Magnesium in biology
Encyclopedia
Magnesium is an essential element in biological systems. Magnesium
occurs typically as the Mg2+ ion. It is an essential mineral nutrient
for life and is present in every cell
type in every organism. For example, ATP
(adenosine triphosphate), the main source of energy in cells, must be bound to a magnesium ion in order to be biologically active. What is called ATP is often actually Mg-ATP. Similarly, magnesium plays a role in the stability of all polyphosphate compounds in the cells, including those associated with DNA and RNA synthesis.
Over 300 enzyme
s require the presence of magnesium ions for their catalytic action, including all enzymes utilizing or synthesizing ATP
, or those that use other nucleotides to synthesize DNA
and RNA
. In plants, magnesium is necessary for synthesis of chlorophyll
and photosynthesis.
and is highly bioavailable in the hydrosphere
. This availability, in combination with a useful and very unusual chemistry, may have led to its usefulness in evolution as an ion for signaling, enzyme activation and catalysis
. However, the unusual nature of ionic magnesium has also led to a major challenge in the use of the ion in biological systems. Biological membranes are impermeable to magnesium (and other ions) so transport proteins must facilitate the flow of magnesium , both into and out of cells and intracellular compartments.
s it has been shown that different cell types maintain different concentrations of magnesium. It seems likely that the same is true for plants. This suggests that different cell types may regulate influx and efflux of magnesium in different ways based on their unique metabolic needs. Interstitial and systemic concentrations of free magnesium must be delicately maintained by the combined processes of buffering (binding of ions to proteins and other molecules) and muffling (the transport of ions to storage or extracellular spaces).
In plants, and more recently in animals, magnesium has been recognized as an important signaling ion, both activating and mediating many biochemical reactions. The best example of this is perhaps the regulation of carbon
fixation in chloroplast
s in the Calvin cycle
.
The importance of magnesium to proper cellular function cannot be overstated. Deficiency of the nutrient
results in disease in the affected organism. In single-celled organisms such as bacteria
and yeast
, low levels of magnesium manifests in greatly reduced growth rates. In magnesium transport knockout
strains of bacteria, healthy rates are maintained only with exposure to very high external concentrations of the ion. In yeast, mitochondrial magnesium deficiency also leads to disease.
Plants deficient in magnesium show stress responses. The first observable signs of both magnesium starvation and overexposure in plants is a decrease in the rate of photosynthesis
. This is due to the central position of the Mg++ ion in the chlorophyll
molecule. The later effects of magnesium deficiency on plants are a significant reduction in growth and reproductive viability. Magnesium can also be toxic to plants, although this is typically seen only in drought
conditions.
 In animals, magnesium deficiency (hypomagnesemia
In animals, magnesium deficiency (hypomagnesemia
) is seen when the environmental availability of magnesium is low. In ruminant animals, particularly vulnerable to magnesium availability in pasture grasses, the condition is known as ‘grass tetany’. Hypomagnesemia is identified by a loss of balance due to muscle weakness. A number of genetically attributable hypomagnesemia disorders have also been identified in humans.
Overexposure to magnesium may be toxic to individual cells, though these effects have been difficult to show experimentally. In humans the condition is termed hypermagnesemia, and is well documented, though it is usually caused by loss of kidney
function. In healthy individuals, excess magnesium is rapidly excreted in the urine (Harrison’s Principles of Internal Medicine, Online Edition).
s, and has been associated with cardiovascular disease
, diabetes, high blood pressure, anxiety
disorders, migraine
s, osteoporosis
and cerebral infarction
. Acute deficiency (see hypomagnesemia
) is rare, and is more common as a drug side effect (such as chronic alcohol or diuretic use) than from low food intake per se, but it can also occur within people fed intravenously for extended periods of time. The incidence of chronic deficiency resulting in less than optimal health is debated.
The DRI
upper tolerated limit for supplemental
magnesium is 350 mg/day (calculated as milligrams (mg) of elemental magnesium in the salt). (Supplements based on amino acid chelates, such as glycinate, lysinate etc., are much better tolerated by the digestive system and do not have the side effects of the older compounds used, while sustained release supplements prevent the occurrence of diarrhea.) The most common symptom of excess oral magnesium intake is diarrhea
. Since the kidneys of adult humans excrete excess magnesium efficiently, oral magnesium poisoning in adults with normal renal function is very rare. Infants, which have less ability to excrete excess magnesium even when healthy, should not be given magnesium supplements, except under a physician's care.
Magnesium salts (usually in the form of magnesium sulfate or chloride when given parenterally) are used therapeutically for a number of medical conditions, see Epsom salts for a list of conditions which have been treated with supplemental magnesium ion. Magnesium is absorbed with reasonable efficiency (30% to 40%) by the body from any soluble magnesium salt, such as the chloride or citrate. Magnesium is similarly absorbed from Epsom salts, although the sulfate in these salts adds to their laxative effect at higher doses. Magnesium absorption from the insoluble oxide and hydroxide salts (milk of magnesia) is erratic and of poorer efficiency, since it depends on the neutralization and solution of the salt by the acid of the stomach, which may not be (and usually is not) complete.
Magnesium orotate
may be used as adjuvant therapy in patients on optimal treatment for severe congestive heart failure
, increasing survival rate and improving clinical symptoms and patient's quality of life.
, which conduct a positively charged calcium ion
into the neuron
. With an excess of magnesium, more channels will be blocked and the nerve will have less activity.
. Even if the case is not eclampsia, there may be antihypertensive
effects of having a substantial portion of the intake of sodium chloride
(NaCl) exchanged for e.g. magnesium chloride; NaCl is an osmolite and increases arginine vasopressin (AVP) release, which increases extracellular volume and thus results in increased blood pressure. However, not all osmolites have this effect on AVP release, so with magnesium chloride, the increase in osmolarity may not cause such a hypertensive response.
 Green vegetables such as spinach
Green vegetables such as spinach
provide magnesium because of the abundance of chlorophyll
molecules which contain the ion. Nut
s (especially cashew
s and almond
s), seed
s, dark chocolate, roasted soybeans, bran, and some whole grain
s are also good sources of magnesium.
Although many foods contain magnesium, it is usually found in low levels. As with most nutrients, daily needs for magnesium are unlikely to be met by one serving of any single food. Eating a wide variety of fruits, vegetables, and grains will help ensure adequate intake of magnesium.
Because magnesium readily dissolves in water, refined foods, which are often processed or cooked in water and dried, are generally poor sources of the nutrient. For example, whole wheat bread
has twice as much magnesium as white bread because the magnesium-rich germ and bran are removed when white flour is processed. The table of food sources of magnesium suggests many dietary sources of magnesium.
"Hard" water
can also provide magnesium, but "soft" water does not contain the ion. Dietary surveys do not assess magnesium intake from water, which may lead to underestimating total magnesium intake and its variability.
Too much magnesium may make it difficult for the body to absorb calcium
. Not enough magnesium can lead to hypomagnesemia
as described above, with irregular heartbeats, high blood pressure (a sign in humans but not some experimental animals such as rodents), insomnia and muscle spasms (fasciculation
). However, as noted, symptoms of low magnesium from pure dietary deficiency are thought to be rarely encountered.
Following are some foods and the amount of magnesium in them:
ion
in cells (in moles) and the most abundant free divalent cation — as a result it is deeply and intrinsically woven into cellular metabolism
. Indeed, Mg2+-dependent enzymes appear in virtually every metabolic pathway: specific binding of Mg2+ to biological membranes is frequently observed, Mg2+ is also used as a signalling molecule, and much of nucleic acid biochemistry requires Mg2+, including all reactions which require release of energy from ATP. In nucleotides, the triple phosphate moiety of the compound is invariably stabilized by association with Mg2+ in all enzymic processes.
11.4) is used to allow both hydrolysis and condensation reactions (most commonly phosphate ester hydrolysis and phosphoryl transfer) that would otherwise require pH values greatly removed from physiological values.
