
Quenching (fluorescence)
Encyclopedia
Quenching refers to any process which decreases the fluorescence
intensity of a given substance. A variety of processes can result in quenching, such as excited state
reactions, energy transfer, complex-formation and collisional quenching. As a consequence, quenching is often heavily dependent on pressure
and temperature
. Molecular oxygen
and the iodide
ion are common chemical quenchers. The chloride ion is a well known quencher for quinine fluorescence. Quenching poses a problem for non-instant spectroscopic methods, such as laser-induced fluorescence
.
Quenching is made use of in optode
sensors; for instance the quenching effect of oxygen on certain ruthenium
complexes allows the measurement of oxygen saturation
in solution. Quenching is the basis for fluorescence resonance energy transfer
(FRET) assays. Quenching and dequenching upon interaction with a specific molecular biological target is the basis for activatable optical contrast agents for molecular imaging
.
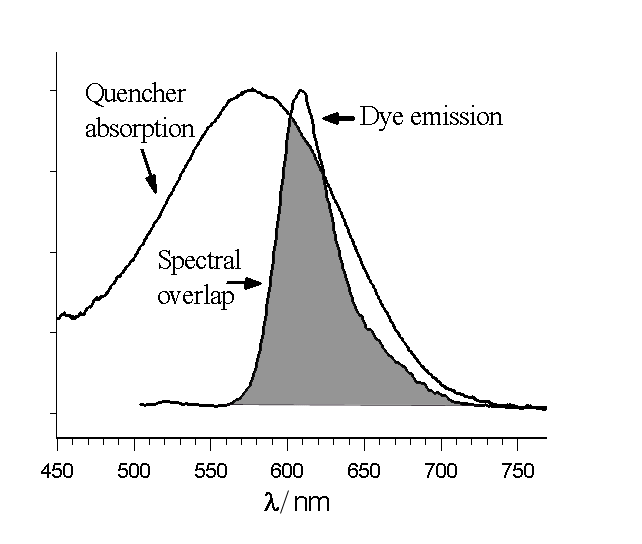 There are a few distinct mechanisms by which energy can be transferred nonradiatively (without absorption or emission of photons) between two dyes, a donor and an acceptor. Förster resonance energy transfer (FRET or FET) is a dynamic quenching mechanism because energy transfer occurs while the donor is in the excited state. FRET is based on classical dipole–dipole interactions between the transition dipole
There are a few distinct mechanisms by which energy can be transferred nonradiatively (without absorption or emission of photons) between two dyes, a donor and an acceptor. Förster resonance energy transfer (FRET or FET) is a dynamic quenching mechanism because energy transfer occurs while the donor is in the excited state. FRET is based on classical dipole–dipole interactions between the transition dipole
s of the donor and acceptor and is extremely dependent on the donor–acceptor distance, R, falling off at a rate of 1/R6. FRET also depends on the donor–acceptor spectral overlap (see figure) and the relative orientation of the donor and acceptor transition dipole moments. FRET can typically occur over distances up to 100 Å.
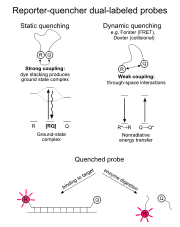
Dexter
(also known as exchange or collisional energy transfer) is another dynamic quenching mechanism. Dexter energy transfer is a short-range phenomenon that decreases with e−R and depends on spatial overlap of donor and quencher molecular orbitals. In most donor-fluorophore–quencher-acceptor situations, the Förster mechanism is more important than the Dexter mechanism. With both Förster and Dexter energy transfer, the shapes of the absorption and fluorescence spectra of the dyes are unchanged.
Exciplex (excited state complex) formation is a third dynamic quenching mechanism.
The remaining energy transfer mechanism is static quenching (also referred to as contact quenching). Static quenching can be a dominant mechanism for some reporter-quencher probes. Unlike dynamic quenching, static quenching occurs when the donor and acceptor molecules are in the ground state. The donor and acceptor molecules bind together to form a ground state complex, an intramolecular dimer with its own unique properties, such as being nonfluorescent and having a unique absorption
spectrum
. Dye aggregation is often due to hydrophobic effects—the dye molecules stack together to minimize contact with water. Planar aromatic dyes that are matched for association through hydrophobic, electrostatic and steric forces can enhance static quenching. High temperatures and addition of surfactants tend to disrupt ground state complex formation.
Fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation of a different wavelength. It is a form of luminescence. In most cases, emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation...
intensity of a given substance. A variety of processes can result in quenching, such as excited state
Excited state
Excitation is an elevation in energy level above an arbitrary baseline energy state. In physics there is a specific technical definition for energy level which is often associated with an atom being excited to an excited state....
reactions, energy transfer, complex-formation and collisional quenching. As a consequence, quenching is often heavily dependent on pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
and temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
. Molecular oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
and the iodide
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
ion are common chemical quenchers. The chloride ion is a well known quencher for quinine fluorescence. Quenching poses a problem for non-instant spectroscopic methods, such as laser-induced fluorescence
Laser-induced fluorescence
Laser-induced fluorescence is a spectroscopic method used for studying structure of molecules, detection of selective species and flow visualization and measurements....
.
Quenching is made use of in optode
Optode
An optode or optrode is an optical sensor device that optically measures a specific substance usually with the aid of a chemical transducer.-Construction:...
sensors; for instance the quenching effect of oxygen on certain ruthenium
Ruthenium
Ruthenium is a chemical element with symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most chemicals. The Russian scientist Karl Ernst Claus discovered the element...
complexes allows the measurement of oxygen saturation
Oxygen saturation
Oxygen saturation or dissolved oxygen is a relative measure of the amount of oxygen that is dissolved or carried in a given medium. It can be measured with a dissolved oxygen probe such as an oxygen sensor or an optode in liquid media, usually water.It has particular significance in medicine and...
in solution. Quenching is the basis for fluorescence resonance energy transfer
Fluorescence resonance energy transfer
Förster resonance energy transfer , also known as fluorescence resonance energy transfer, resonance energy transfer or electronic energy transfer , is a mechanism describing energy transfer between two chromophores.A donor chromophore, initially in its electronic excited state, may transfer energy...
(FRET) assays. Quenching and dequenching upon interaction with a specific molecular biological target is the basis for activatable optical contrast agents for molecular imaging
Molecular imaging
Molecular imaging originated from the field of radiopharmacology due to the need to better understand the fundamental molecular pathways inside organisms in a noninvasive manner.- Overview :...
.
Quenching mechanisms

Dipole
In physics, there are several kinds of dipoles:*An electric dipole is a separation of positive and negative charges. The simplest example of this is a pair of electric charges of equal magnitude but opposite sign, separated by some distance. A permanent electric dipole is called an electret.*A...
s of the donor and acceptor and is extremely dependent on the donor–acceptor distance, R, falling off at a rate of 1/R6. FRET also depends on the donor–acceptor spectral overlap (see figure) and the relative orientation of the donor and acceptor transition dipole moments. FRET can typically occur over distances up to 100 Å.
Dexter
Dexter electron transfer
Dexter electron transfer is a quenching mechanism in which an excited electron state transfers from one molecule to a second . This requires a wavefunction overlap between the donor and acceptor, so can only occur at short distances; typically of the order 15-20Å...
(also known as exchange or collisional energy transfer) is another dynamic quenching mechanism. Dexter energy transfer is a short-range phenomenon that decreases with e−R and depends on spatial overlap of donor and quencher molecular orbitals. In most donor-fluorophore–quencher-acceptor situations, the Förster mechanism is more important than the Dexter mechanism. With both Förster and Dexter energy transfer, the shapes of the absorption and fluorescence spectra of the dyes are unchanged.
Exciplex (excited state complex) formation is a third dynamic quenching mechanism.
The remaining energy transfer mechanism is static quenching (also referred to as contact quenching). Static quenching can be a dominant mechanism for some reporter-quencher probes. Unlike dynamic quenching, static quenching occurs when the donor and acceptor molecules are in the ground state. The donor and acceptor molecules bind together to form a ground state complex, an intramolecular dimer with its own unique properties, such as being nonfluorescent and having a unique absorption
Absorption (electromagnetic radiation)
In physics, absorption of electromagnetic radiation is the way by which the energy of a photon is taken up by matter, typically the electrons of an atom. Thus, the electromagnetic energy is transformed to other forms of energy for example, to heat. The absorption of light during wave propagation is...
spectrum
Spectrum
A spectrum is a condition that is not limited to a specific set of values but can vary infinitely within a continuum. The word saw its first scientific use within the field of optics to describe the rainbow of colors in visible light when separated using a prism; it has since been applied by...
. Dye aggregation is often due to hydrophobic effects—the dye molecules stack together to minimize contact with water. Planar aromatic dyes that are matched for association through hydrophobic, electrostatic and steric forces can enhance static quenching. High temperatures and addition of surfactants tend to disrupt ground state complex formation.

See also
- Dark quencherDark quencherA dark quencher is a substance that absorbs excitation energy from a fluorophore and dissipates the energy as heat; while a typical quencher re-emits much of this energy as light . Dark quenchers are used in molecular biology in conjunction with fluorophores...
, for use in molecular biology. - Förster resonance energy transfer, a phenomenon on which some quenching techniques rely

