Hydroxyl radical
Encyclopedia
Radical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
. Most notably hydroxyl radicals are produced from the decomposition of hydroperoxides
Organic peroxide
Organic peroxides are organic compounds containing the peroxide functional group . If the R' is hydrogen, the compound is called an organic hydroperoxide. Peresters have general structure RCOOR. The O-O bond easily breaks and forms free radicals of the form RO·...
(ROOH) or, in atmospheric chemistry
Atmospheric chemistry
Atmospheric chemistry is a branch of atmospheric science in which the chemistry of the Earth's atmosphere and that of other planets is studied. It is a multidisciplinary field of research and draws on environmental chemistry, physics, meteorology, computer modeling, oceanography, geology and...
, by the reaction of excited
Excited state
Excitation is an elevation in energy level above an arbitrary baseline energy state. In physics there is a specific technical definition for energy level which is often associated with an atom being excited to an excited state....
atomic oxygen with water. It is also an important radical formed in radiation chemistry, since it leads to the formation of hydrogen peroxide and oxygen, which can enhance corrosion
Corrosion
Corrosion is the disintegration of an engineered material into its constituent atoms due to chemical reactions with its surroundings. In the most common use of the word, this means electrochemical oxidation of metals in reaction with an oxidant such as oxygen...
and SCC
Stress corrosion cracking
Stress corrosion cracking is the unexpected sudden failure of normally ductile metals subjected to a tensile stress in a corrosive environment, especially at elevated temperature in the case of metals. SCC is highly chemically specific in that certain alloys are likely to undergo SCC only when...
in coolant systems subjected to radioactive environments. Hydroxyl radicals are also produced during UV-light dissociation of H2O2 (suggested in 1879) and likely in Fenton chemistry
Fenton's reagent
Fenton's reagent is a solution of hydrogen peroxide and an iron catalyst that is used to oxidize contaminants or waste waters. Fenton's reagent can be used to destroy organic compounds such as trichloroethylene and tetrachloroethylene ....
, where trace amounts of reduced transition metals catalyze peroxide-mediated oxidations of organic compounds.
In organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
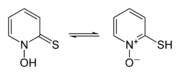
hydroxyl radicals are most commonly generated by photolysis of 1-Hydroxy-2(1H)-pyridinethione.
The hydroxyl radical is often referred to as the "detergent" of the troposphere
Troposphere
The troposphere is the lowest portion of Earth's atmosphere. It contains approximately 80% of the atmosphere's mass and 99% of its water vapor and aerosols....
because it reacts with many pollutants, often acting as the first step to their removal. It also has an important role in eliminating some greenhouse gas
Greenhouse gas
A greenhouse gas is a gas in an atmosphere that absorbs and emits radiation within the thermal infrared range. This process is the fundamental cause of the greenhouse effect. The primary greenhouse gases in the Earth's atmosphere are water vapor, carbon dioxide, methane, nitrous oxide, and ozone...
es like methane
Methane
Methane is a chemical compound with the chemical formula . It is the simplest alkane, the principal component of natural gas, and probably the most abundant organic compound on earth. The relative abundance of methane makes it an attractive fuel...
and ozone
Ozone
Ozone , or trioxygen, is a triatomic molecule, consisting of three oxygen atoms. It is an allotrope of oxygen that is much less stable than the diatomic allotrope...
. The rate of reaction with the hydroxyl radical often determines how long many pollutants last in the atmosphere, if they do not undergo photolysis or are rained out. For instance methane, which reacts relatively slowly with hydroxyl radical, has an average lifetime of >5 years and many CFC
Chlorofluorocarbon
A chlorofluorocarbon is an organic compound that contains carbon, chlorine, and fluorine, produced as a volatile derivative of methane and ethane. A common subclass are the hydrochlorofluorocarbons , which contain hydrogen, as well. They are also commonly known by the DuPont trade name Freon...
s have lifetimes of 50+ years. Pollutants, such as larger hydrocarbons, can have very short average lifetimes of less than a few hours.
The first reaction with many volatile organic compounds (VOCs) is the removal of an hydrogen atom, forming water and an alkyl radical (R•).
- •OH + RH → H2O + R•
The alkyl radical will typically react rapidly with oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
forming a peroxy radical.
