequence). The technique is extremely useful in current laboratory practice because it provides a rapid and inexpensive access to custom-made oligonucleotides of the desired sequence. Whereas enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
s synthesize DNA
DNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
and RNA
RNA
Ribonucleic acid , or RNA, is one of the three major macromolecules that are essential for all known forms of life....
in a 5' to 3' direction, chemical oligonucleotide synthesis is carried out in the opposite, 3' to 5' direction. Currently, the process is implemented as solid-phase synthesis
Solid-phase synthesis
In chemistry, solid-phase synthesis is a method in which molecules are bound on a bead and synthesized step-by-step in a reactant solution; compared with normal synthesis in a liquid state, it is easier to remove excess reactant or byproduct from the product. In this method, building blocks are...
using phosphoramidite method and phosphoramidite building blocks derived from protected 2'-deoxynucleosides
Nucleoside
Nucleosides are glycosylamines consisting of a nucleobase bound to a ribose or deoxyribose sugar via a beta-glycosidic linkage...
(dA
Deoxyadenosine
Deoxyadenosine is a deoxyribonucleoside. It is a derivative of the nucleoside adenosine, differing from the latter by the replacement of a hydroxyl group by hydrogen at the 2' position of its ribose sugar moiety. Deoxyadenosine is the DNA nucleoside A, which pairs with deoxythymidine in...
, dC
Deoxycytidine
Deoxycytidine is a deoxyribonucleoside. It is like cytidine, but with one oxygen atom removed....
, dG
Deoxyguanosine
Deoxyguanosine is composed of the purine nucleoside guanine linked by its N9 nitrogen to the C1 carbon of deoxyribose. It is similar to guanosine, but with one hydroxyl group removed from the 2' position of the ribose sugar . If a phosphate group is attached at the 5' position, it becomes...
, and T
Thymidine
Thymidine is a chemical compound, more precisely a pyrimidine deoxynucleoside. Deoxythymidine is the DNA nucleoside T, which pairs with deoxyadenosine in double-stranded DNA...
), ribonucleosides
Nucleoside
Nucleosides are glycosylamines consisting of a nucleobase bound to a ribose or deoxyribose sugar via a beta-glycosidic linkage...
(A, C
Cytidine
Cytidine is a nucleoside molecule that is formed when cytosine is attached to a ribose ring via a β-N1-glycosidic bond...
, G
Guanosine
Guanosine is a purine nucleoside comprising guanine attached to a ribose ring via a β-N9-glycosidic bond. Guanosine can be phosphorylated to become guanosine monophosphate , cyclic guanosine monophosphate , guanosine diphosphate , and guanosine triphosphate...
, and U
Uridine
Uridine is a molecule that is formed when uracil is attached to a ribose ring via a β-N1-glycosidic bond.If uracil is attached to a deoxyribose ring, it is known as a deoxyuridine....
), or chemically modified nucleosides, e.g. LNA
Locked nucleic acid
A locked nucleic acid , often referred to as inaccessible RNA, is a modified RNA nucleotide. The ribose moiety of an LNA nucleotide is modified with an extra bridge connecting the 2' oxygen and 4' carbon. The bridge "locks" the ribose in the 3'-endo conformation, which is often found in the A-form...
. To obtain the desired oligonucleotide, the building blocks are sequentially coupled to the growing oligonucleotide chain in the order required by the sequence of the product (see Synthetic cycle below). The process has been fully automated since the late 1970s. Upon the completion of the chain assembly, the product is released from the solid phase to solution, deprotected, and collected. The occurrence of side reactions sets practical limits for the length of synthetic oligonucleotides (up to about 200 nucleotide
Nucleotide
Nucleotides are molecules that, when joined together, make up the structural units of RNA and DNA. In addition, nucleotides participate in cellular signaling , and are incorporated into important cofactors of enzymatic reactions...
residues) because the number of errors accumulates with the length of the oligonucleotide being synthesized. Products are often isolated by HPLC
High-performance liquid chromatography
High-performance liquid chromatography , HPLC, is a chromatographic technique that can separate a mixture of compounds and is used in biochemistry and analytical chemistry to identify, quantify and purify the individual components of the mixture.HPLC typically utilizes different types of stationary...
to obtain the desired oligonucleotides in high purity. Typically, synthetic oligonucleotides are single-stranded DNA or RNA molecules around 15–25 bases in length. They are most commonly used as antisense oligonucleotides, small interfering RNA
Small interfering RNA
Small interfering RNA , sometimes known as short interfering RNA or silencing RNA, is a class of double-stranded RNA molecules, 20-25 nucleotides in length, that play a variety of roles in biology. The most notable role of siRNA is its involvement in the RNA interference pathway, where it...
, primers
Primer (molecular biology)
A primer is a strand of nucleic acid that serves as a starting point for DNA synthesis. They are required for DNA replication because the enzymes that catalyze this process, DNA polymerases, can only add new nucleotides to an existing strand of DNA...
for DNA sequencing
DNA sequencing
DNA sequencing includes several methods and technologies that are used for determining the order of the nucleotide bases—adenine, guanine, cytosine, and thymine—in a molecule of DNA....
and amplification
Polymerase chain reaction
The polymerase chain reaction is a scientific technique in molecular biology to amplify a single or a few copies of a piece of DNA across several orders of magnitude, generating thousands to millions of copies of a particular DNA sequence....
