
Material properties of diamond
Encyclopedia
Diamond is the allotrope of carbon
in which the carbon
atoms are arranged in the specific type of cubic lattice
called diamond cubic
. Diamond is an optically isotropic crystal
that is transparent to opaque. Owing to its strong covalent bond
ing, diamond is the hardest naturally occurring material known. Yet, due to important structural weaknesses, diamond's toughness
is only fair to good. The precise tensile strength
of diamond is unknown, however strength up to 60 GPa
has been observed, and it could be as high as 90–225 GPa depending on the crystal orientation. The anisotropy
of diamond hardness is carefully considered during diamond cutting
. Diamond has a high refractive index
(2.417) and moderate dispersion
(0.044) properties which give cut diamonds their brilliance. Scientists classify diamonds into four main types according to the nature of crystallographic defect
s present. Trace impurities substitutionally replacing carbon atoms in a diamond's crystal lattice, and in some cases structural defects, are responsible for the wide range of colors seen in diamond. Most diamonds are electrical insulator
s but extremely efficient thermal conductor
s. Unlike many other minerals, the specific gravity
of diamond crystals (3.52) has rather small variation from diamond to diamond.
, diamond is the hardest known naturally occurring material, scoring 10 on the Mohs scale of mineral hardness
. Diamond is extremely strong due to the structure of its carbon atoms, where each carbon atom has four neighbors joined to it with covalent bonds. The material boron nitride
, when in a form structurally identical to diamond (zincblende structure), is nearly as hard as diamond; a currently hypothetical material, beta carbon nitride
, may also be as hard or harder in one form. It has been shown that some diamond aggregates having nanometer grain size are harder and tougher than conventional large diamond crystals, thus they perform better as abrasive material. Due to the use of those new ultra-hard materials for diamond testing, more accurate values are now known for diamond hardness. A surface perpendicular to the [111] crystallographic direction (that is the longest diagonal of a cube) of a pure (i.e. type IIa) diamond has a hardness value of 167 GPa when scratched with an nanodiamond tip, while the nanodiamond sample itself has a value of 310 GPa when tested with another nanodiamond tip. Because the test only works properly with a tip made of harder material than the sample being tested, the true value for nanodiamond is likely somewhat lower than 310 GPa.
The precise tensile strength
of diamond is unknown, however strength up to 60 GPa
has been observed, and it could be as high as 90–225 GPa depending on the perfection of diamond lattice and on its orientation: Tensile strength is the highest for the [100] crystal direction
(normal to the cubic face), smaller for the [110] and the smallest for the [111] axis (along the longest cube diagonal).
Cubic
diamonds have a perfect and easy octahedral
cleavage
, which means that they only have four planes—weak directions following the face
s of the octahedron where there are fewer bonds—along which diamond can easily split upon blunt impact to leave a smooth surface. Similarly, diamond's hardness is markedly directional: the hardest direction is the diagonal on the cube
face, 100 times harder than the softest direction, which is the dodecahedral plane. The octahedral plane is intermediate between the two extremes. The diamond cutting
process relies heavily on this directional hardness, as without it a diamond would be nearly impossible to fashion. Cleavage
also plays a helpful role, especially in large stones where the cutter wishes to remove flawed material or to produce more than one stone from the same piece of rough (see, e.g., Cullinan Diamond
).
Diamonds crystallize in the diamond cubic crystal system
(space group
Fdm) and consist of tetrahedrally
, covalently bonded carbon atoms. A second form called lonsdaleite
, with hexagonal symmetry, has also been found, but it is extremely rare and forms only in meteor
ites or in laboratory synthesis. The local environment of each atom is identical in the two structures. From theoretical considerations, lonsdaleite is expected to be harder than diamond, but the size and quality of the available stones are insufficient to test this hypothesis. In terms of crystal habit
, diamonds occur most often as euhedral
(well-formed) or rounded octahedra and twinned
, flattened octahedra with a triangular outline. Other forms include dodecahedra and (rarely) cubes. There is evidence that nitrogen
impurities play an important role in the formation of well-shaped euhedral crystals. The largest diamonds found, such as the Cullinan Diamond
, were shapeless. These diamonds are pure (i.e. type II) and therefore contain little if any nitrogen.
The faces of diamond octahedrons are highly lustrous
due to their hardness; triangular shaped growth defects (trigons) or etch pits are often present on the faces. A diamond's fracture
may be step-like, conchoidal
(shell-like, similar to glass
) or irregular. Diamonds which are nearly round, due to the formation of multiple steps on octahedral faces, are commonly coated in a gum-like skin (nyf). The combination of stepped faces, growth defects, and nyf produces a "scaly" or corrugated appearance. Many diamonds are so distorted that few crystal faces are discernible. Some diamonds found in Brazil
and the Democratic Republic of the Congo
are polycrystalline and occur as opaque, darkly colored, spherical, radial masses of tiny crystals; these are known as ballas and are important to industry as they lack the cleavage planes of single-crystal diamond. Carbonado
is a similar opaque microcrystalline
form which occurs in shapeless masses. Like ballas diamond, carbonado lacks cleavage planes and its specific gravity varies widely from 2.9 to 3.5. Bort
diamonds, found in Brazil, Venezuela
, and Guyana
, are the most common type of industrial-grade diamond. They are also polycrystalline and often poorly crystallized; they are translucent and cleave easily.
Due to its great hardness and strong molecular bonding, a cut diamond's facet
s and facet edges appear the flattest and sharpest. A curious side effect of diamond's surface perfection is hydrophobia combined with lipophilia. The former property means a drop of water placed on a diamond will form a coherent droplet, whereas in most other minerals the water would spread out to cover the surface. Similarly, diamond is unusually lipophilic, meaning grease
and oil
readily collect on a diamond's surface. Whereas on other minerals oil would form coherent drops, on a diamond the oil would spread. This property is exploited in the use of so-called "grease pens," which apply a line of grease to the surface of a suspect diamond simulant
. Diamond surfaces are hydrophobic
when the surface carbon atoms terminate with a hydrogen atom and hydrophilic
when the surface atoms terminate with an oxygen atom or hydroxyl radical
. Treatment with gases or plasma
s containing the appropriate gas, at temperatures of 450 °C or higher, can change the surface property completely. Naturally occurring diamonds have a surface with less than a half monolayer coverage of oxygen, the balance being hydrogen and the behavior is moderately hydrophobic. This allows for separation from other minerals at the mine using the so-called "grease-belt".
 Unlike hardness, which only denotes resistance to scratching, diamond's toughness
Unlike hardness, which only denotes resistance to scratching, diamond's toughness
or tenacity is only fair to good. Toughness relates to the ability to resist breakage from falls or impacts. Due to diamond's perfect and easy cleavage, it is vulnerable to breakage. A diamond will shatter if hit with an ordinary hammer. The toughness of natural diamond has been measured as 2.0 MPa m1/2, which is good compared to other gemstones, but poor compared to most engineering materials. As with any material, the macroscopic geometry of a diamond contributes to its resistance to breakage. Diamond has a cleavage plane and is therefore more fragile in some orientations than others. Diamond cutters use this attribute to cleave some stones, prior to faceting.
Ballas and carbonado diamond are exceptional, as they are polycrystalline and therefore much tougher than single-crystal diamond; they are used for deep-drilling bits and other demanding industrial applications. Particular faceting shapes of diamonds are more prone to breakage and thus may be uninsurable by reputable insurance companies. The brilliant cut
of gemstones is designed specifically to reduce the likelihood of breakage or splintering.
Solid foreign crystals are commonly present in diamond. They are mostly minerals, such as olivine
, garnet
s, ruby
, and many others. These and other inclusions, such as internal fractures or "feathers", can compromise the structural integrity of a diamond. Cut diamonds that have been enhanced
to improve their clarity
via glass infilling of fractures or cavities are especially fragile, as the glass will not stand up to ultrasonic
cleaning or the rigors of the jeweler's torch. Fracture-filled diamonds may shatter if treated improperly.