(adenosine triphosphate), the main source of energy in cells, must be bound to a magnesium ion in order to be biologically active. What is called ATP is often actually Mg-ATP.
s have an important range of interactions with Mg2+. The binding of Mg2+ to DNA
and RNA
stabilises structure; this can be observed in the increased melting temperature (Tm) of double-stranded DNA in the presence of Mg2+. Additionally, ribosome
s contain large amounts of Mg2+ and the stabilisation provided is essential to the complexation of this ribo-protein. A large number of enzymes involved in the biochemistry of nucleic acids bind Mg2+ for activity, using the ion for both activation and catalysis. Finally, the autocatalysis of many ribozymes (enzymes containing only RNA) is Mg2+ dependent (e.g. the yeast mitochondrial group II self splicing introns).
Magnesium ions can be critical in maintaining the positional integrity of closely clustered phosphate groups. These clusters appear in numerous and distinct parts of the cell nucleus
and cytoplasm
. For instance hexahydrated Mg2+ ions bind in the deep major groove
and at the outer mouth of A-form nucleic acid duplex
es.
s and cell wall
s are polyanionic surfaces. This has important implications for the transport of ions, particularly because it has been shown that different membranes preferentially bind different ions. Both Mg2+ and Ca2+ regularly stabilise membranes by the cross-linking of carboxylated and phosphorylated head groups of lipids. However, the envelope membrane of E. coli has also been shown to bind Na+, K+, Mn2+ and Fe3+. The transport of ions is dependent on both the concentration gradient of the ion and the electric potential (ΔΨ) across the membrane, which will be affected by the charge on the membrane surface. For example, the specific binding of Mg2+ to the chloroplast
envelope has been implicated in a loss of photosynthetic efficiency by the blockage of K+ uptake and the subsequent acidification of the chloroplast stroma.
s (Ka
≤ 105) and this can be exploited by the cell to switch enzymatic activity on and off by changes in the local concentration of Mg2+. Although the concentration of free cytoplasmic Mg2+ is on the order of 1 mmol/L, the total Mg2+ content of animal cells is 30 mmol/L and in plants the content of leaf endodermal cells has been measured at values as high as 100 mmol/L (Stelzer et al., 1990), much of which is buffered in storage compartments. The cytoplasmic concentration of free Mg2+ is buffered by binding to chelators (e.g. ATP), but also more importantly by storage of Mg2+ in intracellular compartments. The transport of Mg2+ between intracellular compartments may be a major part of regulating enzyme activity. The interaction of Mg2+ with proteins must also be considered for the transport of the ion across biological membranes.
(Mn2+) is readily capable of replacing Mg2+, but only in a limited set of circumstances. Mn2+ is very similar to Mg2+ in terms of its chemical properties, including inner and outer shell complexation. Mn2+ effectively binds ATP and allows hydrolysis of the energy molecule by most ATPases. Mn2+ can also replace Mg2+ as the activating ion for a number of Mg2+-dependent enzymes, although some enzyme activity is usually lost. Sometimes such enzyme metal preferences vary among closely related species: for example, the reverse transcriptase
enzyme of lentivirus
es like HIV
, SIV
and FIV is typically dependent on Mg2+, whereas the analogous enzyme for other retrovirus
es prefers Mn2+.
Second, the technique of two-electrode voltage-clamp allows the direct measurement of the ion flux across the membrane of a cell. The membrane is held at an electric potential and the responding current is measured. All ions passing across the membrane contribute to the measured current.
Third, the technique of patch-clamp which uses isolated sections of natural or artificial membrane in much the same manner as voltage-clamp but without the secondary effects of a cellular system. Under ideal conditions the conductance of individual channels can be quantified. This methodology gives the most direct measurement of the action of ion channels.
Inductively coupled plasma (ICP) using either the mass spectrometry (MS) or atomic emission spectroscopy (AES) modifications also allows the determination of the total ion content of biological samples. These techniques are more sensitive than flame AAS and are capable of measuring the quantities of multiple ions simultaneously. However, they are also significantly more expensive.
, these steps are probably more difficult than for most other ions. To date, only the ZntA protein of Paramecium has been shown to be a Mg2+ channel. The mechanisms of Mg2+ transport by the remaining proteins are beginning to be uncovered with the first three dimensional structure of a Mg2+ transport complex being solved in 2004.
The hydration shell of the Mg2+ ion has a very tightly bound inner shell of six water molecules and a relatively tightly bound second shell containing 12 – 14 water molecules (Markham et al., 2002). Thus recognition of the Mg2+ ion probably requires some mechanism to interact initially with the hydration shell of Mg2+, followed by a direct recognition/binding of the ion to the protein. Due to the strength of the inner sphere complexation between Mg2+ and any ligand, multiple simultaneous interactions with the transport protein at this level might significantly retard the ion in the transport pore. Hence, it is possible that much of the hydration water is retained during transport, allowing the weaker (but still specific) outer sphere coordination.
In spite of the mechanistic difficulty, Mg2+ must be transported across membranes, and a large number of Mg2+ fluxes across membranes from a variety of systems have been described. However, only a small selection of Mg2+ transporters have been characterised at the molecular level.
ion
s (Mg2+) in cellular biology are usually in almost all senses opposite to Ca2+
ions, because they are bivalent too, but have greater electronegativity and thus hold on to water molecules stronger, preventing passage through the channel (even though magnesium is smaller). Thus Mg2+ ions block Ca2+ channels (NMDA channels) for example, etc.
Mg2+ is taken up into plants via the roots. Interactions with other cations in the rhizosphere
can have a significant effect on the uptake of the ion.(Kurvits and Kirkby, 1980; The structure of root cell walls is highly permeable to water and ions, and hence ion uptake into root cells, can occur anywhere from the root hairs to cells located almost in the centre of the root (limited only by the Casparian strip
). Plant cell walls and membranes carry a great number of negative charges and the interactions of cations with these charges is key to the uptake of cations by root cells allowing a local concentrating effect. Mg2+ binds relatively weakly to these charges, and can be displaced by other cations, impeding uptake and causing deficiency in the plant.
Within individual plant cells the Mg2+ requirements are largely the same as for all cellular life; Mg2+ is used to stabilise membranes, is vital to the utilisation of ATP, is extensively involved in the nucleic acid biochemistry, and is a cofactor for many enzymes (including the ribosome). Also, Mg2+ is the coordinating ion in the chlorophyll molecule. It is the intracellular compartmentalisation of Mg2+ in plant cells that leads to additional complexity. Four compartments within the plant cell have reported interactions with Mg2+. Initially Mg2+ will enter the cell into the cytoplasm (by an as yet unidentified system), but free Mg2+ concentrations in this compartment are tightly regulated at relatively low levels (≈2 mmol/L) and so any excess Mg2+ is either quickly exported or stored in the second intracellular compartment, the vacuole. The requirement for Mg2+ in mitochondria has been demonstrated in yeast and it seems highly likely that the same will apply in plants. The chloroplasts also require significant amounts of internal Mg2+, and low concentrations of cytoplasmic Mg2+. In addition, it seems likely that the other subcellular organelles (e.g. Golgi, endoplasmic reticulum, etc.) also require Mg2+.
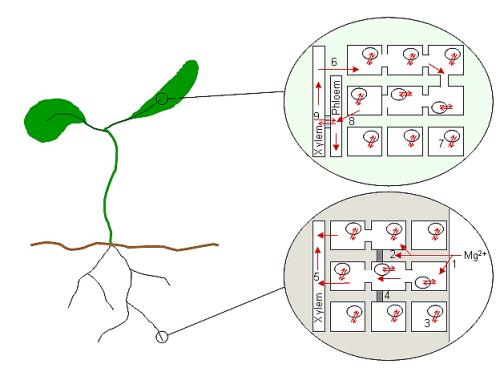
The diagram shows a schematic of a plant and the putative processes of Mg2+ transport at the root and leaf where Mg2+ is loaded and unloaded from the vascular tissues. Mg2+ is taken up into the root cell wall space (1) and interacts with the negative charges associated with the cell walls and membranes. Mg2+ may be taken up into cells immediately (symplastic pathway) or may travel as far as the Casparian band (4) before being absorbed into cells (apoplastic pathway; 2). The concentration of Mg2+ in the root cells is probably buffered by storage in root cell vacuoles (3). Note that cells in the root tip do not contain vacuoles. Once in the root cell cytoplasm Mg2+ travels towards the centre of the root by plasmodesmata
, where it is loaded into the xylem (5) for transport to the upper parts of the plant. When the Mg2+ reaches the leaves it is unloaded from the xylem into cells (6) and again is buffered in vacuoles (7). Whether cycling of Mg2+ into the phloem occurs via general cells in the leaf (8) or directly from xylem to phloem via transfer cells
(9) is unknown. Mg2+ may return to the roots in the phloem sap.