, probes
Hybridization probe
In molecular biology, a hybridization probe is a fragment of DNA or RNA of variable length , which is used in DNA or RNA samples to detect the presence of nucleotide sequences that are complementary to the sequence in the probe...
for detecting complementary DNA or RNA via molecular hybridization, tools for the targeted introduction of mutation
Mutation
In molecular biology and genetics, mutations are changes in a genomic sequence: the DNA sequence of a cell's genome or the DNA or RNA sequence of a virus. They can be defined as sudden and spontaneous changes in the cell. Mutations are caused by radiation, viruses, transposons and mutagenic...
s and restriction sites, and for the synthesis of artificial gene
Gene
A gene is a molecular unit of heredity of a living organism. It is a name given to some stretches of DNA and RNA that code for a type of protein or for an RNA chain that has a function in the organism. Living beings depend on genes, as they specify all proteins and functional RNA chains...
s.
History
The evolution of oligonucleotide synthesis saw four major methods of the formation of internucleosidic linkages and has been reviewed in the literature in great detail.Early work and contemporary H-phosphonate synthesis

Alexander R. Todd, Baron Todd
Alexander Robertus Todd, Baron Todd, OM, PRS FRSE was a Scottish biochemist whose research on the structure and synthesis of nucleotides, nucleosides, and nucleotide coenzymes gained him the 1957 Nobel Prize for Chemistry.Todd was born near Glasgow, attended Allan Glen's School and graduated from...
’s group pioneered H-phosphonate and phosphate
Phosphate
A phosphate, an inorganic chemical, is a salt of phosphoric acid. In organic chemistry, a phosphate, or organophosphate, is an ester of phosphoric acid. Organic phosphates are important in biochemistry and biogeochemistry or ecology. Inorganic phosphates are mined to obtain phosphorus for use in...
triester methods of oligonucleotide synthesis. The reaction of compounds 1 and 2 to form H-phosphonate diester 3 is an H-phosphonate coupling in solution while that of compounds 4 and 5 to give 6 is a phosphotriester coupling (see Phosphotriester synthesis below).

Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
in aqueous pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
is carried out at the end of the chain assembly rather than as a step in the synthetic cycle. Alternatively, 8 can be converted to phosphorothioate 9 (X = S).
Phosphodiester synthesis

4-Toluenesulfonyl chloride
4-Toluenesulfonyl chloride is an organic compound with the formula CH3C6H4SO2Cl. This colourless, malodorous solid is a reagent widely used in organic synthesis...
(Ts-Cl). The activated species were reacted with a 5’-O-protected nucleoside 1 to give a protected dinucleoside monophosphate 3. Upon the removal of 3’-O-acetyl group using base-catalyzed hydrolysis, further chain elongation was carried out. Following this methodology, sets of tri- and tetradeoxyribonucleotides were synthesized and were enzymatically converted to longer oligonucleotides, which allowed elucidation of the genetic code
Genetic code
The genetic code is the set of rules by which information encoded in genetic material is translated into proteins by living cells....
. The major limitation of the phosphodiester method consisted in the formation of pyrophosphate oligomers and oligonucleotides branched at the internucleosidic phosphate. The method seems to be a step back from the more selective chemistry described earlier; however, at that time, most phosphate-protecting groups available now had not yet been introduced. The lack of the convenient protection strategy necessitated taking a retreat to a slower and less selective chemistry to achieve the ultimate goal of the study.
Phosphotriester synthesis

Phosphite triester synthesis
In the 1970s, substantially more reactive P(III) derivatives of nucleosides, 3'-O-chlorophosphites, were successfully used for the formation of internucleosidic linkages. This led to the discovery of the phosphite triester methodology. The group led by M. Caruthers took the advantage of less aggressive and more selective 1H-tetrazolidophosphites and implemented the method on solid phase. Very shortly after, the workers from the same group further improved the method by using more stable nucleoside phosphoramidites as building blocks. The use of 2-cyanoethyl phosphite-protecting group in place of a less user-friendly methyl group led to the nucleoside phosphoramidites currently used in oligonucleotide synthesis (see Phosphoramidite building blocks below). Many later improvements to the manufacturing of building blocks, instrumentation, and synthetic protocols made the phosphoramidite chemistry a very reliable and expedite method of choice for the preparation of synthetic oligonucleotides.Nucleoside phosphoramidites

- The 5'-hydroxyl group is protected by an acid-labile DMT (4,4'-dimethoxytrityl) group.
- ThymineThymineThymine is one of the four nucleobases in the nucleic acid of DNA that are represented by the letters G–C–A–T. The others are adenine, guanine, and cytosine. Thymine is also known as 5-methyluracil, a pyrimidine nucleobase. As the name suggests, thymine may be derived by methylation of uracil at...
and uracilUracilUracil is one of the four nucleobases in the nucleic acid of RNA that are represented by the letters A, G, C and U. The others are adenine, cytosine, and guanine. In RNA, uracil binds to adenine via two hydrogen bonds. In DNA, the uracil nucleobase is replaced by thymine.Uracil is a common and...
, nucleic bases of thymidineThymidineThymidine is a chemical compound, more precisely a pyrimidine deoxynucleoside. Deoxythymidine is the DNA nucleoside T, which pairs with deoxyadenosine in double-stranded DNA...
and uridineUridineUridine is a molecule that is formed when uracil is attached to a ribose ring via a β-N1-glycosidic bond.If uracil is attached to a deoxyribose ring, it is known as a deoxyuridine....
, respectively, do not have exocyclic amino groups and hence do not require any protection. Although the nucleic base of guanosine and 2'-deoxyguanosine does have an exocyclic amino group, its basicity is low to an extent that it does not react with phosphoramidites under the conditions of the coupling reaction. However, a phosphoramidite derived from the N2-unprotected 5'-O-DMT-2'-deoxyguanosine is poorly soluble in acetonitrileAcetonitrileAcetonitrile is the chemical compound with formula . This colourless liquid is the simplest organic nitrile. It is produced mainly as a byproduct of acrylonitrile manufacture...