 Diamonds occur in various colors — black, brown, yellow, gray, white, blue, orange, purple to pink and red. Colored diamonds contain crystallographic defect
Diamonds occur in various colors — black, brown, yellow, gray, white, blue, orange, purple to pink and red. Colored diamonds contain crystallographic defect
s, including substitutional impurities and structural defects, that cause the coloration. Theoretically, pure diamonds would be transparent and colorless. Diamonds are scientifically classed into two main types and several subtypes, according to the nature of defects present and how they affect light absorption:
Type I diamond has nitrogen
(N) atoms as the main impurity, at a concentration of up to 1%. If the N atoms are in pairs or larger aggregates, they do not affect the diamond's color; these are Type Ia. About 98% of gem diamonds are type Ia: these diamonds belong to the Cape series, named after the diamond-rich region formerly known as Cape Province
in South Africa
, whose deposits are largely Type Ia. If the nitrogen atoms are dispersed throughout the crystal in isolated sites (not paired or grouped), they give the stone an intense yellow or occasionally brown tint (type Ib); the rare canary diamonds belong to this type, which represents only ~0.1% of known natural diamonds. Synthetic diamond containing nitrogen is usually of type Ib. Type Ia and Ib diamonds absorb in both the infrared
and ultraviolet
region of the electromagnetic spectrum
, from 320 nm. They also have a characteristic fluorescence and visible absorption spectrum (see Optical properties).
Type II diamonds have very few if any nitrogen impurities. Pure (type IIa) diamond can be colored pink, red, or brown due to structural anomalies arising through plastic deformation during crystal growth — these diamonds are rare (1.8% of gem diamonds), but constitute a large percentage of Australian diamonds. Type IIb diamonds, which account for ~0.1% of gem diamonds, are usually a steely blue or gray due to boron atoms scattered within the crystal matrix. These diamonds are also semiconductor
s, unlike other diamond types (see Electrical properties). Most blue-gray diamonds coming from the Argyle mine
of Australia are not of type IIb, but of Ia type. Those diamonds contain large concentrations of defects and impurities (especially hydrogen and nitrogen) and the origin of their color is yet uncertain. Type II diamonds weakly absorb in a different region of the infrared (the absorption is due to the diamond lattice rather than impurities), and transmit in the ultraviolet below 225 nm, unlike type I diamonds. They also have differing fluorescence characteristics, but no discernible visible absorption spectrum.
Certain diamond enhancement
techniques are commonly used to artificially produce an array of colors, including blue, green, yellow, red, and black. Color enhancement techniques usually involve irradiation
, including proton
bombardment via cyclotron
s; neutron
bombardment in the piles of nuclear reactor
s; and electron
bombardment by Van de Graaff generator
s. These high-energy particles physically alter the diamond's crystal lattice, knocking carbon atoms out of place and producing color center
s. The depth of color penetration depends on the technique and its duration, and in some cases the diamond may be left radioactive to some degree.
Some irradiated diamonds are completely natural — one famous example is the Dresden Green Diamond
. In these natural stones the color is imparted by "radiation burns" (natural irradiation by alpha particle
s originating from uranium ore) in the form of small patches, usually only microns
deep. Additionally, Type IIa diamonds can have their structural deformations "repaired" via a high-pressure high-temperature (HPHT) process, removing much or all of the diamond's color.
 The luster
The luster
of a diamond is described as 'adamantine', which simply means diamond-like. Reflections on a properly cut diamond's facets are undistorted, due to their flatness. The refractive index
of diamond (as measured via sodium light, 589.3 nm) is 2.417. Because it is cubic in structure, diamond is also isotropic. Its high dispersion
of 0.044 (variation of refractive index across the visible spectrum) manifests in the perceptible fire of cut diamonds. This fire—flashes of prismatic
colors seen in transparent stones—is perhaps diamond's most important optical property from a jewelry perspective. The prominence or amount of fire seen in a stone is heavily influenced by the choice of diamond cut
and its associated proportions (particularly crown height), although the body color of fancy (i.e., unusual) diamonds may hide their fire to some degree.
Many other minerals have higher dispersion (that is difference in refractive index for blue and red light) than diamond: sphene
0.051, andradite
0.057, cassiterite
0.071, SrTiO3
0.109, sphalerite
0.156, synthetic rutile
0.330. However, the combination of dispersion with extreme hardness, wear and chemical resistivity, as well as clever marketing, determines the exceptional value of diamond as a gemstone.
, that is, they emit light of various colors and intensities under long-wave ultra-violet light (365 nm): Cape series stones (type Ia) usually fluoresce blue, and these stones may also phosphoresce
yellow, a unique property among gemstones. Other possible long-wave fluorescence colors are green (usually in brown stones), yellow, mauve, or red (in type IIb diamonds). In natural diamonds, there is typically little if any response to short-wave ultraviolet, but the reverse is true of synthetic diamonds. Some natural type IIb diamonds phosphoresce blue after exposure to short-wave ultraviolet. In natural diamonds, fluorescence under X-ray
s is generally bluish-white, yellowish or greenish. Some diamonds, particularly Canadian diamonds, show no fluorescence.
The origin of the luminescence colors is often unclear and not unique. Blue emission from type IIa and IIb diamonds is reliably identified with dislocations by directly correlating the emission with dislocations in an electron microscope
. However, blue emission in type Ia diamond could be either due to dislocations or the N3 defects (three nitrogen atoms bordering a vacancy). Green emission in natural diamond is usually due to the H3 center (two substitutional nitrogen atoms separated by a vacancy), whereas in synthetic diamond it usually originates from nickel
used as a catalyst (see figure). Orange or red emission could be due to various reasons, one being the nitrogen-vacancy center
which is present in sufficient quantities in all types of diamond, even type IIb.
All those lines are labeled as N3 and N2 optical centers and associated with a defect consisting of three nitrogen atoms bordering a vacancy. Other stones show additional bands: brown, green, or yellow diamonds show a band in the green at 504 nm (H3 center, see above), sometimes accompanied by two additional weak bands at 537 nm and 495 nm (H4 center, a large complex presumably involving 4 substitutional nitrogen atoms and 2 lattice vacancies). Type IIb diamonds may absorb in the far red due to the substitutional boron, but otherwise show no observable visible absorption spectrum.
Gemological
laboratories make use of spectrophotometer machines that can distinguish natural, artificial, and color-enhanced diamonds
. The spectrophotometers analyze the infrared
, visible, and ultraviolet
absorption and luminescence spectra of diamonds cooled with liquid nitrogen
to detect tell-tale absorption lines that are not normally discernible.
s due to substitutional boron
impurities replacing carbon atoms, diamond is a good electrical insulator
. Natural blue or blue-gray diamonds, common for the Argyle diamond mine
in Australia
, are rich in hydrogen
; these diamonds are not semiconductors and it is unclear whether hydrogen is actually responsible for their blue-gray color. Natural blue diamonds containing boron and synthetic diamonds doped
with boron are p-type semiconductor
s. N-type
diamond films are reproducibly synthesized by phosphorus doping during chemical vapor deposition
. Diode p-n junction
s and UV light emitting diodes (LED
s, at 235 nm) have been produced by sequential deposition of p-type (boron-doped) and n-type (phosphorus-doped) layers. Diamond transistors have been produced.
In April 2004, the journal Nature
reported that below the superconducting transition temperature 4 K
, boron-doped diamond synthesized at high temperature and high pressure is a bulk superconductor. Superconductivity was later observed in heavily boron-doped films grown by various chemical vapor deposition
techniques, and the highest reported transition temperature (by 2009) is 11.4 K.
atoms which replace carbon atoms in the crystal matrix, and also have high thermal conductance. Thermal conductivity of natural diamond was measured to be about 22 W/(cm·K). Monocrystalline synthetic diamond enriched in 12C isotope (99.9%) has the highest thermal conductivity
of any known solid at room temperature: 33.2 W/(cm·K), five times more than copper
. Because diamond has such high thermal conductance it is already used in semiconductor manufacture to prevent silicon
and other semiconducting materials from overheating. At lower temperatures conductivity becomes even better as its Fermi electrons
can match the phonon
ic normal transport mode near the Debye point, and thermal conductivity reaches 410 W/(cm·K) at 104 K (12C-enriched diamond).