- R• + O2 → RO2
The fate of this radical in the troposphere
Troposphere
The troposphere is the lowest portion of Earth's atmosphere. It contains approximately 80% of the atmosphere's mass and 99% of its water vapor and aerosols....
is dependent on factors such as the amount of sunlight, pollution in the atmosphere and the nature of the alkyl radical that formed it (See chapters 12 & 13 in External Links "University Lecture notes on Atmospheric chemistry)
Biological significance
The hydroxyl radical can damage virtually all types of macromolecules: carbohydrates, nucleic acids (mutationMutation
In molecular biology and genetics, mutations are changes in a genomic sequence: the DNA sequence of a cell's genome or the DNA or RNA sequence of a virus. They can be defined as sudden and spontaneous changes in the cell. Mutations are caused by radiation, viruses, transposons and mutagenic...
s), lipids (lipid peroxidation
Lipid peroxidation
Lipid peroxidation refers to the oxidative degradation of lipids. It is the process in which free radicals "steal" electrons from the lipids in cell membranes, resulting in cell damage. This process proceeds by a free radical chain reaction mechanism...
) and amino acids (e.g. conversion of Phe
PHE
PHE may refer to:* Population Health and Environment , an approach to development that integrates health or family planning with conservation efforts* Paramount Home Entertainment* BitTorrent protocol encryption...
to m-Tyrosine
Tyrosine
Tyrosine or 4-hydroxyphenylalanine, is one of the 22 amino acids that are used by cells to synthesize proteins. Its codons are UAC and UAU. It is a non-essential amino acid with a polar side group...
and o-Tyrosine
Tyrosine
Tyrosine or 4-hydroxyphenylalanine, is one of the 22 amino acids that are used by cells to synthesize proteins. Its codons are UAC and UAU. It is a non-essential amino acid with a polar side group...
). PMID 7776173. The hydroxyl radical has a very short in vivo
In vivo
In vivo is experimentation using a whole, living organism as opposed to a partial or dead organism, or an in vitro controlled environment. Animal testing and clinical trials are two forms of in vivo research...
half-life
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
of approximately 10−9 seconds and a high reactivity. This makes it a very dangerous compound to the organism. PMID 7776173. PMID 9288572
Unlike superoxide
Superoxide
A superoxide, also known by the obsolete name hyperoxide, is a compound that possesses the superoxide anion with the chemical formula O2−. The systematic name of the anion is dioxide. It is important as the product of the one-electron reduction of dioxygen O2, which occurs widely in nature...
, which can be detoxified by superoxide dismutase
Superoxide dismutase
Superoxide dismutases are a class of enzymes that catalyze the dismutation of superoxide into oxygen and hydrogen peroxide. As such, they are an important antioxidant defense in nearly all cells exposed to oxygen...
, the hydroxyl radical cannot be eliminated by an enzymatic
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
reaction. Mechanisms for scavenging peroxyl radicals for the protection of cellular
Cell (biology)
The cell is the basic structural and functional unit of all known living organisms. It is the smallest unit of life that is classified as a living thing, and is often called the building block of life. The Alberts text discusses how the "cellular building blocks" move to shape developing embryos....
structures includes endogenous antioxidants such as melatonin
Melatonin
Melatonin , also known chemically as N-acetyl-5-methoxytryptamine, is a naturally occurring compound found in animals, plants, and microbes...
and glutathione
Glutathione
Glutathione is a tripeptide that contains an unusual peptide linkage between the amine group of cysteine and the carboxyl group of the glutamate side-chain...
, and dietary antioxidants such as mannitol
Mannitol
Mannitol is a white, crystalline organic compound with the formula . This polyol is used as an osmotic diuretic agent and a weak renal vasodilator...
and vitamin E
Vitamin E
Vitamin E is used to refer to a group of fat-soluble compounds that include both tocopherols and tocotrienols. There are many different forms of vitamin E, of which γ-tocopherol is the most common in the North American diet. γ-Tocopherol can be found in corn oil, soybean oil, margarine and dressings...
. PMID 7776173.
Importance in the Earth atmosphere
The hydroxyl •OH radicals is one of the main chemical species controlling the oxidizing capacity of the global Earth atmosphere. This oxidizing reactive species has a major impact on the concentrations and distribution of greenhouse gases and pollutants in the Earth atmosphere. It is the most widespread oxidizer in the troposphereTroposphere
The troposphere is the lowest portion of Earth's atmosphere. It contains approximately 80% of the atmosphere's mass and 99% of its water vapor and aerosols....
