Phosphorus
Overview
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
that has the symbol P and atomic number
Atomic number
In chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element...
15. A multivalent nonmetal
Nonmetal
Nonmetal, or non-metal, is a term used in chemistry when classifying the chemical elements. On the basis of their general physical and chemical properties, every element in the periodic table can be termed either a metal or a nonmetal...
of the nitrogen group
Nitrogen group
The nitrogen group is a periodic table group consisting of nitrogen , phosphorus , arsenic , antimony , bismuth and ununpentium ....
, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks
Phosphate minerals
Phosphate minerals are those minerals that contain the tetrahedrally coordinated phosphate anion along with the freely substituting arsenate and vanadate...
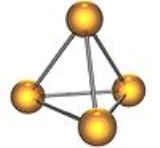
. Elemental phosphorus exists in two major forms—white phosphorus and red phosphorus—but due to its high reactivity, phosphorus is never found as a free element on Earth.
The first form of elemental phosphorus to be produced (white phosphorus, in 1669) emits a faint glow upon exposure to oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
– hence its name given from Greek mythology, meaning "light-bearer" (Latin Lucifer
Lucifer
Traditionally, Lucifer is a name that in English generally refers to the devil or Satan before being cast from Heaven, although this is not the original meaning of the term. In Latin, from which the English word is derived, Lucifer means "light-bearer"...
), referring to the "Morning Star
Hesperus
In Greek mythology, Hesperus is the Evening Star, the planet Venus in the evening. He is the son of the dawn goddess Eos and is the brother of Eosphorus , the Morning Star. Hesperus' Roman equivalent is Vesper...
", the planet Venus
Venus
Venus is the second planet from the Sun, orbiting it every 224.7 Earth days. The planet is named after Venus, the Roman goddess of love and beauty. After the Moon, it is the brightest natural object in the night sky, reaching an apparent magnitude of −4.6, bright enough to cast shadows...
.
Unanswered Questions