 When a Mg2+ ion has been absorbed by a cell requiring it for metabolic processes, it is generally assumed that the ion stays in that cell for as long as the cell is active. In vascular cells this is not always the case; in times of plenty Mg2+ is stored in the vacuole, takes no part in the day-to-day metabolic processes of the cell (Stelzer et al., 1990) , and is released at need. But for most cells it is death by senescence or injury that releases Mg2+ and many of the other ionic constituents, recycling them into healthy parts of the plant. Additionally, when Mg2+ in the environment is limiting some species are able to mobilise Mg2+ from older tissues. These processes involve the release of Mg2+ from its bound and stored states and its transport back into the vascular tissue, where it can be distributed to the rest of the plant. In times of growth and development Mg2+ is also remobilised within the plant as source and sink relationships change.
When a Mg2+ ion has been absorbed by a cell requiring it for metabolic processes, it is generally assumed that the ion stays in that cell for as long as the cell is active. In vascular cells this is not always the case; in times of plenty Mg2+ is stored in the vacuole, takes no part in the day-to-day metabolic processes of the cell (Stelzer et al., 1990) , and is released at need. But for most cells it is death by senescence or injury that releases Mg2+ and many of the other ionic constituents, recycling them into healthy parts of the plant. Additionally, when Mg2+ in the environment is limiting some species are able to mobilise Mg2+ from older tissues. These processes involve the release of Mg2+ from its bound and stored states and its transport back into the vascular tissue, where it can be distributed to the rest of the plant. In times of growth and development Mg2+ is also remobilised within the plant as source and sink relationships change.
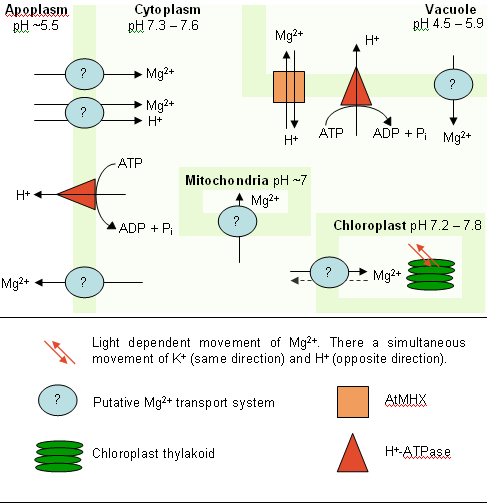
The homeostasis of Mg2+ within single plant cells is maintained by processes occurring at the plasma membrane and at the vacuole membrane (see Figure 2). The major driving force for the translocation of ions in plant cells is ΔpH. H+-ATPases pump H+ ions against their concentration gradient to maintain the pH differential that can be used for the transport of other ions and molecules. H+ ions are pumped out of the cytoplasm into the extracellular space or into the vacuole. The entry of Mg2+ into cells may occur through one of two pathways, via channels using the ΔΨ (negative inside) across this membrane or by symport with H+ ions. To transport the Mg2+ ion into the vacuole requires a Mg2+/H+ antiport transporter (such as AtMHX). It is interesting to note that the H+-ATPases are dependent on Mg2+ (bound to ATP) for activity, so that Mg2+ is required to maintain its own homeostasis.
A schematic of a plant cell is shown including the four major compartments currently recognised as interacting with Mg2+. H+-ATPases maintain a constant ΔpH across the plasma membrane and the vacuole membrane. Mg2+ is transported into the vacuole using the energy of ΔpH (in A. thaliana by AtMHX). Transport of Mg2+ into cells may use either the negative ΔΨ or the ΔpH. The transport of Mg2+ into mitochondria probably uses ΔΨ as in the mitochondria of yeast, and it is likely that chloroplasts take Mg2+ by a similar system. The mechanism and the molecular basis for the release of Mg2+ from vacuoles and from the cell is not known. Likewise the light-regulated Mg2+ concentration changes in chloroplasts are not fully understood, but do require the transport of H+ ions across the thylakoid membrane.
Mg2+ is probably taken up into chloroplasts to the greatest extent during the light induced development from proplastid to chloroplast or etioplast to chloroplast. At these times the synthesis of chlorophyll and the biogenesis of the thylakoid membrane stacks absolutely require the divalent cation.
Whether Mg2+ is able to move into and out of chloroplasts after this initial developmental phase has been the subject of several conflicting reports. Deshaies et al. (1984) found that Mg2+ did move in and out of isolated chloroplasts from young pea plants, but Gupta and Berkowitz (1989) were unable to reproduce the result using older spinach chloroplasts. Deshaies et al. had stated in their paper that older pea chloroplasts showed less significant changes in Mg2+ content than those used to form their conclusions. Perhaps the relative proportion of immature chloroplasts present in the preparations might explain these observations.
The metabolic state of the chloroplast changes considerably between night and day. During the day the chloroplast is actively harvesting the energy of light and converting it into chemical energy. The activation of the metabolic pathways involved comes from the changes in the chemical nature of the stroma on the addition of light. H+ is pumped out of the stroma (into both the cytoplasm and the lumen) leading to an alkaline pH. Mg2+ (along with K+) is released from the lumen into the stroma, in an electroneutralisation process to balance the flow of H+. Finally, thiol groups on enzymes are reduced by a change in the redox state of the stroma. Examples of enzymes activated in response to these changes are fructose 1,6-bisphosphatase, sedoheptulose bisphosphatase and ribulose-1,5-bisphosphate carboxylase. During the dark period, if these enzymes were active a wasteful cycling of products and substrates would occur.
Two major classes of the enzymes that interact with Mg2+ in the stroma during the light phase can be identified. Firstly, enzymes in the glycolytic pathway most often interact with two atoms of Mg2+. The first atom is as an allosteric modulator of the enzymes’ activity, while the second forms part of the active site and is directly involved in the catalytic reaction. The second class of enzymes include those where the Mg2+ is complexed to nucleotide di- and tri-phosphates (ADP and ATP) and the chemical change involves phosphoryl transfer. Mg2+ may also serve in a structural maintenance role in these enzymes (e.g. enolase).
A Mg2+ deficit can be caused by the lack of the ion in the media (soil), but more commonly comes from inhibition of its uptake. Mg2+ binds quite weakly to the negatively charged groups in the root cell walls, so that excesses of other cations such as K+, NH4+, Ca2+ and Mn2+ can all impede uptake.(Kurvits and Kirkby, 1980; In acid soils Al3+ is a particularly strong inhibitor of Mg2+ uptake. The inhibition by Al3+ and Mn2+ is more severe than can be explained by simple displacement, hence it is possible that these ions bind to the Mg2+ uptake system directly. In bacteria and yeast, such binding by Mn2+ has already been observed. Stress responses in the plant develop as cellular processes halt due to a lack of Mg2+ (e.g. maintenance of ΔpH across the plasma and vacuole membranes). Interestingly, in Mg2+-starved plants under low light conditions the percentage of Mg2+ bound to chlorophyll has been recorded at 50%. Presumably, this imbalance has detrimental effects on other cellular processes.
Mg2+ toxicity stress is more difficult to develop. When Mg2+ is plentiful the plants generally take up the ion and store it (Stelzer et al., 1990). However, if this is followed by drought then ionic concentrations within the cell can increase dramatically. High cytoplasmic Mg2+ concentrations block a K+ channel in the inner envelope membrane of the chloroplast, in turn inhibiting the removal of H+ ions from the chloroplast stroma. This leads to an acidification of the stroma that inactivates key enzymes in carbon fixation, which all leads to the production of oxygen free radicals in the chloroplast that then cause oxidative damage.