, the solvent commonly used in oligonucleotide synthesis. In contrast, the N2-protected versions of the same compound dissolve in acetonitrile well and hence are widely used. Nucleic bases adenineAdenineAdenine is a nucleobase with a variety of roles in biochemistry including cellular respiration, in the form of both the energy-rich adenosine triphosphate and the cofactors nicotinamide adenine dinucleotide and flavin adenine dinucleotide , and protein synthesis, as a chemical component of DNA...
and cytosineCytosineCytosine is one of the four main bases found in DNA and RNA, along with adenine, guanine, and thymine . It is a pyrimidine derivative, with a heterocyclic aromatic ring and two substituents attached . The nucleoside of cytosine is cytidine...
bear the exocyclic amino groups reactive with the activated phosphoramidites under the conditions of the coupling reaction. Although, at the expense of additional steps in the synthetic cycle, the oligonucleotide chain assembly may be carried out using dA and dC phosphoramidites with unprotected amino groups, most often these are kept permanently protected over the entire length of the oligonucleotide chain assembly. The protection of the exocyclic amino groups have to be orthogonal to that of the 5'-hydroxy group because the latter is removed at the end of each synthetic cycle. The simplest to implement and hence the most widely accepted is the strategy where the exocyclic amino groups bear a base-labile protection. Most often, two protection schemes are used.
- In the first, the standard and more robust scheme (Figure), Bz (benzoyl) protection is used for A, dA, C, and dC, while G and dG are protected with isobutyryl group. More recently, Ac (acetyl) group is often used to protect C and dC as shown in Figure.
- In the second, mild protection scheme, A and dA are protected with isobutyryl or phenoxyacetyl groups (PAC). C and dC bear acetyl protection, and G and dG are protected with 4-isopropylphenoxyacetyl (iPr-PAC) or dimethylformamidino (dmf) groups. Mild protecting groups are removed more readily than the standard protecting groups. However, the phosphoramidites bearing these groups are less stable when stored in solution.
- The phosphite group is protected by a base-labile 2-cyanoethyl group. Once a phosphoramidite has been coupled to the solid support-bound oligonucleotide and the phosphite moieties have been converted to the P(V) species, the presence of the phosphate protection is not mandatory for the successful conducting of further coupling reactions.

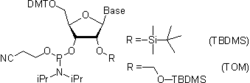
- In RNA synthesis, the 2'-hydroxy group is protected with TBDMS (t-butyldimethylsilyl) group. or with TOM (tri-iso-propylsilyloxymethyl) group, both being removable by treatment with fluoride ion.
- The phosphite moiety also bears a diisopropylamino (iPr2N) group reactive under acidic conditions. Upon activation, the diisopropylamino group leaves to be substituted by the 5'-hydroxy group of the support-bound oligonucleotide (see "Step 2: Coupling" below).
Non-nucleoside phosphoramidites
Non-nucleoside phosphoramidites are the phosphoramidite reagents designed to introduce various functionalities at the termini of synthetic oligonucleotides or between nucleotide residues in the middle of the sequence. In order to be introduced inside the sequence, a non-nucleosidic modifier has to possess at least two hydroxy groups, one of which is often protected with the DMT group while the other bears the reactive phosphoramidite moiety.Non-nucleosidic phosphoramidites are used to introduce desired groups that are not available in natural nucleosides or that can be introduced more readily using simpler chemical designs. A very short selection of commercial phosphoramidite reagents is shown in Scheme for the demonstration of the available structural and functional diversity. These reagents serve for the attachment of 5'-terminal phosphate (1), NH2 (2), SH (3), aldehydo (4), and carboxylic groups (5), CC triple bonds (6), non-radioactive labels and quenchers
Quenching (fluorescence)
Quenching refers to any process which decreases the fluorescence intensity of a given substance. A variety of processes can result in quenching, such as excited state reactions, energy transfer, complex-formation and collisional quenching. As a consequence, quenching is often heavily dependent on...
(exemplified by 6-FAM amidite
Fluorescein amidite
Fluorescein amidite, abbreviated as FAM , is an important synthetic equivalent of fluorescein dye used in oligonucleotide synthesis and molecular biology...
7 for the attachment of fluorescein
Fluorescein
Fluorescein is a synthetic organic compound available as a dark orange/red powder soluble in water and alcohol. It is widely used as a fluorescent tracer for many applications....
and dabcyl amidite 8, respectively), hydrophilic and hydrophobic modifiers (exemplified by hexaethyleneglycol amidite 9 and cholesterol
Cholesterol
Cholesterol is a complex isoprenoid. Specifically, it is a waxy steroid of fat that is produced in the liver or intestines. It is used to produce hormones and cell membranes and is transported in the blood plasma of all mammals. It is an essential structural component of mammalian cell membranes...
amidite 10, respectively), and biotin
Biotin
Biotin, also known as Vitamin H or Coenzyme R, is a water-soluble B-complex vitamin discovered by Bateman in 1916. It is composed of a ureido ring fused with a tetrahydrothiophene ring. A valeric acid substituent is attached to one of the carbon atoms of the tetrahydrothiophene ring...
amidite 11.
Synthetic cycle

Step 1: De-blocking (detritylation)
The DMT group is removed with a solution of an acid, such as 2% TCATrichloroacetic acid
Trichloroacetic acid is an analogue of acetic acid in which the three hydrogen atoms of the methyl group have all been replaced by chlorine atoms....
or 3% Dichloroacetic acid
Dichloroacetic acid
Dichloroacetic acid, often abbreviated DCA, is the chemical compound with formula . It is an acid, an analogue of acetic acid, in which two of the three hydrogen atoms of the methyl group have been replaced by chlorine atoms. The salts and esters of dichloroacetic acid are called dichloroacetates...