Diamond's high thermal conductivity is used by jewelers and gemologists who may employ an electronic thermal probe to separate diamonds from their imitations. These probes consist of a pair of battery-powered thermistor
s mounted in a fine copper tip. One thermistor functions as a heating device while the other measures the temperature of the copper tip: if the stone being tested is a diamond, it will conduct the tip's thermal energy rapidly enough to produce a measurable temperature drop. This test takes about 2–3 seconds. However, older probes will be fooled by moissanite
, a crystalline mineral form of silicon carbide
introduced in 1998 as an alternative to diamonds, which has a similar thermal conductivity.
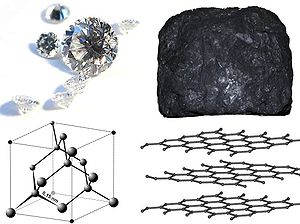 Being a form of carbon, diamond oxidizes in air if heated over 700 °C. In absence of oxygen, e.g. in a flow of high-purity argon
Being a form of carbon, diamond oxidizes in air if heated over 700 °C. In absence of oxygen, e.g. in a flow of high-purity argon
gas, diamond can be heated up to about 1700 °C. Its surface blackens, but can be recovered by re-polishing. At high pressure (~20 GPa) diamond can be heated up to 2500 °C, and a report published in 2009 suggests that diamond can withstand temperatures of 3000 °C and above.
Diamonds are carbon crystal
s that form deep within the Earth under high temperatures and extreme pressures. At surface air pressure (one atmosphere), diamonds are not as stable as graphite, and so the decay of diamond is thermodynamically
favorable (δH = −2 kJ / mol). So, contrary to De Beers
' ad campaign extending from 1948 to at least 2006 under the slogan "A diamond is forever", diamonds are definitely not forever. However, owing to a very large kinetic energy
barrier, diamonds are metastable; they will not decay into graphite under normal conditions.
Allotropes of carbon
This is a list of the allotropes of carbon.-Diamond:Diamond is one of the most well known allotropes of carbon. The hardness and high dispersion of light of diamond make it useful for both industrial applications and jewellery. Diamond is the hardest known natural mineral. This makes it an...
in which the carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
atoms are arranged in the specific type of cubic lattice
Cubic crystal system
In crystallography, the cubic crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals....
called diamond cubic
Diamond cubic
The diamond cubic crystal structure is a repeating pattern of 8 atoms that certain materials may adopt as they solidify. While the first known example was diamond, other elements in group IV also adopt this structure, including tin, the semiconductors silicon and germanium, and silicon/germanium...
. Diamond is an optically isotropic crystal
Crystal
A crystal or crystalline solid is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. The scientific study of crystals and crystal formation is known as crystallography...
that is transparent to opaque. Owing to its strong covalent bond
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
ing, diamond is the hardest naturally occurring material known. Yet, due to important structural weaknesses, diamond's toughness
Fracture toughness
In materials science, fracture toughness is a property which describes the ability of a material containing a crack to resist fracture, and is one of the most important properties of any material for virtually all design applications. The fracture toughness of a material is determined from the...
is only fair to good. The precise tensile strength
Tensile strength
Ultimate tensile strength , often shortened to tensile strength or ultimate strength, is the maximum stress that a material can withstand while being stretched or pulled before necking, which is when the specimen's cross-section starts to significantly contract...
of diamond is unknown, however strength up to 60 GPa
Pascal (unit)
The pascal is the SI derived unit of pressure, internal pressure, stress, Young's modulus and tensile strength, named after the French mathematician, physicist, inventor, writer, and philosopher Blaise Pascal. It is a measure of force per unit area, defined as one newton per square metre...
has been observed, and it could be as high as 90–225 GPa depending on the crystal orientation. The anisotropy
Anisotropy
Anisotropy is the property of being directionally dependent, as opposed to isotropy, which implies identical properties in all directions. It can be defined as a difference, when measured along different axes, in a material's physical or mechanical properties An example of anisotropy is the light...
of diamond hardness is carefully considered during diamond cutting
Diamond cutting
Diamond cutting is the art, skill and, increasingly, science of changing a diamond from a rough stone into a faceted gem. Cutting diamond requires specialized knowledge, tools, equipment, and techniques because of its extreme difficulty....
. Diamond has a high refractive index
Refractive index
In optics the refractive index or index of refraction of a substance or medium is a measure of the speed of light in that medium. It is expressed as a ratio of the speed of light in vacuum relative to that in the considered medium....
(2.417) and moderate dispersion
Dispersion (optics)
In optics, dispersion is the phenomenon in which the phase velocity of a wave depends on its frequency, or alternatively when the group velocity depends on the frequency.Media having such a property are termed dispersive media...
(0.044) properties which give cut diamonds their brilliance. Scientists classify diamonds into four main types according to the nature of crystallographic defect
Crystallographic defect
Crystalline solids exhibit a periodic crystal structure. The positions of atoms or molecules occur on repeating fixed distances, determined by the unit cell parameters. However, the arrangement of atom or molecules in most crystalline materials is not perfect...
s present. Trace impurities substitutionally replacing carbon atoms in a diamond's crystal lattice, and in some cases structural defects, are responsible for the wide range of colors seen in diamond. Most diamonds are electrical insulator
Electrical insulation
thumb|250px|[[Coaxial Cable]] with dielectric insulator supporting a central coreThis article refers to electrical insulation. For insulation of heat, see Thermal insulation...
s but extremely efficient thermal conductor
Thermal conductivity
In physics, thermal conductivity, k, is the property of a material's ability to conduct heat. It appears primarily in Fourier's Law for heat conduction....
s. Unlike many other minerals, the specific gravity
Specific gravity
Specific gravity is the ratio of the density of a substance to the density of a reference substance. Apparent specific gravity is the ratio of the weight of a volume of the substance to the weight of an equal volume of the reference substance. The reference substance is nearly always water for...
of diamond crystals (3.52) has rather small variation from diamond to diamond.
Hardness and crystal structure
Known to the ancient Greeks as ἀδάμας – adámas ("proper", "unalterable", "unbreakable") and sometimes called adamantAdamant
Adamant and similar words are used to refer to any especially hard substance, whether composed of diamond, some other gemstone, or some type of metal. Both adamant and diamond derive from the Greek word αδαμας , meaning "untameable"...
, diamond is the hardest known naturally occurring material, scoring 10 on the Mohs scale of mineral hardness
Mohs scale of mineral hardness
The Mohs scale of mineral hardness characterizes the scratch resistance of various minerals through the ability of a harder material to scratch a softer material. It was created in 1812 by the German geologist and mineralogist Friedrich Mohs and is one of several definitions of hardness in...
. Diamond is extremely strong due to the structure of its carbon atoms, where each carbon atom has four neighbors joined to it with covalent bonds. The material boron nitride
Boron nitride
Boron nitride is a chemical compound with chemical formula BN, consisting of equal numbers of boron and nitrogen atoms. BN is isoelectronic to a similarly structured carbon lattice and thus exists in various crystalline forms...
, when in a form structurally identical to diamond (zincblende structure), is nearly as hard as diamond; a currently hypothetical material, beta carbon nitride
Beta carbon nitride
Beta carbon nitride is a material predicted to be harder than diamond.The material was first proposed in 1985 by Marvin Cohen and Amy Liu. Examining the nature of crystalline bonds they theorised that carbon and nitrogen atoms could form a particularly short and strong bond in a stable crystal...
, may also be as hard or harder in one form. It has been shown that some diamond aggregates having nanometer grain size are harder and tougher than conventional large diamond crystals, thus they perform better as abrasive material. Due to the use of those new ultra-hard materials for diamond testing, more accurate values are now known for diamond hardness. A surface perpendicular to the [111] crystallographic direction (that is the longest diagonal of a cube) of a pure (i.e. type IIa) diamond has a hardness value of 167 GPa when scratched with an nanodiamond tip, while the nanodiamond sample itself has a value of 310 GPa when tested with another nanodiamond tip. Because the test only works properly with a tip made of harder material than the sample being tested, the true value for nanodiamond is likely somewhat lower than 310 GPa.