Magnesium
Magnesium is a chemical element with the symbol Mg, atomic number 12, and common oxidation number +2. It is an alkaline earth metal and the eighth most abundant element in the Earth's crust and ninth in the known universe as a whole...
occurs typically as the Mg2+ ion. It is an essential mineral nutrient
Nutrient
A nutrient is a chemical that an organism needs to live and grow or a substance used in an organism's metabolism which must be taken in from its environment. They are used to build and repair tissues, regulate body processes and are converted to and used as energy...
for life and is present in every cell
Cell (biology)
The cell is the basic structural and functional unit of all known living organisms. It is the smallest unit of life that is classified as a living thing, and is often called the building block of life. The Alberts text discusses how the "cellular building blocks" move to shape developing embryos....
type in every organism. For example, ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
(adenosine triphosphate), the main source of energy in cells, must be bound to a magnesium ion in order to be biologically active. What is called ATP is often actually Mg-ATP. Similarly, magnesium plays a role in the stability of all polyphosphate compounds in the cells, including those associated with DNA and RNA synthesis.
Over 300 enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
s require the presence of magnesium ions for their catalytic action, including all enzymes utilizing or synthesizing ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
, or those that use other nucleotides to synthesize DNA
DNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
and RNA
RNA
Ribonucleic acid , or RNA, is one of the three major macromolecules that are essential for all known forms of life....
. In plants, magnesium is necessary for synthesis of chlorophyll
Chlorophyll
Chlorophyll is a green pigment found in almost all plants, algae, and cyanobacteria. Its name is derived from the Greek words χλωρος, chloros and φύλλον, phyllon . Chlorophyll is an extremely important biomolecule, critical in photosynthesis, which allows plants to obtain energy from light...
and photosynthesis.
Function
A balance of magnesium is vital to the well being of all organisms. Magnesium is a relatively abundant ion in the lithosphereLithosphere
The lithosphere is the rigid outermost shell of a rocky planet. On Earth, it comprises the crust and the portion of the upper mantle that behaves elastically on time scales of thousands of years or greater.- Earth's lithosphere :...
and is highly bioavailable in the hydrosphere
Hydrosphere
A hydrosphere in physical geography describes the combined mass of water found on, under, and over the surface of a planet....
. This availability, in combination with a useful and very unusual chemistry, may have led to its usefulness in evolution as an ion for signaling, enzyme activation and catalysis
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
. However, the unusual nature of ionic magnesium has also led to a major challenge in the use of the ion in biological systems. Biological membranes are impermeable to magnesium (and other ions) so transport proteins must facilitate the flow of magnesium , both into and out of cells and intracellular compartments.
Biological range, distribution, and regulation
In animalAnimal
Animals are a major group of multicellular, eukaryotic organisms of the kingdom Animalia or Metazoa. Their body plan eventually becomes fixed as they develop, although some undergo a process of metamorphosis later on in their life. Most animals are motile, meaning they can move spontaneously and...
s it has been shown that different cell types maintain different concentrations of magnesium. It seems likely that the same is true for plants. This suggests that different cell types may regulate influx and efflux of magnesium in different ways based on their unique metabolic needs. Interstitial and systemic concentrations of free magnesium must be delicately maintained by the combined processes of buffering (binding of ions to proteins and other molecules) and muffling (the transport of ions to storage or extracellular spaces).
In plants, and more recently in animals, magnesium has been recognized as an important signaling ion, both activating and mediating many biochemical reactions. The best example of this is perhaps the regulation of carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
fixation in chloroplast
Chloroplast
Chloroplasts are organelles found in plant cells and other eukaryotic organisms that conduct photosynthesis. Chloroplasts capture light energy to conserve free energy in the form of ATP and reduce NADP to NADPH through a complex set of processes called photosynthesis.Chloroplasts are green...
s in the Calvin cycle
Calvin cycle
The Calvin cycle or Calvin–Benson-Bassham cycle or reductive pentose phosphate cycle or C3 cycle or CBB cycle is a series of biochemical redox reactions that take place in the stroma of chloroplasts in photosynthetic organisms...
.
The importance of magnesium to proper cellular function cannot be overstated. Deficiency of the nutrient
Nutrient
A nutrient is a chemical that an organism needs to live and grow or a substance used in an organism's metabolism which must be taken in from its environment. They are used to build and repair tissues, regulate body processes and are converted to and used as energy...
results in disease in the affected organism. In single-celled organisms such as bacteria
Bacteria
Bacteria are a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria have a wide range of shapes, ranging from spheres to rods and spirals...
and yeast
Yeast
Yeasts are eukaryotic micro-organisms classified in the kingdom Fungi, with 1,500 species currently described estimated to be only 1% of all fungal species. Most reproduce asexually by mitosis, and many do so by an asymmetric division process called budding...
, low levels of magnesium manifests in greatly reduced growth rates. In magnesium transport knockout
Knockout
A knockout is a fight-ending, winning criterion in several full-contact combat sports, such as boxing, kickboxing, Muay Thai, mixed martial arts, Karate and others sports involving striking...
strains of bacteria, healthy rates are maintained only with exposure to very high external concentrations of the ion. In yeast, mitochondrial magnesium deficiency also leads to disease.
Plants deficient in magnesium show stress responses. The first observable signs of both magnesium starvation and overexposure in plants is a decrease in the rate of photosynthesis
Photosynthesis
Photosynthesis is a chemical process that converts carbon dioxide into organic compounds, especially sugars, using the energy from sunlight. Photosynthesis occurs in plants, algae, and many species of bacteria, but not in archaea. Photosynthetic organisms are called photoautotrophs, since they can...
. This is due to the central position of the Mg++ ion in the chlorophyll
Chlorophyll
Chlorophyll is a green pigment found in almost all plants, algae, and cyanobacteria. Its name is derived from the Greek words χλωρος, chloros and φύλλον, phyllon . Chlorophyll is an extremely important biomolecule, critical in photosynthesis, which allows plants to obtain energy from light...
molecule. The later effects of magnesium deficiency on plants are a significant reduction in growth and reproductive viability. Magnesium can also be toxic to plants, although this is typically seen only in drought
Drought
A drought is an extended period of months or years when a region notes a deficiency in its water supply. Generally, this occurs when a region receives consistently below average precipitation. It can have a substantial impact on the ecosystem and agriculture of the affected region...
conditions.

Hypomagnesemia
Hypomagnesemia is an electrolyte disturbance in which there is an abnormally low level of magnesium in the blood. Usually a serum level less than 0.7 mmol/L is used as reference. The prefix hypo- means low . The middle 'magnes' refers to magnesium...
) is seen when the environmental availability of magnesium is low. In ruminant animals, particularly vulnerable to magnesium availability in pasture grasses, the condition is known as ‘grass tetany’. Hypomagnesemia is identified by a loss of balance due to muscle weakness. A number of genetically attributable hypomagnesemia disorders have also been identified in humans.
Overexposure to magnesium may be toxic to individual cells, though these effects have been difficult to show experimentally. In humans the condition is termed hypermagnesemia, and is well documented, though it is usually caused by loss of kidney
Kidney
The kidneys, organs with several functions, serve essential regulatory roles in most animals, including vertebrates and some invertebrates. They are essential in the urinary system and also serve homeostatic functions such as the regulation of electrolytes, maintenance of acid–base balance, and...
function. In healthy individuals, excess magnesium is rapidly excreted in the urine (Harrison’s Principles of Internal Medicine, Online Edition).
Human health
Magnesium deficiency in humans was first described in the medical literature in 1934. The adult human daily nutritional requirement, which is affected by various factors including gender, weight and size, is 300-400 mg/day. Inadequate magnesium intake frequently causes muscle spasmSpasm
In medicine a spasm is a sudden, involuntary contraction of a muscle, a group of muscles, or a hollow organ, or a similarly sudden contraction of an orifice. It is sometimes accompanied by a sudden burst of pain, but is usually harmless and ceases after a few minutes...
s, and has been associated with cardiovascular disease
Cardiovascular disease
Heart disease or cardiovascular disease are the class of diseases that involve the heart or blood vessels . While the term technically refers to any disease that affects the cardiovascular system , it is usually used to refer to those related to atherosclerosis...