(DCA), in an inert solvent (dichloromethane
Dichloromethane
Dichloromethane is an organic compound with the formula CH2Cl2. This colorless, volatile liquid with a moderately sweet aroma is widely used as a solvent. Although it is not miscible with water, it is miscible with many organic solvents...
or toluene
Toluene
Toluene, formerly known as toluol, is a clear, water-insoluble liquid with the typical smell of paint thinners. It is a mono-substituted benzene derivative, i.e., one in which a single hydrogen atom from the benzene molecule has been replaced by a univalent group, in this case CH3.It is an aromatic...
). The orange-colored DMT cation formed is washed out; the step results in the solid support-bound oligonucleotide precursor bearing a free 5'-terminal hydroxyl group.
It is worth remembering that conducting detritylation for an extended time or with stronger than recommended solutions of acids leads to depurination
Depurination
In molecular genetics, depurination is an alteration of DNA in which the purine base is removed from the deoxyribose sugar by hydrolysis of the beta-N-glycosidic link between them. After depurination, an apurinic site is formed where the sugar phosphate backbone remains and the sugar ring has a...
of solid support-bound oligonucleotide and thus reduces the yield of the desired full-length product.
Step 2: Coupling
A 0.02 - 0.2M solution of nucleoside phosphoramidite (or a mixture of several phosphoramidites) in acetonitrileAcetonitrile
Acetonitrile is the chemical compound with formula . This colourless liquid is the simplest organic nitrile. It is produced mainly as a byproduct of acrylonitrile manufacture...
is activated by a 0.2 - 0.7M solution of an acidic azole
Azole
An azole is a class of five-membered nitrogen heterocyclic ring compounds containing at least one other non-carbon atom of either nitrogen, sulfur, or oxygen. The parent compounds are aromatic and have two double bonds; there are successively reduced analogs with fewer...
catalyst, 1H-tetrazole
Tetrazole
Tetrazoles are a class of synthetic organic heterocyclic compound, consisting of a 5-member ring of four nitrogen and one carbon atom . The simplest is tetrazole itself, CN4H2. They are unknown in nature...
, 2-ethylthiotetrazole, 2-benzylthiotetrazole, 4,5-dicyanoimidazole
Imidazole
Imidazole is an organic compound with the formula C3H4N2. This aromatic heterocyclic is a diazole and is classified as an alkaloid. Imidazole refers to the parent compound, whereas imidazoles are a class of heterocycles with similar ring structure, but varying substituents...
, or a number of similar compounds. The mixing is usually very brief and occurs in fluid lines of oligonucleotide synthesizers (see below) while the components are being delivered to the reactors containing solid support. The activated phosphoramidite in 1.5 - 20-fold excess over the support-bound material is then brought in contact with the starting solid support (first coupling) or a support-bound oligonucleotide precursor (following couplings) whose 5'-hydroxy group reacts with the activated phosphoramidite moiety of the incoming nucleoside phosphoramidite to form a phosphite triester linkage. The phosphoramidite coupling is very rapid and requires, on small scale, about 20 s for its completion. The reaction is also highly sensitive to the presence of water, particularly when dilute solutions of phosphoramidites are used, and is commonly carried out in anhydrous acetonitrile. Generally, the larger the scale of the synthesis, the lower the excess and the higher the concentration of the phosphoramidites is used. In contrast, the concentration of the activator is primarily determined by its solubility in acetonitrile and is irrespective of the scale of the synthesis. Upon the completion of the coupling, any unbound reagents and by-products are removed by washing.
Step 3: Capping
The capping step is performed by treating the solid support-bound material with a mixture of acetic anhydride and 1-methylimidazole1-Methylimidazole
1-Methylimidazole or N-Methylimidazole is an aromatic heterocyclic organic compound with the formula CH3C3H3N2. Its N-methylation removes the possibility of tautomerization, which occurs in imidazole and many imidazole derivatives. 1-Methylimidazole maintains the pyridine-like nitrogen of...
or, less often, DMAP
4-Dimethylaminopyridine
4-Dimethylaminopyridine is a derivative of pyridine with the chemical formula 2NC5H4N. This colourless solid is a useful nucleophilic catalyst for a variety of reactions such as esterifications with anhydrides, the Baylis-Hillman reaction, hydrosilylations, tritylation, the Steglich...
as catalysts and, in the phosphoramidite method, serves two purposes.
- After the completion of the coupling reaction, a small percentage of the solid support-bound 5'-OH groups (0.1 to 1%) remains unreacted and needs to be permanently blocked from further chain elongation to prevent the formation of oligonucleotides with an internal base deletion commonly referred to as (n-1) shortmers. The unreacted 5'-hydroxy groups are, to a large extent, acetylated by the capping mixture.
- It has also been reported that phosphoramidites activated with 1H-tetrazole react, to a small extent, with the O6 position of guanosine. Upon oxidation with I2 /water, this side product, possibly via O6-N7 migration, undergoes depurinationDepurinationIn molecular genetics, depurination is an alteration of DNA in which the purine base is removed from the deoxyribose sugar by hydrolysis of the beta-N-glycosidic link between them. After depurination, an apurinic site is formed where the sugar phosphate backbone remains and the sugar ring has a...
. The apurinic sites thus formed are readily cleaved in the course of the final deprotection of the oligonucleotide under the basic conditions (see below) to give two shorter oligonucleotides thus reducing the yield of the full-length product. The O6 modifications are rapidly removed by treatment with the capping reagent as long as the capping step is performed prior to oxidation with I2/water.