The precise tensile strength
Tensile strength
Ultimate tensile strength , often shortened to tensile strength or ultimate strength, is the maximum stress that a material can withstand while being stretched or pulled before necking, which is when the specimen's cross-section starts to significantly contract...
of diamond is unknown, however strength up to 60 GPa
Pascal (unit)
The pascal is the SI derived unit of pressure, internal pressure, stress, Young's modulus and tensile strength, named after the French mathematician, physicist, inventor, writer, and philosopher Blaise Pascal. It is a measure of force per unit area, defined as one newton per square metre...
has been observed, and it could be as high as 90–225 GPa depending on the perfection of diamond lattice and on its orientation: Tensile strength is the highest for the [100] crystal direction
Miller index
Miller indices form a notation system in crystallography for planes and directions in crystal lattices.In particular, a family of lattice planes is determined by three integers h, k, and ℓ, the Miller indices. They are written , and each index denotes a plane orthogonal to a direction in the...
(normal to the cubic face), smaller for the [110] and the smallest for the [111] axis (along the longest cube diagonal).
Cubic
Cubic crystal system
In crystallography, the cubic crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals....
diamonds have a perfect and easy octahedral
Octahedron
In geometry, an octahedron is a polyhedron with eight faces. A regular octahedron is a Platonic solid composed of eight equilateral triangles, four of which meet at each vertex....
cleavage
Cleavage (crystal)
Cleavage, in mineralogy, is the tendency of crystalline materials to split along definite crystallographic structural planes. These planes of relative weakness are a result of the regular locations of atoms and ions in the crystal, which create smooth repeating surfaces that are visible both in the...
, which means that they only have four planes—weak directions following the face
Face (geometry)
In geometry, a face of a polyhedron is any of the polygons that make up its boundaries. For example, any of the squares that bound a cube is a face of the cube...
s of the octahedron where there are fewer bonds—along which diamond can easily split upon blunt impact to leave a smooth surface. Similarly, diamond's hardness is markedly directional: the hardest direction is the diagonal on the cube
Cube
In geometry, a cube is a three-dimensional solid object bounded by six square faces, facets or sides, with three meeting at each vertex. The cube can also be called a regular hexahedron and is one of the five Platonic solids. It is a special kind of square prism, of rectangular parallelepiped and...
face, 100 times harder than the softest direction, which is the dodecahedral plane. The octahedral plane is intermediate between the two extremes. The diamond cutting
Diamond cutting
Diamond cutting is the art, skill and, increasingly, science of changing a diamond from a rough stone into a faceted gem. Cutting diamond requires specialized knowledge, tools, equipment, and techniques because of its extreme difficulty....
process relies heavily on this directional hardness, as without it a diamond would be nearly impossible to fashion. Cleavage
Cleavage (crystal)
Cleavage, in mineralogy, is the tendency of crystalline materials to split along definite crystallographic structural planes. These planes of relative weakness are a result of the regular locations of atoms and ions in the crystal, which create smooth repeating surfaces that are visible both in the...
also plays a helpful role, especially in large stones where the cutter wishes to remove flawed material or to produce more than one stone from the same piece of rough (see, e.g., Cullinan Diamond
Cullinan Diamond
The Cullinan diamond is the largest rough gem-quality diamond ever found, at .The largest polished gem from the stone is named Cullinan I or the Great Star of Africa, and at was the largest polished diamond in the world until the 1985 discovery of the Golden Jubilee Diamond, , also from the...
).
Diamonds crystallize in the diamond cubic crystal system
Crystal system
In crystallography, the terms crystal system, crystal family, and lattice system each refer to one of several classes of space groups, lattices, point groups, or crystals...
(space group
Space group
In mathematics and geometry, a space group is a symmetry group, usually for three dimensions, that divides space into discrete repeatable domains.In three dimensions, there are 219 unique types, or counted as 230 if chiral copies are considered distinct...
Fdm) and consist of tetrahedrally
Tetrahedron
In geometry, a tetrahedron is a polyhedron composed of four triangular faces, three of which meet at each vertex. A regular tetrahedron is one in which the four triangles are regular, or "equilateral", and is one of the Platonic solids...
, covalently bonded carbon atoms. A second form called lonsdaleite
Lonsdaleite
Lonsdaleite , also called hexagonal diamond in reference to the crystal structure, is an allotrope of carbon with a hexagonal lattice. In nature, it forms when meteorites containing graphite strike the Earth. The great heat and stress of the impact transforms the graphite into diamond, but retains...
, with hexagonal symmetry, has also been found, but it is extremely rare and forms only in meteor
METEOR
METEOR is a metric for the evaluation of machine translation output. The metric is based on the harmonic mean of unigram precision and recall, with recall weighted higher than precision...
ites or in laboratory synthesis. The local environment of each atom is identical in the two structures. From theoretical considerations, lonsdaleite is expected to be harder than diamond, but the size and quality of the available stones are insufficient to test this hypothesis. In terms of crystal habit
Crystal habit
Crystal habit is an overall description of the visible external shape of a mineral. This description can apply to an individual crystal or an assembly of crystals or aggregates....
, diamonds occur most often as euhedral
Euhedral
Euhedral crystals are those that are well-formed with sharp, easily recognised faces. Normally, crystals do not form smooth faces or sharp crystal outlines. Many crystals grow from cooling liquid magma...
(well-formed) or rounded octahedra and twinned
Crystal twinning
Crystal twinning occurs when two separate crystals share some of the same crystal lattice points in a symmetrical manner. The result is an intergrowth of two separate crystals in a variety of specific configurations. A twin boundary or composition surface separates the two crystals....
, flattened octahedra with a triangular outline. Other forms include dodecahedra and (rarely) cubes. There is evidence that nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
impurities play an important role in the formation of well-shaped euhedral crystals. The largest diamonds found, such as the Cullinan Diamond
Cullinan Diamond
The Cullinan diamond is the largest rough gem-quality diamond ever found, at .The largest polished gem from the stone is named Cullinan I or the Great Star of Africa, and at was the largest polished diamond in the world until the 1985 discovery of the Golden Jubilee Diamond, , also from the...
, were shapeless. These diamonds are pure (i.e. type II) and therefore contain little if any nitrogen.
The faces of diamond octahedrons are highly lustrous
Lustre (mineralogy)
Lustre is a description of the way light interacts with the surface of a crystal, rock, or mineral. The word lustre traces its origins back to the Latin word lux, meaning "light", and generally implies radiance, gloss, or brilliance....
due to their hardness; triangular shaped growth defects (trigons) or etch pits are often present on the faces. A diamond's fracture
Fracture
A fracture is the separation of an object or material into two, or more, pieces under the action of stress.The word fracture is often applied to bones of living creatures , or to crystals or crystalline materials, such as gemstones or metal...
may be step-like, conchoidal
Conchoidal fracture
Conchoidal fracture describes the way that brittle materials break when they do not follow any natural planes of separation. Materials that break in this way include flint and other fine-grained minerals, as well as most amorphous solids, such as obsidian and other types of glass.Conchoidal...
(shell-like, similar to glass
Glass
Glass is an amorphous solid material. Glasses are typically brittle and optically transparent.The most familiar type of glass, used for centuries in windows and drinking vessels, is soda-lime glass, composed of about 75% silica plus Na2O, CaO, and several minor additives...