, diabetes, high blood pressure, anxiety
Anxiety
Anxiety is a psychological and physiological state characterized by somatic, emotional, cognitive, and behavioral components. The root meaning of the word anxiety is 'to vex or trouble'; in either presence or absence of psychological stress, anxiety can create feelings of fear, worry, uneasiness,...
disorders, migraine
Migraine
Migraine is a chronic neurological disorder characterized by moderate to severe headaches, and nausea...
s, osteoporosis
Osteoporosis
Osteoporosis is a disease of bones that leads to an increased risk of fracture. In osteoporosis the bone mineral density is reduced, bone microarchitecture is deteriorating, and the amount and variety of proteins in bone is altered...
and cerebral infarction
Cerebral infarction
A cerebral infarction is the ischemic kind of stroke due to a disturbance in the blood vessels supplying blood to the brain. It can be atherothrombotic or embolic. Stroke caused by cerebral infarction should be distinguished from two other kinds of stroke: cerebral hemorrhage and subarachnoid...
. Acute deficiency (see hypomagnesemia
Hypomagnesemia
Hypomagnesemia is an electrolyte disturbance in which there is an abnormally low level of magnesium in the blood. Usually a serum level less than 0.7 mmol/L is used as reference. The prefix hypo- means low . The middle 'magnes' refers to magnesium...
) is rare, and is more common as a drug side effect (such as chronic alcohol or diuretic use) than from low food intake per se, but it can also occur within people fed intravenously for extended periods of time. The incidence of chronic deficiency resulting in less than optimal health is debated.
The DRI
Dietary Reference Intake
The Dietary Reference Intake is a system of nutrition recommendations from the Institute of Medicine of the U.S. National Academy of Sciences. The DRI system is used by both the United States and Canada and is intended for the general public and health professionals...
upper tolerated limit for supplemental
Dietary supplement
A dietary supplement, also known as food supplement or nutritional supplement, is a preparation intended to supplement the diet and provide nutrients, such as vitamins, minerals, fiber, fatty acids, or amino acids, that may be missing or may not be consumed in sufficient quantities in a person's diet...
magnesium is 350 mg/day (calculated as milligrams (mg) of elemental magnesium in the salt). (Supplements based on amino acid chelates, such as glycinate, lysinate etc., are much better tolerated by the digestive system and do not have the side effects of the older compounds used, while sustained release supplements prevent the occurrence of diarrhea.) The most common symptom of excess oral magnesium intake is diarrhea
Diarrhea
Diarrhea , also spelled diarrhoea, is the condition of having three or more loose or liquid bowel movements per day. It is a common cause of death in developing countries and the second most common cause of infant deaths worldwide. The loss of fluids through diarrhea can cause dehydration and...
. Since the kidneys of adult humans excrete excess magnesium efficiently, oral magnesium poisoning in adults with normal renal function is very rare. Infants, which have less ability to excrete excess magnesium even when healthy, should not be given magnesium supplements, except under a physician's care.
Magnesium salts (usually in the form of magnesium sulfate or chloride when given parenterally) are used therapeutically for a number of medical conditions, see Epsom salts for a list of conditions which have been treated with supplemental magnesium ion. Magnesium is absorbed with reasonable efficiency (30% to 40%) by the body from any soluble magnesium salt, such as the chloride or citrate. Magnesium is similarly absorbed from Epsom salts, although the sulfate in these salts adds to their laxative effect at higher doses. Magnesium absorption from the insoluble oxide and hydroxide salts (milk of magnesia) is erratic and of poorer efficiency, since it depends on the neutralization and solution of the salt by the acid of the stomach, which may not be (and usually is not) complete.
Magnesium orotate
Magnesium orotate
Magnesium orotate, the magnesium salt of orotic acid, is a mineral supplement....
may be used as adjuvant therapy in patients on optimal treatment for severe congestive heart failure
Congestive heart failure
Heart failure often called congestive heart failure is generally defined as the inability of the heart to supply sufficient blood flow to meet the needs of the body. Heart failure can cause a number of symptoms including shortness of breath, leg swelling, and exercise intolerance. The condition...
, increasing survival rate and improving clinical symptoms and patient's quality of life.
Nerve Conduction
Magnesium can affect muscle relaxation through direct action on the cell membrane. Mg++ ions close certain types of calcium channelsNMDA
N-Methyl-D-aspartic acid or N-Methyl-D-aspartate is an amino acid derivative which acts as a specific agonist at the NMDA receptor mimicking the action of glutamate, the neurotransmitter which normally acts at that receptor...
, which conduct a positively charged calcium ion
Calcium in biology
Calcium plays a pivotal role in the physiology and biochemistry of organisms and the cell. It plays an important role in signal transduction pathways, where it acts as a second messenger, in neurotransmitter release from neurons, contraction of all muscle cell types, and fertilization...
into the neuron
Neuron
A neuron is an electrically excitable cell that processes and transmits information by electrical and chemical signaling. Chemical signaling occurs via synapses, specialized connections with other cells. Neurons connect to each other to form networks. Neurons are the core components of the nervous...
. With an excess of magnesium, more channels will be blocked and the nerve will have less activity.
Hypertension
Magnesium-containing Epsom salts are especially used in treating the hypertension of eclampsiaEclampsia
Eclampsia , an acute and life-threatening complication of pregnancy, is characterized by the appearance of tonic-clonic seizures, usually in a patient who had developed pre-eclampsia...
. Even if the case is not eclampsia, there may be antihypertensive
Antihypertensive
The antihypertensives are a class of drugs that are used to treat hypertension . Evidence suggests that reduction of the blood pressure by 5 mmHg can decrease the risk of stroke by 34%, of ischaemic heart disease by 21%, and reduce the likelihood of dementia, heart failure, and mortality from...
effects of having a substantial portion of the intake of sodium chloride
Sodium chloride
Sodium chloride, also known as salt, common salt, table salt or halite, is an inorganic compound with the formula NaCl. Sodium chloride is the salt most responsible for the salinity of the ocean and of the extracellular fluid of many multicellular organisms...
(NaCl) exchanged for e.g. magnesium chloride; NaCl is an osmolite and increases arginine vasopressin (AVP) release, which increases extracellular volume and thus results in increased blood pressure. However, not all osmolites have this effect on AVP release, so with magnesium chloride, the increase in osmolarity may not cause such a hypertensive response.
Food sources

Spinach
Spinach is an edible flowering plant in the family of Amaranthaceae. It is native to central and southwestern Asia. It is an annual plant , which grows to a height of up to 30 cm. Spinach may survive over winter in temperate regions...
provide magnesium because of the abundance of chlorophyll
Chlorophyll
Chlorophyll is a green pigment found in almost all plants, algae, and cyanobacteria. Its name is derived from the Greek words χλωρος, chloros and φύλλον, phyllon . Chlorophyll is an extremely important biomolecule, critical in photosynthesis, which allows plants to obtain energy from light...
molecules which contain the ion. Nut
Nut (fruit)
A nut is a hard-shelled fruit of some plants having an indehiscent seed. While a wide variety of dried seeds and fruits are called nuts in English, only a certain number of them are considered by biologists to be true nuts...
s (especially cashew
Cashew
The cashew is a tree in the family Anacardiaceae. Its English name derives from the Portuguese name for the fruit of the cashew tree, caju, which in turn derives from the indigenous Tupi name, acajú. It is now widely grown in tropical climates for its cashew nuts and cashew apples.-Etymology:The...
s and almond
Almond
The almond , is a species of tree native to the Middle East and South Asia. Almond is also the name of the edible and widely cultivated seed of this tree...
s), seed
Seed
A seed is a small embryonic plant enclosed in a covering called the seed coat, usually with some stored food. It is the product of the ripened ovule of gymnosperm and angiosperm plants which occurs after fertilization and some growth within the mother plant...
s, dark chocolate, roasted soybeans, bran, and some whole grain
Whole grain
Whole grains are cereal grains that contain cereal germ, endosperm, and bran, in contrast to refined grains, which retain only the endosperm. Whole grains can generally be sprouted while refined grains generally will not sprout. Whole-meal products are made by grinding whole grains in order to make...
s are also good sources of magnesium.