- The synthesis of oligonucleotide phosphorothioates (OPS, see below) does not involve the oxidation with I2/water, and, respectively, does not suffer from the side reaction described above. On the other hand, if the capping step is performed prior to sulfurization, the solid support may contain the residual acetic anhydride and N-methylimidazole left after the capping step. The capping mixture interferes with the sulfur transfer reaction, which results in the extensive formation of the phosphate triester internucleosidic linkages in place of the desired PS triesters. Therefore, for the synthesis of OPS, it is advisable to conduct the sulfurization step prior to the capping step.
Step 4: Oxidation
The newly formed tricoordinated phosphite triester linkage is not natural and is of limited stability under the conditions of oligonucleotide synthesis. The treatment of the support-bound material with iodine and water in the presence of a weak base (pyridine, lutidine, or collidineCollidine
Collidine is the trivial name used to describe the chemical compounds trimethylpyridine. Their chemical properties resemble those of pyridine, although the presence of the methyl groups will prohibit some of the more straightforward reactions...
) oxidizes the phosphite triester into a tetracoordinated phosphate triester, a protected precursor of the naturally occurring phosphate diester internucleosidic linkage. This step is substituted with a sulfurization step to obtain oligonucleotide phosphorothioates. In the latter case, the sulfurization step is best carried out prior to capping.
Solid supports
In solid-phase synthesis, an oligonucleotide being assembled is covalentlyCovalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
bound, via its 3'-terminal hydroxy group, to a solid support material and remains attached to it over the entire course of the chain assembly. The solid support is contained in columns whose dimensions depend on the scale of synthesis and may vary between 0.05 mL and several litres. The overwhelming majority of oligonucleotides are synthesized on small scale ranging from 40 nmol
Mole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
to 1 μmol. More recently, high-throughput oligonucleotide synthesis where the solid support is contained in the wells of multi-well plates (most often, 96 or 384 wells per plate) became a method of choice for parallel synthesis of oligonucleotides on small scale. At the end of the chain assembly, the oligonucleotide is released from the solid support and eluted from the column or the well.
Solid Support Material
In contrast to organic solid-phase synthesis and peptide synthesisPeptide synthesis
In organic chemistry, peptide synthesis is the production of peptides, which are organic compounds in which multiple amino acids are linked via amide bonds which are also known as peptide bonds...
, the synthesis of oligonucleotides proceeds best on non-swellable or low-swellable solid supports. The two most often used solid-phase materials are Controlled Pore Glass
Glass
Glass is an amorphous solid material. Glasses are typically brittle and optically transparent.The most familiar type of glass, used for centuries in windows and drinking vessels, is soda-lime glass, composed of about 75% silica plus Na2O, CaO, and several minor additives...
(CPG) and macroporous polystyrene
Polystyrene
Polystyrene ) also known as Thermocole, abbreviated following ISO Standard PS, is an aromatic polymer made from the monomer styrene, a liquid hydrocarbon that is manufactured from petroleum by the chemical industry...
(MPPS).
- CPG is commonly defined by its pore size. In oligonucleotide chemistry, pore sizes of 500, 1000, 1500, 2000, and 3000 ÅÅÅ represents various sounds in several languages. Å is part of the alphabets used for the Alemannic and the Bavarian-Austrian dialects of German...
are used to allow the preparation of about 50, 80, 100, 150, and 200-mer oligonucleotides, respectively. To make native CPG suitable for further processing, the surface of the material is treated with (3-aminopropyl)triethoxysilane to give Aminopropyl CPG. The aminopropyl arm may be further extended to result in Long Chain Aminoalkyl (LCAA) CPG. The amino group is then used as an anchoring point for linkers suitable for oligonucleotide synthesis (see below). - MPPS suitable for oligonucleotide synthesis is a low-swellable, highly cross-linked polystyrene obtained by polymerization of divinylbenzeneDivinylbenzeneDivinylbenzene consists of a benzene ring bonded to two vinyl groups. It is related to styrene by the addition of a second vinyl group. Divinylbenzene, as it is usually encountered, is a 2:1 mixture of m- and p-divinylbenzene, containing also the corresponding ethylvinylbenzene isomers. It is...
(min 60%), styrene, and 4-chloromethylstyrene in the presence of a porogeneous agent. The macroporous chloromethyl MPPS obtained is converted to aminomethyl MPPS.
Linker chemistry


Acetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula 2O. Commonly abbreviated Ac2O, it is the simplest isolatable acid anhydride and is a widely used reagent in organic synthesis...
. Typically, three conceptually different groups of solid supports are used.
- Universal supports. In a more recent and more convenient method, the synthesis starts with the universal support where a non-nucleosidic linker is attached to the solid support material (Compounds 1 and 2). A phosphoramidite respective to the 3'-terminal nucleoside residue is coupled to the universal solid support in the first synthetic cycle of oligonucleotide chain assembly using the standard protocols. The chain assembly is then continued until the completion, after which the solid support-bound oligonucleotide is deprotected. The characteristic feature of the universal solid supports is that the release of the oligonucleotides occurs by the hydrolytic cleavage of a P-O bond that attaches the 3’-O of the 3’-terminal nucleotide residue to the universal linker as shown in Scheme 5. The critical advantage of this approach is that the same solid support is used irrespectively of the sequence of the oligonucleotide to be synthesized. For the complete removal of the linker and the 3'-terminal phosphate from the assembled oligonucleotide, the solid support 1 and several similar solid supports require gaseous ammonia, aqueous ammonium hydroxide, aqueous methylamine, or their mixture and are commercially available. The solid support 2 requires a solution of ammoniaAmmoniaAmmonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
in anhydrous methanolMethanolMethanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
and is also commercially available. - Nucleosidic solid supports. In a historically first and still popular approach, the 3'-hydroxy group of the 3'-terminal nucleoside residue is attached to the solid support via, most often, 3’-O-succinyl arm as in compound 3. The oligonucleotide chain assembly starts with the coupling of a phosphoramidite building block respective to the nucleotideNucleotideNucleotides are molecules that, when joined together, make up the structural units of RNA and DNA. In addition, nucleotides participate in cellular signaling , and are incorporated into important cofactors of enzymatic reactions...