) or irregular. Diamonds which are nearly round, due to the formation of multiple steps on octahedral faces, are commonly coated in a gum-like skin (nyf). The combination of stepped faces, growth defects, and nyf produces a "scaly" or corrugated appearance. Many diamonds are so distorted that few crystal faces are discernible. Some diamonds found in Brazil
Brazil
Brazil , officially the Federative Republic of Brazil , is the largest country in South America. It is the world's fifth largest country, both by geographical area and by population with over 192 million people...
and the Democratic Republic of the Congo
Democratic Republic of the Congo
The Democratic Republic of the Congo is a state located in Central Africa. It is the second largest country in Africa by area and the eleventh largest in the world...
are polycrystalline and occur as opaque, darkly colored, spherical, radial masses of tiny crystals; these are known as ballas and are important to industry as they lack the cleavage planes of single-crystal diamond. Carbonado
Carbonado
Carbonado, commonly known as the "Black Diamond", is a natural polycrystalline diamond found in alluvial deposits in the Central African Republic and Brazil. Its natural colour is black or dark grey, and it is more porous than other diamonds....
is a similar opaque microcrystalline
Microcrystalline
A microcrystalline material is a crystallized substance or rock that contains small crystals visible only through microscopic examination.-See also:* Macrocrystalline* Microcrystalline silicon* Protocrystalline* Rock microstructure...
form which occurs in shapeless masses. Like ballas diamond, carbonado lacks cleavage planes and its specific gravity varies widely from 2.9 to 3.5. Bort
Bort
Bort or boart is a term used in the diamond industry to refer to shards of gem-grade/quality diamonds. In the manufacturing and heavy industries, "bort" is used to describe dark, imperfectly formed/crystallized diamonds of varying levels of opacity. The lowest grade, "crushing bort", is crushed by...
diamonds, found in Brazil, Venezuela
Venezuela
Venezuela , officially called the Bolivarian Republic of Venezuela , is a tropical country on the northern coast of South America. It borders Colombia to the west, Guyana to the east, and Brazil to the south...
, and Guyana
Guyana
Guyana , officially the Co-operative Republic of Guyana, previously the colony of British Guiana, is a sovereign state on the northern coast of South America that is culturally part of the Anglophone Caribbean. Guyana was a former colony of the Dutch and of the British...
, are the most common type of industrial-grade diamond. They are also polycrystalline and often poorly crystallized; they are translucent and cleave easily.
Due to its great hardness and strong molecular bonding, a cut diamond's facet
Facet
Facets are flat faces on geometric shapes. The organization of naturally occurring facets was key to early developments in crystallography, since they reflect the underlying symmetry of the crystal structure...
s and facet edges appear the flattest and sharpest. A curious side effect of diamond's surface perfection is hydrophobia combined with lipophilia. The former property means a drop of water placed on a diamond will form a coherent droplet, whereas in most other minerals the water would spread out to cover the surface. Similarly, diamond is unusually lipophilic, meaning grease
Petroleum
Petroleum or crude oil is a naturally occurring, flammable liquid consisting of a complex mixture of hydrocarbons of various molecular weights and other liquid organic compounds, that are found in geologic formations beneath the Earth's surface. Petroleum is recovered mostly through oil drilling...
and oil
Oil
An oil is any substance that is liquid at ambient temperatures and does not mix with water but may mix with other oils and organic solvents. This general definition includes vegetable oils, volatile essential oils, petrochemical oils, and synthetic oils....
readily collect on a diamond's surface. Whereas on other minerals oil would form coherent drops, on a diamond the oil would spread. This property is exploited in the use of so-called "grease pens," which apply a line of grease to the surface of a suspect diamond simulant
Diamond simulant
The high price of gem-grade diamonds, as well as significant ethical concerns of the diamond trade, have created a large demand for materials with similar gemological characteristics, known as diamond simulants or imitations. Simulants are distinct from synthetic diamond, which unlike simulants is...
. Diamond surfaces are hydrophobic
Hydrophobe
In chemistry, hydrophobicity is the physical property of a molecule that is repelled from a mass of water....
when the surface carbon atoms terminate with a hydrogen atom and hydrophilic
Hydrophile
A hydrophile, from the Greek "water" and φιλια "love," is a molecule or other molecular entity that is attracted to, and tends to be dissolved by water. A hydrophilic molecule or portion of a molecule is one that has a tendency to interact with or be dissolved by, water and other polar substances...
when the surface atoms terminate with an oxygen atom or hydroxyl radical
Hydroxyl radical
The hydroxyl radical, •OH, is the neutral form of the hydroxide ion . Hydroxyl radicals are highly reactive and consequently short-lived; however, they form an important part of radical chemistry. Most notably hydroxyl radicals are produced from the decomposition of hydroperoxides or, in...
. Treatment with gases or plasma
Chalcedony
Chalcedony is a cryptocrystalline form of silica, composed of very fine intergrowths of the minerals quartz and moganite. These are both silica minerals, but they differ in that quartz has a trigonal crystal structure, while moganite is monoclinic...
s containing the appropriate gas, at temperatures of 450 °C or higher, can change the surface property completely. Naturally occurring diamonds have a surface with less than a half monolayer coverage of oxygen, the balance being hydrogen and the behavior is moderately hydrophobic. This allows for separation from other minerals at the mine using the so-called "grease-belt".
Toughness

Fracture toughness
In materials science, fracture toughness is a property which describes the ability of a material containing a crack to resist fracture, and is one of the most important properties of any material for virtually all design applications. The fracture toughness of a material is determined from the...
or tenacity is only fair to good. Toughness relates to the ability to resist breakage from falls or impacts. Due to diamond's perfect and easy cleavage, it is vulnerable to breakage. A diamond will shatter if hit with an ordinary hammer. The toughness of natural diamond has been measured as 2.0 MPa m1/2, which is good compared to other gemstones, but poor compared to most engineering materials. As with any material, the macroscopic geometry of a diamond contributes to its resistance to breakage. Diamond has a cleavage plane and is therefore more fragile in some orientations than others. Diamond cutters use this attribute to cleave some stones, prior to faceting.
Ballas and carbonado diamond are exceptional, as they are polycrystalline and therefore much tougher than single-crystal diamond; they are used for deep-drilling bits and other demanding industrial applications. Particular faceting shapes of diamonds are more prone to breakage and thus may be uninsurable by reputable insurance companies. The brilliant cut
Diamond cut
A diamond cut is a style or design guide used when shaping a diamond for polishing such as the brilliant cut. Cut does not refer to shape , but the symmetry, proportioning and polish of a diamond...
of gemstones is designed specifically to reduce the likelihood of breakage or splintering.
Solid foreign crystals are commonly present in diamond. They are mostly minerals, such as olivine
Olivine
The mineral olivine is a magnesium iron silicate with the formula 2SiO4. It is a common mineral in the Earth's subsurface but weathers quickly on the surface....
, garnet
Garnet
The garnet group includes a group of minerals that have been used since the Bronze Age as gemstones and abrasives. The name "garnet" may come from either the Middle English word gernet meaning 'dark red', or the Latin granatus , possibly a reference to the Punica granatum , a plant with red seeds...
s, ruby
Ruby
A ruby is a pink to blood-red colored gemstone, a variety of the mineral corundum . The red color is caused mainly by the presence of the element chromium. Its name comes from ruber, Latin for red. Other varieties of gem-quality corundum are called sapphires...
, and many others. These and other inclusions, such as internal fractures or "feathers", can compromise the structural integrity of a diamond. Cut diamonds that have been enhanced
Diamond enhancement
Diamond enhancements are specific treatments, performed on natural diamonds , which are designed to improve the gemological characteristics — and therefore the value — of the stone in one or more ways...
to improve their clarity
Diamond clarity
Diamond clarity is a quality of diamonds relating to the existence and visual appearance of internal characteristics of a diamond called inclusions, and surface defects called blemishes. Clarity is one of the four Cs of diamond grading, the others being carat, color, and cut...
via glass infilling of fractures or cavities are especially fragile, as the glass will not stand up to ultrasonic
Ultrasound
Ultrasound is cyclic sound pressure with a frequency greater than the upper limit of human hearing. Ultrasound is thus not separated from "normal" sound based on differences in physical properties, only the fact that humans cannot hear it. Although this limit varies from person to person, it is...
cleaning or the rigors of the jeweler's torch. Fracture-filled diamonds may shatter if treated improperly.