Although many foods contain magnesium, it is usually found in low levels. As with most nutrients, daily needs for magnesium are unlikely to be met by one serving of any single food. Eating a wide variety of fruits, vegetables, and grains will help ensure adequate intake of magnesium.
Because magnesium readily dissolves in water, refined foods, which are often processed or cooked in water and dried, are generally poor sources of the nutrient. For example, whole wheat bread
Whole wheat bread
Whole wheat bread is a type of bread that is made using flour which is partly or entirely made from whole or almost whole wheat grains, see whole wheat flour and whole grain. It is one kind of brown bread. Synonyms or near-synonyms for whole wheat bread elsewhere in the world are whole grain bread...
has twice as much magnesium as white bread because the magnesium-rich germ and bran are removed when white flour is processed. The table of food sources of magnesium suggests many dietary sources of magnesium.
"Hard" water
Hard water
Hard water is water that has high mineral content . Hard water has high concentrations of Ca2+ and Mg2+ ions. Hard water is generally not harmful to one's health but can pose serious problems in industrial settings, where water hardness is monitored to avoid costly breakdowns in boilers, cooling...
can also provide magnesium, but "soft" water does not contain the ion. Dietary surveys do not assess magnesium intake from water, which may lead to underestimating total magnesium intake and its variability.
Too much magnesium may make it difficult for the body to absorb calcium
Calcium
Calcium is the chemical element with the symbol Ca and atomic number 20. It has an atomic mass of 40.078 amu. Calcium is a soft gray alkaline earth metal, and is the fifth-most-abundant element by mass in the Earth's crust...
. Not enough magnesium can lead to hypomagnesemia
Hypomagnesemia
Hypomagnesemia is an electrolyte disturbance in which there is an abnormally low level of magnesium in the blood. Usually a serum level less than 0.7 mmol/L is used as reference. The prefix hypo- means low . The middle 'magnes' refers to magnesium...
as described above, with irregular heartbeats, high blood pressure (a sign in humans but not some experimental animals such as rodents), insomnia and muscle spasms (fasciculation
Fasciculation
A fasciculation , or "muscle twitch", is a small, local, involuntary muscle contraction and relaxation visible under the skin arising from the spontaneous discharge of a bundle of skeletal muscle fibers...
). However, as noted, symptoms of low magnesium from pure dietary deficiency are thought to be rarely encountered.
Following are some foods and the amount of magnesium in them:
- Black-eyed peas (1/2 cup) = 45 mg
- Buckwheat Flour (100g (4 oz)) = 250 mg
- Halibut (100g (4 oz)) = 107 mg
- Milk: low fat (1 cup) = 40 mg
- Oats (100g (4 oz)) = 235 mg
- Peanut butter (2 tablespoons) = 50 mg
- Spinach (1/2 cup) = 80 mg
- Wholemeal Bread (1 Slice) = 25 mg
Biological chemistry
Mg2+ is the fourth most abundant metalMetal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
in cells (in moles) and the most abundant free divalent cation — as a result it is deeply and intrinsically woven into cellular metabolism
Metabolism
Metabolism is the set of chemical reactions that happen in the cells of living organisms to sustain life. These processes allow organisms to grow and reproduce, maintain their structures, and respond to their environments. Metabolism is usually divided into two categories...
. Indeed, Mg2+-dependent enzymes appear in virtually every metabolic pathway: specific binding of Mg2+ to biological membranes is frequently observed, Mg2+ is also used as a signalling molecule, and much of nucleic acid biochemistry requires Mg2+, including all reactions which require release of energy from ATP. In nucleotides, the triple phosphate moiety of the compound is invariably stabilized by association with Mg2+ in all enzymic processes.
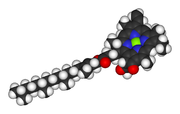
Chlorophyll
In photosynthetic organisms Mg2+ has the additional vital role of being the coordinating ion in the chlorophyll molecule. This role was discovered by R. M. Willstätter, who received the Nobel Prize in Chemistry 1915 for the purification and structure of chlorophyll.Enzymes
The chemistry of the Mg2+ ion, as applied to enzymes, uses the full range of this ion’s unusual reaction chemistry to fulfill a range of functions. Mg2+ interacts with substrates, enzymes and occasionally both (Mg2+ may form part of the active site). Mg2+ generally interacts with substrates through inner sphere coordination, stabilising anions or reactive intermediates, also including binding to ATP and activating the molecule to nucleophilic attack. When interacting with enzymes and other proteins Mg2+ may bind using inner or outer sphere coordination, to either alter the conformation of the enzyme or take part in the chemistry of the catalytic reaction. In either case, because Mg2+ is only rarely fully dehydrated during ligand binding, it may be a water molecule associated with the Mg2+ that is important rather than the ion itself. The Lewis acidity of Mg2+ (pKaAcid dissociation constant
An acid dissociation constant, Ka, is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction known as dissociation in the context of acid-base reactions...
11.4) is used to allow both hydrolysis and condensation reactions (most commonly phosphate ester hydrolysis and phosphoryl transfer) that would otherwise require pH values greatly removed from physiological values.
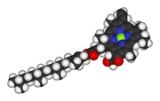
Essential role in the biological activity of ATP
ATPAdenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
(adenosine triphosphate), the main source of energy in cells, must be bound to a magnesium ion in order to be biologically active. What is called ATP is often actually Mg-ATP.
Nucleic acids
Nucleic acidNucleic acid
Nucleic acids are biological molecules essential for life, and include DNA and RNA . Together with proteins, nucleic acids make up the most important macromolecules; each is found in abundance in all living things, where they function in encoding, transmitting and expressing genetic information...
s have an important range of interactions with Mg2+. The binding of Mg2+ to DNA
DNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
and RNA
RNA
Ribonucleic acid , or RNA, is one of the three major macromolecules that are essential for all known forms of life....
stabilises structure; this can be observed in the increased melting temperature (Tm) of double-stranded DNA in the presence of Mg2+. Additionally, ribosome
Ribosome
A ribosome is a component of cells that assembles the twenty specific amino acid molecules to form the particular protein molecule determined by the nucleotide sequence of an RNA molecule....
s contain large amounts of Mg2+ and the stabilisation provided is essential to the complexation of this ribo-protein. A large number of enzymes involved in the biochemistry of nucleic acids bind Mg2+ for activity, using the ion for both activation and catalysis. Finally, the autocatalysis of many ribozymes (enzymes containing only RNA) is Mg2+ dependent (e.g. the yeast mitochondrial group II self splicing introns).
Magnesium ions can be critical in maintaining the positional integrity of closely clustered phosphate groups. These clusters appear in numerous and distinct parts of the cell nucleus
Cell nucleus
In cell biology, the nucleus is a membrane-enclosed organelle found in eukaryotic cells. It contains most of the cell's genetic material, organized as multiple long linear DNA molecules in complex with a large variety of proteins, such as histones, to form chromosomes. The genes within these...
and cytoplasm
Cytoplasm
The cytoplasm is a small gel-like substance residing between the cell membrane holding all the cell's internal sub-structures , except for the nucleus. All the contents of the cells of prokaryote organisms are contained within the cytoplasm...
. For instance hexahydrated Mg2+ ions bind in the deep major groove
DNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
and at the outer mouth of A-form nucleic acid duplex
Duplex
Duplex commonly means double or twofold.It may also refer to:* Duplex , a two-unit apartment building or condominium* Duplex, a common electrical receptacle with two NEMA type 5 plugs* Duplex locomotive, a type of steam locomotive...
es.