residue second from the 3’-terminus. The 3’-terminal hydroxy group in oligonucleotides synthesized on nucleosidic solid supports is deprotected under the conditions somewhat milder than those applicable for universal solid supports. However, the fact that a nucleosidic solid support has to be selected in a sequence-specific manner reduces the throughput of the entire synthetic process and increases the likelihood of human error. - Special solid supports are used for the attachment of desired functional or reporter groups at the 3’-terminus of synthetic oligonucleotides. For example, the commercial solid support 4 allows the preparation of oligonucleotides bearing 3’-terminal 3-aminopropyl linker. Similarly to non-nucleosidic phosphoramidites, many other special solid supports designed for the attachment of reactive functional groups, non-radioactive reporter groups, and terminal modifiers (e.c., hydrophobic tethers) and suited for various applications are commercially available.
Oligonucleotide phosphorothioates and their synthesis

Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
at phosphorus
Phosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
. In a simple case of a dinucleotide, this results in the formation of a diastereomeric pair of Sp- and Rp-dinucleoside monophosphorothioates whose structures are shown in Figure. In a n-mer oligonucleotide where all (n – 1) internucleosidic linkages are phosphorothioate linkages, the number of diastereomers m is calculated as m = 2(n – 1). Being non-natural analogs of nucleic acids, OPS are substantially more stable towards hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
by nucleases, the class of enzymes that destoy nucleic acids by breaking the bridging P-O bond of the phosphodiester moiety. This property determines the use of OPS as antisense oligonucleotides in in vitro
In vitro
In vitro refers to studies in experimental biology that are conducted using components of an organism that have been isolated from their usual biological context in order to permit a more detailed or more convenient analysis than can be done with whole organisms. Colloquially, these experiments...
and in vivo
In vivo
In vivo is experimentation using a whole, living organism as opposed to a partial or dead organism, or an in vitro controlled environment. Animal testing and clinical trials are two forms of in vivo research...
applications where the extensive exposure to nucleases is inevitable. Similarly, to improve the stability of siRNA
Sírna
Sírna Sáeglach , son of Dian mac Demal, son of Demal mac Rothechtaid, son of Rothechtaid mac Main, was, according to medieval Irish legend and historical tradition, a High King of Ireland...
, at least one phosphorothioate linkage is often introduced at the 3'-terminus of both sense
Sense
Senses are physiological capacities of organisms that provide inputs for perception. The senses and their operation, classification, and theory are overlapping topics studied by a variety of fields, most notably neuroscience, cognitive psychology , and philosophy of perception...
and antisense strands. In chirally pure OPS, all-Sp diastereomers are more stable to enzymatic degradation than their all-Rp analogs. However, the preparation of chirally pure OPS remains a synthetic challenge. In laboratory practice, mixtures of diastereomers of OPS are commonly used.
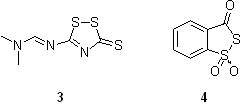
Synthesis of OPS is very similar to that of natural oligonucleotides. The difference is that the oxidation step is replaced by sulfur transfer reaction (sulfurization) and that the capping step is performed after the sulfurization. Of many reported reagents capable of the efficient sulfur transfer, only three are commercially available:

- 3-((Dimethylaminomethylidene)amino)-3H-1,2,4-dithiazole-3-thione, DDTT (3, Fig. 4) provides rapid kinetics of sulfurization and high stability in solution. The reagent is available from several sources.
- 3H-1,2-benzodithiol-3-one 1,1-dioxide (4, Fig. 4) also known as Beaucage Reagent displays a better solubility in acetonitrile and short reaction times. However, the reagent is of limited stability in solution and is less efficient in sulfurizing RNA linkages.
- N,N,N'N-Tetraethylthiuram disulfideDisulfiramDisulfiram is a drug discovered in the 1920s and used to support the treatment of chronic alcoholism by producing an acute sensitivity to alcohol. Trade names for disulfiram in different countries are Antabuse and Antabus manufactured by Odyssey Pharmaceuticals...
(TETD) is soluble in acetonitrile and is commercially available. However, the sulfurization reaction of an internucleosidic DNA linkage with TETD requires 15 min, which is more than 10 times as slow as that with compounds 3 and 4.
Automation
In the past, oligonucleotide synthesis was carried out manually in solution or on solid phase. The solid phase synthesis was implemented using, as containers for the solid phase, miniature glass columns similar in their shape to low-pressure chromatography columns or syringes equipped with porous filters.Currently, solid-phase oligonucleotide synthesis is carried out automatically using computer-controlled instruments (oligonucleotide synthesizers) and is technically implemented in column, multi-well plate, and array formats. The column format is best suited for research and large scale applications where a high-throughput is not required. Multi-well plate format is designed specifically for high-throughput synthesis on small scale to satisfy the growing demand of industry and academia for synthetic oligonucleotides. A number of oligonucleotide synthesizers for small scale synthesis and medium to large scale synthesis are available commercially.
Synthesis of oligonucleotide microarrays
One may visualize an oligonucleotide microarray as a miniature multi-well plate where physical dividers between the wells (plastic walls) are intentionally removed. With respect to the chemistry, synthesis of oligonucleotide microarrays is different from the conventional oligonucleotide synthesis in two respects:
- Oligonucleotides remain permanently attached to the solid phase, which requires the use of linkers that are stable under the conditions of the final deprotection procedure.