Color and its causes


Crystallographic defect
Crystalline solids exhibit a periodic crystal structure. The positions of atoms or molecules occur on repeating fixed distances, determined by the unit cell parameters. However, the arrangement of atom or molecules in most crystalline materials is not perfect...
s, including substitutional impurities and structural defects, that cause the coloration. Theoretically, pure diamonds would be transparent and colorless. Diamonds are scientifically classed into two main types and several subtypes, according to the nature of defects present and how they affect light absorption:
Type I diamond has nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
(N) atoms as the main impurity, at a concentration of up to 1%. If the N atoms are in pairs or larger aggregates, they do not affect the diamond's color; these are Type Ia. About 98% of gem diamonds are type Ia: these diamonds belong to the Cape series, named after the diamond-rich region formerly known as Cape Province
Cape Province
The Province of the Cape of Good Hope was a province in the Union of South Africa and subsequently the Republic of South Africa...
in South Africa
South Africa
The Republic of South Africa is a country in southern Africa. Located at the southern tip of Africa, it is divided into nine provinces, with of coastline on the Atlantic and Indian oceans...
, whose deposits are largely Type Ia. If the nitrogen atoms are dispersed throughout the crystal in isolated sites (not paired or grouped), they give the stone an intense yellow or occasionally brown tint (type Ib); the rare canary diamonds belong to this type, which represents only ~0.1% of known natural diamonds. Synthetic diamond containing nitrogen is usually of type Ib. Type Ia and Ib diamonds absorb in both the infrared
Infrared
Infrared light is electromagnetic radiation with a wavelength longer than that of visible light, measured from the nominal edge of visible red light at 0.74 micrometres , and extending conventionally to 300 µm...
and ultraviolet
Ultraviolet
Ultraviolet light is electromagnetic radiation with a wavelength shorter than that of visible light, but longer than X-rays, in the range 10 nm to 400 nm, and energies from 3 eV to 124 eV...
region of the electromagnetic spectrum
Electromagnetic spectrum
The electromagnetic spectrum is the range of all possible frequencies of electromagnetic radiation. The "electromagnetic spectrum" of an object is the characteristic distribution of electromagnetic radiation emitted or absorbed by that particular object....
, from 320 nm. They also have a characteristic fluorescence and visible absorption spectrum (see Optical properties).
Type II diamonds have very few if any nitrogen impurities. Pure (type IIa) diamond can be colored pink, red, or brown due to structural anomalies arising through plastic deformation during crystal growth — these diamonds are rare (1.8% of gem diamonds), but constitute a large percentage of Australian diamonds. Type IIb diamonds, which account for ~0.1% of gem diamonds, are usually a steely blue or gray due to boron atoms scattered within the crystal matrix. These diamonds are also semiconductor
Semiconductor
A semiconductor is a material with electrical conductivity due to electron flow intermediate in magnitude between that of a conductor and an insulator. This means a conductivity roughly in the range of 103 to 10−8 siemens per centimeter...
s, unlike other diamond types (see Electrical properties). Most blue-gray diamonds coming from the Argyle mine
Argyle diamond mine
The Argyle Diamond Mine is a diamond mine located in the East Kimberley region in the remote north of Western Australia. Argyle is the largest diamond producer in the world by volume, although due to the low proportion of gem-quality diamonds, is not the leader by value. It is the only known...
of Australia are not of type IIb, but of Ia type. Those diamonds contain large concentrations of defects and impurities (especially hydrogen and nitrogen) and the origin of their color is yet uncertain. Type II diamonds weakly absorb in a different region of the infrared (the absorption is due to the diamond lattice rather than impurities), and transmit in the ultraviolet below 225 nm, unlike type I diamonds. They also have differing fluorescence characteristics, but no discernible visible absorption spectrum.
Certain diamond enhancement
Diamond enhancement
Diamond enhancements are specific treatments, performed on natural diamonds , which are designed to improve the gemological characteristics — and therefore the value — of the stone in one or more ways...
techniques are commonly used to artificially produce an array of colors, including blue, green, yellow, red, and black. Color enhancement techniques usually involve irradiation
Irradiation
Irradiation is the process by which an object is exposed to radiation. The exposure can originate from various sources, including natural sources. Most frequently the term refers to ionizing radiation, and to a level of radiation that will serve a specific purpose, rather than radiation exposure to...
, including proton
Proton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
bombardment via cyclotron
Cyclotron
In technology, a cyclotron is a type of particle accelerator. In physics, the cyclotron frequency or gyrofrequency is the frequency of a charged particle moving perpendicularly to the direction of a uniform magnetic field, i.e. a magnetic field of constant magnitude and direction...
s; neutron
Neutron
The neutron is a subatomic hadron particle which has the symbol or , no net electric charge and a mass slightly larger than that of a proton. With the exception of hydrogen, nuclei of atoms consist of protons and neutrons, which are therefore collectively referred to as nucleons. The number of...
bombardment in the piles of nuclear reactor
Nuclear reactor
A nuclear reactor is a device to initiate and control a sustained nuclear chain reaction. Most commonly they are used for generating electricity and for the propulsion of ships. Usually heat from nuclear fission is passed to a working fluid , which runs through turbines that power either ship's...
s; and electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
bombardment by Van de Graaff generator
Van de Graaff generator
A Van de Graaff generator is an electrostatic generator which uses a moving belt to accumulate very high voltages on a hollow metal globe on the top of the stand. It was invented in 1929 by American physicist Robert J. Van de Graaff. The potential differences achieved in modern Van de Graaff...
s. These high-energy particles physically alter the diamond's crystal lattice, knocking carbon atoms out of place and producing color center
F-Center
An F-Center or Farbe center is a type of crystallographic defect in which an anionic vacancy in a crystal is filled by one or more electrons, depending on the charge of the missing ion in the crystal. Electrons in such a vacancy tend to absorb light in the visible spectrum such that a material...
s. The depth of color penetration depends on the technique and its duration, and in some cases the diamond may be left radioactive to some degree.
Some irradiated diamonds are completely natural — one famous example is the Dresden Green Diamond
Dresden Green Diamond
The Dresden Green Diamond, also known as "Dresden Green", is a natural green diamond, which probably originated in the Kollur mine in the state of Andhra Pradesh in the Indian subcontinent....
. In these natural stones the color is imparted by "radiation burns" (natural irradiation by alpha particle
Alpha particle
Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus, which is classically produced in the process of alpha decay, but may be produced also in other ways and given the same name...
s originating from uranium ore) in the form of small patches, usually only microns
Micrometre
A micrometer , is by definition 1×10-6 of a meter .In plain English, it means one-millionth of a meter . Its unit symbol in the International System of Units is μm...
deep. Additionally, Type IIa diamonds can have their structural deformations "repaired" via a high-pressure high-temperature (HPHT) process, removing much or all of the diamond's color.
Luster

Lustre (mineralogy)
Lustre is a description of the way light interacts with the surface of a crystal, rock, or mineral. The word lustre traces its origins back to the Latin word lux, meaning "light", and generally implies radiance, gloss, or brilliance....
of a diamond is described as 'adamantine', which simply means diamond-like. Reflections on a properly cut diamond's facets are undistorted, due to their flatness. The refractive index
Refractive index
In optics the refractive index or index of refraction of a substance or medium is a measure of the speed of light in that medium. It is expressed as a ratio of the speed of light in vacuum relative to that in the considered medium....
of diamond (as measured via sodium light, 589.3 nm) is 2.417. Because it is cubic in structure, diamond is also isotropic. Its high dispersion
Dispersion (optics)
In optics, dispersion is the phenomenon in which the phase velocity of a wave depends on its frequency, or alternatively when the group velocity depends on the frequency.Media having such a property are termed dispersive media...
of 0.044 (variation of refractive index across the visible spectrum) manifests in the perceptible fire of cut diamonds. This fire—flashes of prismatic
Prism (optics)
In optics, a prism is a transparent optical element with flat, polished surfaces that refract light. The exact angles between the surfaces depend on the application. The traditional geometrical shape is that of a triangular prism with a triangular base and rectangular sides, and in colloquial use...
colors seen in transparent stones—is perhaps diamond's most important optical property from a jewelry perspective. The prominence or amount of fire seen in a stone is heavily influenced by the choice of diamond cut
Diamond cut
A diamond cut is a style or design guide used when shaping a diamond for polishing such as the brilliant cut. Cut does not refer to shape , but the symmetry, proportioning and polish of a diamond...
and its associated proportions (particularly crown height), although the body color of fancy (i.e., unusual) diamonds may hide their fire to some degree.