Cell membranes and walls
Biological cell membraneCell membrane
The cell membrane or plasma membrane is a biological membrane that separates the interior of all cells from the outside environment. The cell membrane is selectively permeable to ions and organic molecules and controls the movement of substances in and out of cells. It basically protects the cell...
s and cell wall
Cell wall
The cell wall is the tough, usually flexible but sometimes fairly rigid layer that surrounds some types of cells. It is located outside the cell membrane and provides these cells with structural support and protection, and also acts as a filtering mechanism. A major function of the cell wall is to...
s are polyanionic surfaces. This has important implications for the transport of ions, particularly because it has been shown that different membranes preferentially bind different ions. Both Mg2+ and Ca2+ regularly stabilise membranes by the cross-linking of carboxylated and phosphorylated head groups of lipids. However, the envelope membrane of E. coli has also been shown to bind Na+, K+, Mn2+ and Fe3+. The transport of ions is dependent on both the concentration gradient of the ion and the electric potential (ΔΨ) across the membrane, which will be affected by the charge on the membrane surface. For example, the specific binding of Mg2+ to the chloroplast
Chloroplast
Chloroplasts are organelles found in plant cells and other eukaryotic organisms that conduct photosynthesis. Chloroplasts capture light energy to conserve free energy in the form of ATP and reduce NADP to NADPH through a complex set of processes called photosynthesis.Chloroplasts are green...
envelope has been implicated in a loss of photosynthetic efficiency by the blockage of K+ uptake and the subsequent acidification of the chloroplast stroma.
Proteins
The Mg2+ ion tends to bind only weakly to proteinProtein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s (Ka
Acid dissociation constant
An acid dissociation constant, Ka, is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction known as dissociation in the context of acid-base reactions...
≤ 105) and this can be exploited by the cell to switch enzymatic activity on and off by changes in the local concentration of Mg2+. Although the concentration of free cytoplasmic Mg2+ is on the order of 1 mmol/L, the total Mg2+ content of animal cells is 30 mmol/L and in plants the content of leaf endodermal cells has been measured at values as high as 100 mmol/L (Stelzer et al., 1990), much of which is buffered in storage compartments. The cytoplasmic concentration of free Mg2+ is buffered by binding to chelators (e.g. ATP), but also more importantly by storage of Mg2+ in intracellular compartments. The transport of Mg2+ between intracellular compartments may be a major part of regulating enzyme activity. The interaction of Mg2+ with proteins must also be considered for the transport of the ion across biological membranes.
Manganese
In biological systems, only manganeseManganese
Manganese is a chemical element, designated by the symbol Mn. It has the atomic number 25. It is found as a free element in nature , and in many minerals...
(Mn2+) is readily capable of replacing Mg2+, but only in a limited set of circumstances. Mn2+ is very similar to Mg2+ in terms of its chemical properties, including inner and outer shell complexation. Mn2+ effectively binds ATP and allows hydrolysis of the energy molecule by most ATPases. Mn2+ can also replace Mg2+ as the activating ion for a number of Mg2+-dependent enzymes, although some enzyme activity is usually lost. Sometimes such enzyme metal preferences vary among closely related species: for example, the reverse transcriptase
Reverse transcriptase
In the fields of molecular biology and biochemistry, a reverse transcriptase, also known as RNA-dependent DNA polymerase, is a DNA polymerase enzyme that transcribes single-stranded RNA into single-stranded DNA. It also helps in the formation of a double helix DNA once the RNA has been reverse...
enzyme of lentivirus
Lentivirus
Lentivirus is a genus of slow viruses of the Retroviridae family, characterized by a long incubation period...
es like HIV
HIV
Human immunodeficiency virus is a lentivirus that causes acquired immunodeficiency syndrome , a condition in humans in which progressive failure of the immune system allows life-threatening opportunistic infections and cancers to thrive...
, SIV
SIV
SIV or Siv may refer to:* Simian immunodeficiency virus, a virus found in primates and related to HIV* Siv, a character in the children's fiction book series Guardians of Ga'Hoole...
and FIV is typically dependent on Mg2+, whereas the analogous enzyme for other retrovirus
Retrovirus
A retrovirus is an RNA virus that is duplicated in a host cell using the reverse transcriptase enzyme to produce DNA from its RNA genome. The DNA is then incorporated into the host's genome by an integrase enzyme. The virus thereafter replicates as part of the host cell's DNA...
es prefers Mn2+.
Importance in drug binding
An article investigating the structural basis of interactions between clinically relevant antibiotics and the 50S ribosome appeared in Nature in October 2001. High resolution x-ray crystallography established that these antibiotics only associate with the 23S rRNA of a ribosomal subunit, and no interactions are formed with a subunit's protein portion. The article stresses that the results show "the importance of putative Mg2+ ions for the binding of some drugs".By radioactive isotopes
The use of radioactive tracer elements in ion uptake assays allows the calculation of km, Ki and Vmax and determines the initial change in the ion content of the cells. 28Mg decays by the emission of a high energy beta or gamma particle, which can be measured using a scintillation counter. However, the radioactive half-life of 28Mg, the most stable of the radioactive magnesium isotopes, is only 21 hours. This severely restricts the experiments involving the nuclide. Additionally, since 1990 no facility has routinely produced 28Mg and the price per mCi is now predicted to be approximately US$30,000. The chemical nature of Mg2+ is such that it is closely approximated by few other cations. However, Co2+, Mn2+ and Ni2+ have been used successfully to mimic the properties of Mg2+ in some enzyme reactions, and radioactive forms of these elements have been employed successfully in cation transport studies. The difficulty of using metal ion replacement in the study of enzyme function is that the relationship between the enzyme activities with the replacement ion compared to the original is very difficult to ascertain.By fluorescent indicators
A number of chelators of divalent cations have different fluorescence spectra in the bound and unbound states. Chelators for Ca2+ are well established, have high affinity for the cation, and low interference from other ions. Mg2+ chelators lag behind and the major fluorescence dye for Mg2+ (mag-fura 2) actually has a higher affinity for Ca2+. This limits the application of this dye to cell types where the resting level of Ca2+ is < 1 μM and does not vary with the experimental conditions under which Mg2+ is to be measured. Recently, Otten et al. (2001) have described work into a new class of compounds that may prove more useful, having significantly better binding affinities for Mg2+. The use of the fluorescent dyes is limited to measuring the free Mg2+. If the ion concentration is buffered by the cell by chelation or removal to subcellular compartments, the measured rate of uptake will only give minimum values of km and Vmax.By electrophysiology
First, ion-specific microelectrodes can be used to measure the internal free ion concentration of cells and organelles. The major advantages are that readings can be made from cells over relatively long periods of time, and that unlike dyes very little extra ion buffering capacity is added to the cells.Second, the technique of two-electrode voltage-clamp allows the direct measurement of the ion flux across the membrane of a cell. The membrane is held at an electric potential and the responding current is measured. All ions passing across the membrane contribute to the measured current.
Third, the technique of patch-clamp which uses isolated sections of natural or artificial membrane in much the same manner as voltage-clamp but without the secondary effects of a cellular system. Under ideal conditions the conductance of individual channels can be quantified. This methodology gives the most direct measurement of the action of ion channels.
By absorption spectroscopy
Flame atomic absorption spectroscopy (AAS) determines the total magnesium content of a biological sample. This method is destructive; biological samples must be broken down in concentrated acids to avoid clogging the fine nebulising apparatus. Beyond this the only limitation is that samples need to be in a volume of approximately 2 mL and at a concentration range of 0.1 – 0.4 µmol/L for optimum accuracy. As this technique cannot distinguish between Mg2+ already present in the cell and that taken up during the experiment only content not uptake can be quantified.Inductively coupled plasma (ICP) using either the mass spectrometry (MS) or atomic emission spectroscopy (AES) modifications also allows the determination of the total ion content of biological samples. These techniques are more sensitive than flame AAS and are capable of measuring the quantities of multiple ions simultaneously. However, they are also significantly more expensive.
Magnesium transport
The chemical and biochemical properties of Mg2+ present the cellular system with a significant challenge when transporting the ion across biological membranes. The dogma of ion transport states that the transporter recognises the ion then progressively removes the water of hydration, removing most or all of the water at a selective pore before releasing the ion on the far side of the membrane. Due to the properties of Mg2+, large volume change from hydrated to bare ion, high energy of hydration and very low rate of ligand exchange in the inner coordination sphereCoordination sphere
In coordination chemistry, the coordination sphere refers to a central atom or ion and an array of molecules or anions, the ligands, around.Molecules that are attached noncovalently to the ligands are called the second coordination sphere....