- The absence of physical dividers between the sites occupied by individual oligonucleotides, a very limited space on the surface of the microarray (one oligonucleotide sequence occupies a square 25x25 μm) and the requirement of high fidelity of oligonucleotide synthesis dictate the use of site-selective 5'-deprotection techniques. In one approach, the removal of the 5'-O-DMT group is effected by electrochemical generation of the acid at the required site(s). In another approach, 5'-O-(α-methyl-6-nitropiperonyloxycarbonyl) (NPPOC) protecting group removable by UV-irradiation at 365 nm is used.
Post-synthetic processing
After the completion of the chain assembly, the solid support-bound oligonucleotide is fully protected:- The 5'-terminal 5'-hydroxy group is protected with DMT group;
- The internucleosidic phosphate or phosphorothioate moieties are protected with 2-cyanoethyl groups;
- The exocyclic amino groups in all nucleis bases except for T and U are protected with acyl protecting groups.
To furnish a functional oligonucleotide, all the protecting groups have to be removed. The N-acyl base protection and the 2-cyanoethyl phosphate protection may be, and is often removed simultaneously by treatment with inorganic bases or amines. However, the applicability of this method is limited by the fact that the cleavage of 2-cyanoethyl phosphate protection gives rise to acrylonitrile
Acrylonitrile
Acrylonitrile is the chemical compound with the formula C3H3N. This pungent-smelling colorless liquid often appears yellow due to impurities. It is an important monomer for the manufacture of useful plastics. In terms of its molecular structure, it consists of a vinyl group linked to a nitrile...
as a side product. Under the strong basic conditions required for the removal of N-acyl protection, acrylonitrile is capable of alkylation of nucleic bases, primarily, at the N3-position of thymine and uracil residues to give the respective N3-(2-cyanoethyl) adducts via Michael reaction
Michael reaction
The Michael reaction or Michael addition is the nucleophilic addition of a carbanion or another nucleophile to an alpha, beta unsaturated carbonyl compound. It belongs to the larger class of conjugate additions. This is one of the most useful methods for the mild formation of C-C bonds...
. The formation of these side products may be avoided by treating the solid support-boud oligonucleotides with solutions of bases in an organic solvent, for instance, with 50% triethylamine
Triethylamine
Triethylamine is the chemical compound with the formula N3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine, for which TEA is also a common abbreviation....
in acetonitrile
Acetonitrile
Acetonitrile is the chemical compound with formula . This colourless liquid is the simplest organic nitrile. It is produced mainly as a byproduct of acrylonitrile manufacture...
or 10% diethylamine
Diethylamine
Diethylamine is a secondary amine with the molecular structure CH3CH2NHCH2CH3. It is a flammable, strongly alkaline liquid. It is miscible with water and ethanol. It is a colorless liquid which often appears brown due to impurities...
in acetonitrile. This treatment is strongly recommended for medium- and large scale preparations and is optional for syntheses on small scale where the concentration of acrylonitrile generated in the deprotection mixture is low.
Regardless of whether the phosphate protecting groups were removed first, the solid support-bound oligonucleotides are deprotected using one of the two general approaches.
- (1) Most often, 5'-DMT group is removed at the end of the oligonucleotide chain assembly. The oligonucleotides are then released from the solid phase and deprotected (base and phosphate) by treatment with aqueous ammonium hydroxideAmmonium hydroxideAmmonia solution, also known as ammonium hydroxide, ammonia water, ammonical liquor, ammonia liquor, aqua ammonia, aqueous ammonia, or simply ammonia, is a solution of ammonia in water. It can be denoted by the symbols NH3...
, aqueous methylamineMethylamineMethylamine is the organic compound with a formula of CH3NH2. This colourless gas is a derivative of ammonia, but with one H atom replaced by a methyl group. It is the simplest primary amine. It is sold as a solution in methanol, ethanol, THF, and water, or as the anhydrous gas in pressurized...
, their mixtures, gaseous ammonia or methylamine or, less commonly, solutions of other primary amines or alkalies at ambient or elevated temperature. This removes all remaining protection groups from 2'-deoxyoligonucleotides, resulting in a reaction mixture containing the desired product. If the oligonucleotide contains any 2'-O-protected ribonucleotide residues, the deprotection protocol includes the second step where the 2'-O-protecting silyl groups are removed by treatment with fluoride ion by various methods (see, for instance, Ref.).
The fully deprotected product is used as is, or the desired oligonucleotide can be purified by a number of methods. Most commonly, the crude product is desalted using ethanol precipitation
Ethanol precipitation
Ethanol precipitation is a method used to purify and/or concentrate RNA, DNA and polysaccharides such as pectin and xyloglucan from aqueous solutions.- Theory :DNA is polar due to its highly charged phosphate backbone...
, size exclusion chromatography
Size exclusion chromatography
Size-exclusion chromatography is a chromatographic method in which molecules in solution are separated by their size, and in some cases molecular weight . It is usually applied to large molecules or macromolecular complexes such as proteins and industrial polymers...
, or reverse-phase HPLC. To eliminate unwanted truncation products, the oligonucleotides can be purified via polyacrylamide gel electrophoresis
Gel electrophoresis
Gel electrophoresis is a method used in clinical chemistry to separate proteins by charge and or size and in biochemistry and molecular biology to separate a mixed population of DNA and RNA fragments by length, to estimate the size of DNA and RNA fragments or to separate proteins by charge...
or anion-exchange HPLC followed by desalting.