, the lowest part of the atmosphere. Understanding •OH variability is important to evaluating human impacts on the atmosphere and climate. The •OH species has a lifetime in the Earth atmosphere of less than one second. Understanding the role of •OH in the oxidation process of methane (CH4) present in the atmosphere is important for assessing the residence time of this greenhouse gas and its influence on the process of global warming. The lifetime of •OH radicals in the Earth atmosphere is very short, therefore •OH concentrations in the air are very low and very sensitive techniques are required for its direct detection. Global average hydroxyl radical concentrations have been measured indirectly by analyzing methyl chloroform (CH3CCl3) present in the air. The results obtained by Montzka et al. (2011) shows that the interannual variability in •OH estimated from CH3CCl3 measurements is small, indicating that global •OH is generally well buffered against perturbations. This small variability is consistent with measurements of methane
Methane
Methane is a chemical compound with the chemical formula . It is the simplest alkane, the principal component of natural gas, and probably the most abundant organic compound on earth. The relative abundance of methane makes it an attractive fuel...
and other trace gases primarily oxidized by •OH, as well as global photochemical model calculations.
First detection of interstellar •OH
The first experimental evidence for the presence of 18-cm absorption lines of the hydroxyl (•OH) radical in the radio absorption spectrum of Cassiopeia A was obtained by Weinreb et al. (Nature, Vol. 200, pp. 829, 1963) based on observations made during the period October 15–29, 1963. http://www.nature.com/nature/journal/v201/n4916/abs/201279b0.htmlImportant subsequent report of •OH astronomical detections
| Year | Description | Reference |
|---|---|---|
| 1967 | •OH Molecules in the Interstellar Medium. Robinson and McGee. One of the first observational reviews of •OH observations. •OH had been observed in absorption and emission, but at this time the processes which populate the energy levels are not yet known with certainty, so the article does not give good estimates of •OH densities. | http://adsabs.harvard.edu/abs/1967ARA%26A...5..183R |
| 1967 | Normal •OH Emission and Interstellar Dust Clouds. Heiles. First detection of normal emission from •OH in interstellar dust clouds. | http://adsabs.harvard.edu/abs/1968ApJ...151..919H |
| 1971 | Interstellar molecules and dense clouds.D. M. Rank, C. H. Townes, and W. J. Welch. Review of the epoch about molecular line emission of molecules through dense clouds. | http://www.sciencemag.org/cgi/content/refs/174/4014/1083 |
| 1980 | •OH observations of molecular complexes in Orion and Taurus. Baud and Wouterloot. Map of •OH emission in molecular complexes Orion and Taurus. Derived column densities are in good agreement with previous CO results. | http://adsabs.harvard.edu/abs/1980A%26A....90..297B |
| 1981 | Emission-absorption observations of OH in diffuse interstellar clouds. Dickey, Crovisier and Kazès. Observations of fifty eight regions which show HI absorption were studied. Typical densities and excitation temperature for diffuse clouds are determined in this article. | http://adsabs.harvard.edu/abs/1981A%26A....98..271D |
| 1981 | Magnetic fields in molecular clouds — •OH Zeeman observations. Crutcher, Troland and Heiles. •OH Zeeman observations of the absorption lines produced in interstellar dust clouds toward 3C 133, 3C 123, and W51. | http://adsabs.harvard.edu/abs/1981ApJ...249..134C |
| 1981 | Detection of interstellar OH in the Far-Infrared. J. Storey , D. Watson, C. Townes. Strong absorption lines of •OH were detected at wavelengths of 119.23 and 119.44 microns in the direction of Sgr B2. | http://adsabs.harvard.edu/abs/1981ApJ...244L..27S |
| 1989 | Molecular outflows in powerful OH megamasers. Baan, Haschick and Henkel. Observations of •H and •OH molecular emission through •OH megamasers galaxies, in order to get a FIR luminosity and maser activity relation. | http://adsabs.harvard.edu/abs/1989ApJ...346..680B |