Many other minerals have higher dispersion (that is difference in refractive index for blue and red light) than diamond: sphene
Titanite
Titanite, or sphene , is a calcium titanium nesosilicate mineral, CaTiSiO5. Trace impurities of iron and aluminium are typically present...
0.051, andradite
Andradite
Andradite is a species of the garnet group. It is a nesosilicate, with formula Ca3Fe2Si3O12.Andradite includes three varieties:* Melanite: Black in color, referred to as "titanian andradite"....
0.057, cassiterite
Cassiterite
Cassiterite is a tin oxide mineral, SnO2. It is generally opaque, but it is translucent in thin crystals. Its luster and multiple crystal faces produce a desirable gem...
0.071, SrTiO3
Strontium titanate
Strontium titanate is an oxide of strontium and titanium with the chemical formula SrTiO3. At room temperature, it is a centrosymmetric paraelectric material with a perovskite structure...
0.109, sphalerite
Sphalerite
Sphalerite is a mineral that is the chief ore of zinc. It consists largely of zinc sulfide in crystalline form but almost always contains variable iron. When iron content is high it is an opaque black variety, marmatite. It is usually found in association with galena, pyrite, and other sulfides...
0.156, synthetic rutile
Rutile
Rutile is a mineral composed primarily of titanium dioxide, TiO2.Rutile is the most common natural form of TiO2. Two rarer polymorphs of TiO2 are known:...
0.330. However, the combination of dispersion with extreme hardness, wear and chemical resistivity, as well as clever marketing, determines the exceptional value of diamond as a gemstone.
Fluorescence
Diamonds exhibit fluorescenceFluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation of a different wavelength. It is a form of luminescence. In most cases, emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation...
, that is, they emit light of various colors and intensities under long-wave ultra-violet light (365 nm): Cape series stones (type Ia) usually fluoresce blue, and these stones may also phosphoresce
Phosphorescence
Phosphorescence is a specific type of photoluminescence related to fluorescence. Unlike fluorescence, a phosphorescent material does not immediately re-emit the radiation it absorbs. The slower time scales of the re-emission are associated with "forbidden" energy state transitions in quantum...
yellow, a unique property among gemstones. Other possible long-wave fluorescence colors are green (usually in brown stones), yellow, mauve, or red (in type IIb diamonds). In natural diamonds, there is typically little if any response to short-wave ultraviolet, but the reverse is true of synthetic diamonds. Some natural type IIb diamonds phosphoresce blue after exposure to short-wave ultraviolet. In natural diamonds, fluorescence under X-ray
X-ray
X-radiation is a form of electromagnetic radiation. X-rays have a wavelength in the range of 0.01 to 10 nanometers, corresponding to frequencies in the range 30 petahertz to 30 exahertz and energies in the range 120 eV to 120 keV. They are shorter in wavelength than UV rays and longer than gamma...
s is generally bluish-white, yellowish or greenish. Some diamonds, particularly Canadian diamonds, show no fluorescence.
The origin of the luminescence colors is often unclear and not unique. Blue emission from type IIa and IIb diamonds is reliably identified with dislocations by directly correlating the emission with dislocations in an electron microscope
Electron microscope
An electron microscope is a type of microscope that uses a beam of electrons to illuminate the specimen and produce a magnified image. Electron microscopes have a greater resolving power than a light-powered optical microscope, because electrons have wavelengths about 100,000 times shorter than...
. However, blue emission in type Ia diamond could be either due to dislocations or the N3 defects (three nitrogen atoms bordering a vacancy). Green emission in natural diamond is usually due to the H3 center (two substitutional nitrogen atoms separated by a vacancy), whereas in synthetic diamond it usually originates from nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
used as a catalyst (see figure). Orange or red emission could be due to various reasons, one being the nitrogen-vacancy center
Nitrogen-vacancy center
The nitrogen-vacancy center is one of numerous point defects in diamond. Its most explored and useful property is photoluminescence, which can be easily detected from an individual N-V center...
which is present in sufficient quantities in all types of diamond, even type IIb.
Optical absorption
Cape series (Ia) diamonds have a visible absorption spectrum (as seen through a direct-vision spectroscope) consisting of a fine line in the violet at 415.5 nm; however, this line is often invisible until the diamond has been cooled to very low temperatures. Associated with this are weaker lines at 478 nm, 465 nm, 452 nm, 435 nm, and 423 nm.All those lines are labeled as N3 and N2 optical centers and associated with a defect consisting of three nitrogen atoms bordering a vacancy. Other stones show additional bands: brown, green, or yellow diamonds show a band in the green at 504 nm (H3 center, see above), sometimes accompanied by two additional weak bands at 537 nm and 495 nm (H4 center, a large complex presumably involving 4 substitutional nitrogen atoms and 2 lattice vacancies). Type IIb diamonds may absorb in the far red due to the substitutional boron, but otherwise show no observable visible absorption spectrum.
Gemological
Gemology
Gemology or gemmology is the science dealing with natural and artificial gems and gemstones. It is considered a geoscience and a branch of mineralogy...
laboratories make use of spectrophotometer machines that can distinguish natural, artificial, and color-enhanced diamonds
Diamond enhancement
Diamond enhancements are specific treatments, performed on natural diamonds , which are designed to improve the gemological characteristics — and therefore the value — of the stone in one or more ways...
. The spectrophotometers analyze the infrared
Infrared
Infrared light is electromagnetic radiation with a wavelength longer than that of visible light, measured from the nominal edge of visible red light at 0.74 micrometres , and extending conventionally to 300 µm...
, visible, and ultraviolet
Ultraviolet
Ultraviolet light is electromagnetic radiation with a wavelength shorter than that of visible light, but longer than X-rays, in the range 10 nm to 400 nm, and energies from 3 eV to 124 eV...
absorption and luminescence spectra of diamonds cooled with liquid nitrogen
Liquid nitrogen
Liquid nitrogen is nitrogen in a liquid state at a very low temperature. It is produced industrially by fractional distillation of liquid air. Liquid nitrogen is a colourless clear liquid with density of 0.807 g/mL at its boiling point and a dielectric constant of 1.4...
to detect tell-tale absorption lines that are not normally discernible.
Electrical properties
Except for most natural blue diamonds, which are semiconductorSemiconductor
A semiconductor is a material with electrical conductivity due to electron flow intermediate in magnitude between that of a conductor and an insulator. This means a conductivity roughly in the range of 103 to 10−8 siemens per centimeter...
s due to substitutional boron
Boron
Boron is the chemical element with atomic number 5 and the chemical symbol B. Boron is a metalloid. Because boron is not produced by stellar nucleosynthesis, it is a low-abundance element in both the solar system and the Earth's crust. However, boron is concentrated on Earth by the...
impurities replacing carbon atoms, diamond is a good electrical insulator
Electrical insulation
thumb|250px|[[Coaxial Cable]] with dielectric insulator supporting a central coreThis article refers to electrical insulation. For insulation of heat, see Thermal insulation...
. Natural blue or blue-gray diamonds, common for the Argyle diamond mine
Argyle diamond mine
The Argyle Diamond Mine is a diamond mine located in the East Kimberley region in the remote north of Western Australia. Argyle is the largest diamond producer in the world by volume, although due to the low proportion of gem-quality diamonds, is not the leader by value. It is the only known...
in Australia
Australia
Australia , officially the Commonwealth of Australia, is a country in the Southern Hemisphere comprising the mainland of the Australian continent, the island of Tasmania, and numerous smaller islands in the Indian and Pacific Oceans. It is the world's sixth-largest country by total area...
, are rich in hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
; these diamonds are not semiconductors and it is unclear whether hydrogen is actually responsible for their blue-gray color. Natural blue diamonds containing boron and synthetic diamonds doped
Dopant
A dopant, also called a doping agent, is a trace impurity element that is inserted into a substance in order to alter the electrical properties or the optical properties of the substance. In the case of crystalline substances, the atoms of the dopant very commonly take the place of elements that...
with boron are p-type semiconductor
P-type semiconductor
A P-type semiconductor is obtained by carrying out a process of doping: that is, adding a certain type of atoms to the semiconductor in order to increase the number of free charge carriers ....
s. N-type
N-type semiconductor
N-type semiconductors are a type of extrinsic semiconductor where the dopant atoms are capable of providing extra conduction electrons to the host material . This creates an excess of negative electron charge carriers....
diamond films are reproducibly synthesized by phosphorus doping during chemical vapor deposition
Chemical vapor deposition of diamond
Chemical vapor deposition of diamond or CVD is a method of producing synthetic diamond by creating the circumstances necessary for carbon atoms in a gas to settle on a substrate in crystalline form....