- (2) The second approach is only used when the intended method of purification is reverse-phase HPLC. In this case, the 5'-terminal DMT group that serves as a hydrophobic handle for purification is kept on at the end of the synthesis. The oligonucleotide is deprotected under basic conditions as described above and, upon evaporation, is purified by reverse-phase HPLC. The collected material is then detritylated under aqueous acidic conditions. On small scale (less than 0.01–0.02 mmol), the treatment with 80% aqueous acetic acid for 15–30 min at room temperature is often used followed by evaporation of the reaction mixture to dryness in vacuo. Finally, the product is desalted as described above.
- For some applications, additional reporter groups may be attached to an oligonucleotide using a variety of post-synthetic procedures.
Characterization

Sequencing
In genetics and biochemistry, sequencing means to determine the primary structure of an unbranched biopolymer...
, a relatively inexpensive and routine procedure, the considerations of the cost reduction preclude its use in routine manufacturing of oligonucleotides. In day-by-day practice, it is sufficient to obtain the molecular mass
Molecular mass
The molecular mass of a substance is the mass of one molecule of that substance, in unified atomic mass unit u...
of an oligonucleotide by recording its mass spectrum
Mass spectrum
A mass spectrum is an intensity vs. m/z plot representing a chemical analysis. Hence, the mass spectrum of a sample is a pattern representing the distribution of ions by mass in a sample. It is a histogram usually acquired using an instrument called a mass spectrometer...
. Two methods are currently widely used for characterization of oligonucleotides: electrospray mass spectrometry (ES MS) and matrix-assisted laser desorption/ionization
Matrix-assisted laser desorption/ionization
Matrix-assisted laser desorption/ionization is a soft ionization technique used in mass spectrometry, allowing the analysis of biomolecules and large organic molecules , which tend to be fragile and fragment when ionized by more conventional ionization methods...
time-of-flight mass spectrometry
Time-of-flight mass spectrometry
Time-of-flight mass spectrometry is a method of mass spectrometry in which an ion's mass-to-charge ratio is determined via a time measurement. Ions are accelerated by an electric field of known strength. This acceleration results in an ion having the same kinetic energy as any other ion that has...
(MALDI-TOF). Prior to submitting a sample to the analysis by either of the methods, it is very important to exchange all metal ions that might be present in the sample for ammonium or trialkylammonium (e.c. triethylammonium) ions.
- In ES MS spectrum, a given oligonucleotide generates a set of ions that correspond to different ionization states of the compound. Thus, the oligonucleotide with molecular massMolecular massThe molecular mass of a substance is the mass of one molecule of that substance, in unified atomic mass unit u...
M generates ions with masses (M – nH)/n where M is the molecular mass of the oligonucleotide in the form of a free acid (all negative charges of internucleosidic phosphodiester groups are neutralized with H+), n is the ionization state, and H is the atomic massAtomic massThe atomic mass is the mass of a specific isotope, most often expressed in unified atomic mass units. The atomic mass is the total mass of protons, neutrons and electrons in a single atom....
of hydrogen (1 DaAtomic mass unitThe unified atomic mass unit or dalton is a unit that is used for indicating mass on an atomic or molecular scale. It is defined as one twelfth of the rest mass of an unbound neutral atom of carbon-12 in its nuclear and electronic ground state, and has a value of...
). Most useful for characterization are the ions with n ranging from 2 to 5. Software supplied with the more recently manufactured instruments is capable of performing a deconvolutionDeconvolutionIn mathematics, deconvolution is an algorithm-based process used to reverse the effects of convolution on recorded data. The concept of deconvolution is widely used in the techniques of signal processing and image processing...
procedure that is, it finds peaks of ions that belong to the same set and derives the molecular massMolecular massThe molecular mass of a substance is the mass of one molecule of that substance, in unified atomic mass unit u...
of the oligonucleotide. - To obtain more detailed information on the impurity profile of oligonucleotides, liquid chromatography-mass spectrometryLiquid chromatography-mass spectrometryLiquid chromatography–mass spectrometry is an analytical chemistry technique that combines the physical separation capabilities of liquid chromatography with the mass analysis capabilities of mass spectrometry. LC-MS is a powerful technique used for many applications which has very high...
(LC-MS or HPLC-MS) or capillary electrophoresis mass spectrometry (CEMS) are used.
Further reading
- Comprehensive Natural Products Chemistry, Volume 7: DNA and Aspects of Molecular Biology. Kool, Eric T.; Editor. Neth. (1999), 733 pp. Publisher: (Elsevier, Amsterdam, Neth.)
- Beaucage, S. L.; Iyer, R. P. Advances in the synthesis of oligonucleotides by the phosphoramidite approach. Tetrahedron 1992, 48, 2223–2311.
- Beaucage, S. L.; Iyer, R. P. The functionalization of oligonucleotides via phosphoramidite derivatives. Tetrahedron 1993, 49, 1925–1963.
- Beaucage, S. L.; Iyer, R. P. The synthesis of modified oligonucleotides by the phosphoramidite approach and their applications. Tetrahedron 1993, 49, 6123–6194.
- Beaucage, S L. Oligodeoxyribonucleotides synthesis. Phosphoramidite approach. Methods in Molecular Biology (Totowa, NJ, United States) (1993), 20 (Protocols for Oligonucleotides and Analogs), 33–61.
- Reese, C. B. The chemical synthesis of oligo- and poly-nucleotides: a personal commentary. Tetrahedron 2002, 58, 8893–8920.
See also
- Nucleic acids
- Nucleic acid analoguesNucleic acid analoguesNucleic acid analogues are compounds structurally similar to naturally occurring RNA and DNA, used in medicine and in molecular biology research....
- Peptide nucleic acid