Energy levels
•OH is a diatomic molecule. The electronic angular momentum along the molecular axis is +1 or -1, and the electronic spin angular momentum S=1/2. Because of the orbit-spin coupling, the spin angular momentum can be oriented in parallel or anti parallel directions to the orbital angular momentum, producing the splitting into Π1/2 and Π3/2 states. The 2Π3/2 ground state of •OH is split by lambda doubling interaction (an interaction between the nuclei rotation and the unpaired electron motion around its orbit). Hyperfine interaction with the unpaired spin of the proton further splits the levels.Chemistry of the molecule •OH
In order to study gas phase interstellar chemistry, it is convenient to distinguish two types of interstellar clouds: diffuse clouds, with T=30-100 K, and n=10–1000 cm−3, and dense clouds with T=10-30K and density n=104-103 cm−3. Ion chemical routes in both dense and diffuse clouds have been established for some works (Hartquist 1990).•OH production pathways
The •OH radical is linked with the production of H2O in molecular clouds. Studies of •OH distribution in Taurus Molecular Cloud-1 (TMC-1) (http://adsabs.harvard.edu/abs/2000A%26A...353.1065H Harju et al. 2000) suggest that in dense gas, •OH is mainly formed by dissociative recombination of H3O+. Dissociative recombination is the reaction in which a molecular ion recombines with an electron and dissociates into neutral fragments. Important formation mechanisms for •OH are:H3O+ + e- → •OH + H2 (1a) Dissociative recombination
H3O+ + e- → •OH + •H + •H (1b) Dissociative recombination
HCO2+ + e- → •OH + CO (2a) Dissociative recombination
•O + HCO → •OH + CO (3a) Neutral-neutral
H- + H3O+ → •OH + H2 + •H (4a) Ion-molecular ion neutralization
HCO2 + e- → •OH + CO (5a) Dissociative recombination
•OH destruction pathways
Experimental data on association reactions of •H and •OH suggest that radiative association involving atomic and diatomic neutral radicals may be considered as an effective mechanism for the production of small neutral molecules in the interstellar clouds (http://adsabs.harvard.edu/abs/1980MNRAS.192....1FField et al. 1980). The formation of O2 occurs in the gas phase via the neutral exchange reaction between •O and •OH, which is also the main sink for •OH in dense regions (http://adsabs.harvard.edu/abs/2000A%26A...353.1065H Harju et al. 2000).We can see that atomic oxygen takes part both in the production and destruction of •OH, so the abundance of •OH depends mainly on the H3+ abundance. Then, important chemical pathways leading from •OH radicals are:
•OH + •O → O2 + •H (1A) Neutral-neutral
•OH + C+ → CO+ + •H (2A) Ion-neutral
•OH + •N → NO + •H (3A) Neutral-neutral
•OH + C → CO + •H (4A) Neutral-neutral
•OH + •H → H2O + photon (5A) Neutral-neutral
Rate constants and relative rates for important formation and destruction mechanisms
Rate constants can be derived from the dataset published in the website http://udfa.net. Rate constants have the form:k(T)=alpha*(T/300)beta*exp(-gamma/T)cm3s−1
The following table has the rate constants calculated for a typical temperature in a dense cloud T=10 K.
| Reaction | k (T=10 K) cm3s−1 |
|---|---|
| 1a | 3.29 10−6 |
| 1b | 1.41 10−7 |
| 2a | 4.71 10−7 |
| 3a | 5.0 10−11 |
| 4a | 1.26 10−6 |
| 5a | 2.82 10−6 |
| 1A | 7.7 10−10 |
| 2A | 3.5 10−11 |
| 3A | 1.38 10−10 |
| 4A | 1.0 10−10 |
| 5A | 3.33 10−14 |
Formation rates rix can be obtained using the rate constants k(T) and the abundances of the reactants species C and D:
rix=k(T)ix[C][D]
where [Y] represents the abundance of the specie Y. In this approach, abundances were taken from The UMIST database for astrochemistry 2006, and the values are relatives to the H2 density. Following table shows the ratio rix/r1a in order to get a view of the most important reactions.
| r1a | r1b | r2a | r3a | r4a | r5a | |
|---|---|---|---|---|---|---|
| r1a | 1.0 | 0.043 | 0.013 | 0.035 | 3.6 10−5 | 0.679 |
The results suggest that (1a) reaction is the most prominent reaction in dense clouds. It is in concordance with Harju et al. 2000.