. Diode p-n junction
P-n junction
A p–n junction is formed at the boundary between a P-type and N-type semiconductor created in a single crystal of semiconductor by doping, for example by ion implantation, diffusion of dopants, or by epitaxy .If two separate pieces of material were used, this would...
s and UV light emitting diodes (LED
LEd
LEd is a TeX/LaTeX editing software working under Microsoft Windows. It is a freeware product....
s, at 235 nm) have been produced by sequential deposition of p-type (boron-doped) and n-type (phosphorus-doped) layers. Diamond transistors have been produced.
In April 2004, the journal Nature
Nature (journal)
Nature, first published on 4 November 1869, is ranked the world's most cited interdisciplinary scientific journal by the Science Edition of the 2010 Journal Citation Reports...
reported that below the superconducting transition temperature 4 K
Kelvin
The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all...
, boron-doped diamond synthesized at high temperature and high pressure is a bulk superconductor. Superconductivity was later observed in heavily boron-doped films grown by various chemical vapor deposition
Chemical vapor deposition of diamond
Chemical vapor deposition of diamond or CVD is a method of producing synthetic diamond by creating the circumstances necessary for carbon atoms in a gas to settle on a substrate in crystalline form....
techniques, and the highest reported transition temperature (by 2009) is 11.4 K.
Thermal conductivity
Unlike most electrical insulators, diamond is a good conductor of heat because of the strong covalent bonding within the crystal. Most natural blue diamonds contain boronBoron
Boron is the chemical element with atomic number 5 and the chemical symbol B. Boron is a metalloid. Because boron is not produced by stellar nucleosynthesis, it is a low-abundance element in both the solar system and the Earth's crust. However, boron is concentrated on Earth by the...
atoms which replace carbon atoms in the crystal matrix, and also have high thermal conductance. Thermal conductivity of natural diamond was measured to be about 22 W/(cm·K). Monocrystalline synthetic diamond enriched in 12C isotope (99.9%) has the highest thermal conductivity
Thermal conductivity
In physics, thermal conductivity, k, is the property of a material's ability to conduct heat. It appears primarily in Fourier's Law for heat conduction....
of any known solid at room temperature: 33.2 W/(cm·K), five times more than copper
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
. Because diamond has such high thermal conductance it is already used in semiconductor manufacture to prevent silicon
Silicon
Silicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table...
and other semiconducting materials from overheating. At lower temperatures conductivity becomes even better as its Fermi electrons
Fermi level
The Fermi level is a hypothetical level of potential energy for an electron inside a crystalline solid. Occupying such a level would give an electron a potential energy \epsilon equal to its chemical potential \mu as they both appear in the Fermi-Dirac distribution function,which...
can match the phonon
Phonon
In physics, a phonon is a collective excitation in a periodic, elastic arrangement of atoms or molecules in condensed matter, such as solids and some liquids...
ic normal transport mode near the Debye point, and thermal conductivity reaches 410 W/(cm·K) at 104 K (12C-enriched diamond).
Diamond's high thermal conductivity is used by jewelers and gemologists who may employ an electronic thermal probe to separate diamonds from their imitations. These probes consist of a pair of battery-powered thermistor
Thermistor
A thermistor is a type of resistor whose resistance varies significantly with temperature, more so than in standard resistors. The word is a portmanteau of thermal and resistor...
s mounted in a fine copper tip. One thermistor functions as a heating device while the other measures the temperature of the copper tip: if the stone being tested is a diamond, it will conduct the tip's thermal energy rapidly enough to produce a measurable temperature drop. This test takes about 2–3 seconds. However, older probes will be fooled by moissanite
Moissanite
Moissanite originally referred to a rare mineral discovered by Henri Moissan having a chemical formula SiC and various crystalline polymorphs. Earlier, this material had been synthesized in the laboratory and named silicon carbide .- Background :...
, a crystalline mineral form of silicon carbide
Silicon carbide
Silicon carbide , also known as carborundum, is a compound of silicon and carbon with chemical formula SiC. It occurs in nature as the extremely rare mineral moissanite. Silicon carbide powder has been mass-produced since 1893 for use as an abrasive...
introduced in 1998 as an alternative to diamonds, which has a similar thermal conductivity.
Thermal stability

Argon
Argon is a chemical element represented by the symbol Ar. Argon has atomic number 18 and is the third element in group 18 of the periodic table . Argon is the third most common gas in the Earth's atmosphere, at 0.93%, making it more common than carbon dioxide...
gas, diamond can be heated up to about 1700 °C. Its surface blackens, but can be recovered by re-polishing. At high pressure (~20 GPa) diamond can be heated up to 2500 °C, and a report published in 2009 suggests that diamond can withstand temperatures of 3000 °C and above.
Diamonds are carbon crystal
Crystal
A crystal or crystalline solid is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. The scientific study of crystals and crystal formation is known as crystallography...
s that form deep within the Earth under high temperatures and extreme pressures. At surface air pressure (one atmosphere), diamonds are not as stable as graphite, and so the decay of diamond is thermodynamically
Thermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
favorable (δH = −2 kJ / mol). So, contrary to De Beers
De Beers
De Beers is a family of companies that dominate the diamond, diamond mining, diamond trading and industrial diamond manufacturing sectors. De Beers is active in every category of industrial diamond mining: open-pit, underground, large-scale alluvial, coastal and deep sea...
' ad campaign extending from 1948 to at least 2006 under the slogan "A diamond is forever", diamonds are definitely not forever. However, owing to a very large kinetic energy
Kinetic energy
The kinetic energy of an object is the energy which it possesses due to its motion.It is defined as the work needed to accelerate a body of a given mass from rest to its stated velocity. Having gained this energy during its acceleration, the body maintains this kinetic energy unless its speed changes...
barrier, diamonds are metastable; they will not decay into graphite under normal conditions.
See also
- Crystallographic defects in diamondCrystallographic defects in diamondImperfections in the crystal lattice of diamond are common. Such crystallographic defects in diamond may be the result of lattice irregularities or extrinsic substitutional or interstitial impurities, introduced during or after the diamond growth...
- Synthetic diamondSynthetic diamondSynthetic diamond is diamond produced in a technological process; as opposed to natural diamond, which is created in geological processes. Synthetic diamond is also widely known as HPHT diamond or CVD diamond, denoting the production method, High-Pressure High-Temperature synthesis and Chemical...
- Chemical vapor deposition of diamondChemical vapor deposition of diamondChemical vapor deposition of diamond or CVD is a method of producing synthetic diamond by creating the circumstances necessary for carbon atoms in a gas to settle on a substrate in crystalline form....
- DiamondDiamondIn mineralogy, diamond is an allotrope of carbon, where the carbon atoms are arranged in a variation of the face-centered cubic crystal structure called a diamond lattice. Diamond is less stable than graphite, but the conversion rate from diamond to graphite is negligible at ambient conditions...
- Nitrogen-vacancy centerNitrogen-vacancy centerThe nitrogen-vacancy center is one of numerous point defects in diamond. Its most explored and useful property is photoluminescence, which can be easily detected from an individual N-V center...
Further reading
- Pagel-Theisen, Verena. (2001). Diamond grading ABC: The manual (9th ed.), pp. 84–85. Rubin & Son n.v.; Antwerp, Belgium. ISBN 3-9800434-6-0
- Webster, Robert, and Jobbins, E. Allan (Ed.). (1998). Gemmologist's compendium, p. 21, 25, 31. St Edmundsbury Press Ltd, Bury St Edwards. ISBN 0-7198-0291-1
- Properties of diamond (S. Sque, PhD thesis, 2005, University of Exeter, UK)