Next table shows the results by doing the same procedure for destruction reaction:
| r1A | r2A | r3A | r4A | r5A | |
|---|---|---|---|---|---|
| r1A | 1.0 | 6.14 10−3 | 0.152 | 3.6 10−5 | 4.29 10−3 |
Results shows that, 1A reaction is the main sink for OH in dense clouds.
Importance of interstellar •OH observations
Discoveries of the microwave spectra of a considerable number of molecules prove the existence of rather complex molecules in the interstellar clouds, and provides the possibility to study dense clouds, which are obscured by the dust they contain. The •OH molecule has been observed in the interstellar medium since 1963 through its 18-cm transitions. In the subsequent years •OH was observed by its rotational transitions at far infrared wavelengths, mainly in the Orion region. Because each rotational level of •OH is split in by lambda doubling, astronomers can observe a wide variety of energy states from the ground state.•OH as a tracer of shock conditions
Very high densities are required to thermalize the rotational transitions of •OH, so it is difficult to detect far-infrared emission lines from a quiescent molecular cloud. Even at H2 densities of 106 cm−3, dust must be optically thick at infrared wavelengths. But the passage of a shock wave through a molecular cloud is precisely the process which can bring the molecular gas out of equilibrium with the dust, making observations of far-infrared emission lines possible. A moderately fast shock may produce a transient raise in the •OH abundance relative to hydrogen. So, it is possible that far-infrared emission lines of •OH can be a good diagnostic of shock conditions.In diffuse clouds
Diffuse clouds are of astronomical interest because they play a primary role in the evolution and thermodynamics of ISM. Observation of the abundant atomic hydrogen in 21 cm has shown good signal-to-noise ratio in both emission and absorption. Nevertheless, HI observations have a fundamental difficulty when are directed to low mass regions of the hydrogen nucleus, as the center part of a diffuse cloud: Thermal width of hydrogen lines are the same order as the internal velocities structures of interest, so clouds components of various temperatures and central velocities are indistinguishable in the spectrum. Molecular lines observations in principle doesn't suffer of this problems. Unlike HI, molecules generally have excitation temperatureExcitation temperature
The Excitation Temperature is defined for a population of particles via the Boltzmann factor...
Tex << Tkin, so that emission is very weak even from abundant species. CO and •OH are the most easily studied candidates molecules. CO has transitions in a region of the spectrum (wavelength < 3 mm) where there is not strong background continuum sources, but •OH has the 18 cm emission, line convenient for absorption observations. Observation studies provide the most sensitive means of detections of molecules with subthermal excitation, and can give the opacity of the spectral line, which is a central issue to model the molecular region.
Studies based in the kinematic comparison of •OH and HI absorption lines from diffuse clouds are useful in determining their physical conditions, specially because heavier elements provide higher velocity resolution.
•OH masers
•OH maserMaser
A maser is a device that produces coherent electromagnetic waves through amplification by stimulated emission. Historically, “maser” derives from the original, upper-case acronym MASER, which stands for "Microwave Amplification by Stimulated Emission of Radiation"...
s, a type of astrophysical maser
Astrophysical maser
An astrophysical maser is a naturally occurring source of stimulated spectral line emission, typically in the microwave portion of the electromagnetic spectrum...
, were the first masers to be discovered in space and have been observed in more environments than any other type of maser.
In the Milky Way
Milky Way
The Milky Way is the galaxy that contains the Solar System. This name derives from its appearance as a dim un-resolved "milky" glowing band arching across the night sky...
, •OH masers are found in stellar masers (evolved stars), interstellar masers (regions of massive star formation), or in the interface between supernova remnants and molecular material. Interstellar OH masers are often observed from molecular material surrounding ultracompact H II region
H II region
An H II region is a large, low-density cloud of partially ionized gas in which star formation has recently taken place. The short-lived, blue stars forged in these regions emit copious amounts of ultraviolet light, ionizing the surrounding gas...
s (UC H II). But there are masers associated with very young stars that have yet to create UC H II regions. This class of •OH masers appears to form near the edges of very dense material, place where H2O masers form, and where total densities drop rapidly and UV radiation form young stars can dissociate the H2O molecules. So, observations of •OH masers in these regions, can be an important way to probe the distribution of the important H2O molecule in interstellar shocks at high spacial resolutions.