, these steps are probably more difficult than for most other ions. To date, only the ZntA protein of Paramecium has been shown to be a Mg2+ channel. The mechanisms of Mg2+ transport by the remaining proteins are beginning to be uncovered with the first three dimensional structure of a Mg2+ transport complex being solved in 2004.
The hydration shell of the Mg2+ ion has a very tightly bound inner shell of six water molecules and a relatively tightly bound second shell containing 12 – 14 water molecules (Markham et al., 2002). Thus recognition of the Mg2+ ion probably requires some mechanism to interact initially with the hydration shell of Mg2+, followed by a direct recognition/binding of the ion to the protein. Due to the strength of the inner sphere complexation between Mg2+ and any ligand, multiple simultaneous interactions with the transport protein at this level might significantly retard the ion in the transport pore. Hence, it is possible that much of the hydration water is retained during transport, allowing the weaker (but still specific) outer sphere coordination.
In spite of the mechanistic difficulty, Mg2+ must be transported across membranes, and a large number of Mg2+ fluxes across membranes from a variety of systems have been described. However, only a small selection of Mg2+ transporters have been characterised at the molecular level.
Ligand ion channel blockade
MagnesiumMagnesium
Magnesium is a chemical element with the symbol Mg, atomic number 12, and common oxidation number +2. It is an alkaline earth metal and the eighth most abundant element in the Earth's crust and ninth in the known universe as a whole...
ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s (Mg2+) in cellular biology are usually in almost all senses opposite to Ca2+
Calcium in biology
Calcium plays a pivotal role in the physiology and biochemistry of organisms and the cell. It plays an important role in signal transduction pathways, where it acts as a second messenger, in neurotransmitter release from neurons, contraction of all muscle cell types, and fertilization...
ions, because they are bivalent too, but have greater electronegativity and thus hold on to water molecules stronger, preventing passage through the channel (even though magnesium is smaller). Thus Mg2+ ions block Ca2+ channels (NMDA channels) for example, etc.
Plant physiology of magnesium
The previous sections have dealt in detail with the chemical and biochemical aspects of Mg2+ and its transport across cellular membranes. This section will apply this knowledge to aspects of whole plant physiology, in an attempt to show how these processes interact with the larger and more complex environment of the multicellular organism.Nutritional requirements and interactions
Mg2+ is essential for plant growth and is present in higher plants in amounts on the order of 80 μmol g−1 dry weight. The amounts of Mg2+ vary in different parts of the plant and are dependent upon nutritional status. In times of plenty, excess Mg2+ may be stored in vascular cells (Stelzer et al., 1990; and in times of starvation Mg2+ is redistributed, in many plants, from older to newer leaves.Mg2+ is taken up into plants via the roots. Interactions with other cations in the rhizosphere
Rhizosphere (ecology)
The rhizosphere is the narrow region of soil that is directly influenced by root secretions and associated soil microorganisms. Soil which is not part of the rhizosphere is known as bulk soil. The rhizosphere contains many bacteria that feed on sloughed-off plant cells, termed rhizodeposition, and...
can have a significant effect on the uptake of the ion.(Kurvits and Kirkby, 1980; The structure of root cell walls is highly permeable to water and ions, and hence ion uptake into root cells, can occur anywhere from the root hairs to cells located almost in the centre of the root (limited only by the Casparian strip
Casparian strip
In plant anatomy, the Casparian strip is a band of cell wall material deposited on the radial and transverse walls of the endodermis, which is chemically different from the rest of the cell wall. It is used to block the passive flow of materials, such as water and solutes into the stele of a plant...
). Plant cell walls and membranes carry a great number of negative charges and the interactions of cations with these charges is key to the uptake of cations by root cells allowing a local concentrating effect. Mg2+ binds relatively weakly to these charges, and can be displaced by other cations, impeding uptake and causing deficiency in the plant.
Within individual plant cells the Mg2+ requirements are largely the same as for all cellular life; Mg2+ is used to stabilise membranes, is vital to the utilisation of ATP, is extensively involved in the nucleic acid biochemistry, and is a cofactor for many enzymes (including the ribosome). Also, Mg2+ is the coordinating ion in the chlorophyll molecule. It is the intracellular compartmentalisation of Mg2+ in plant cells that leads to additional complexity. Four compartments within the plant cell have reported interactions with Mg2+. Initially Mg2+ will enter the cell into the cytoplasm (by an as yet unidentified system), but free Mg2+ concentrations in this compartment are tightly regulated at relatively low levels (≈2 mmol/L) and so any excess Mg2+ is either quickly exported or stored in the second intracellular compartment, the vacuole. The requirement for Mg2+ in mitochondria has been demonstrated in yeast and it seems highly likely that the same will apply in plants. The chloroplasts also require significant amounts of internal Mg2+, and low concentrations of cytoplasmic Mg2+. In addition, it seems likely that the other subcellular organelles (e.g. Golgi, endoplasmic reticulum, etc.) also require Mg2+.
Distributing magnesium ions within the plant
Once in the cytoplasmic space of root cells Mg2+, along with the other cations, is probably transported radially into the stele and the vascular tissue. From the cells surrounding the xylem the ions are released or pumped into the xylem and carried up through the plant. In the case of Mg2+, which is highly mobile in both the xylem and phloem, the ions will be transported to the top of the plant and back down again in a continuous cycle of replenishment. Hence, uptake and release from vascular cells is probably a key part of whole plant Mg2+ homeostasis. Figure 1 shows how few processes have been connected to their molecular mechanisms (only vacuolar uptake has been associated with a transport protein, AtMHX).The diagram shows a schematic of a plant and the putative processes of Mg2+ transport at the root and leaf where Mg2+ is loaded and unloaded from the vascular tissues. Mg2+ is taken up into the root cell wall space (1) and interacts with the negative charges associated with the cell walls and membranes. Mg2+ may be taken up into cells immediately (symplastic pathway) or may travel as far as the Casparian band (4) before being absorbed into cells (apoplastic pathway; 2). The concentration of Mg2+ in the root cells is probably buffered by storage in root cell vacuoles (3). Note that cells in the root tip do not contain vacuoles. Once in the root cell cytoplasm Mg2+ travels towards the centre of the root by plasmodesmata
Plasmodesmata
Plasmodesmata are microscopic channels which traverse the cell walls of plant cells and some algal cells, enabling transport and communication between them. Species that have plasmodesmata include members of the Charophyceae, Charales and Coleochaetales , as well as all embryophytes, better known...
, where it is loaded into the xylem (5) for transport to the upper parts of the plant. When the Mg2+ reaches the leaves it is unloaded from the xylem into cells (6) and again is buffered in vacuoles (7). Whether cycling of Mg2+ into the phloem occurs via general cells in the leaf (8) or directly from xylem to phloem via transfer cells
Transfer cells
Transfer cells are specialized parenchyma cells that have an increased surface area, due to infoldings of the plasma membrane. They facilitate the transport of sugars from a sugar source, mainly leaves, to a sugar sink, often developing fruits....
(9) is unknown. Mg2+ may return to the roots in the phloem sap.

The homeostasis of Mg2+ within single plant cells is maintained by processes occurring at the plasma membrane and at the vacuole membrane (see Figure 2). The major driving force for the translocation of ions in plant cells is ΔpH. H+-ATPases pump H+ ions against their concentration gradient to maintain the pH differential that can be used for the transport of other ions and molecules. H+ ions are pumped out of the cytoplasm into the extracellular space or into the vacuole. The entry of Mg2+ into cells may occur through one of two pathways, via channels using the ΔΨ (negative inside) across this membrane or by symport with H+ ions. To transport the Mg2+ ion into the vacuole requires a Mg2+/H+ antiport transporter (such as AtMHX). It is interesting to note that the H+-ATPases are dependent on Mg2+ (bound to ATP) for activity, so that Mg2+ is required to maintain its own homeostasis.
A schematic of a plant cell is shown including the four major compartments currently recognised as interacting with Mg2+. H+-ATPases maintain a constant ΔpH across the plasma membrane and the vacuole membrane. Mg2+ is transported into the vacuole using the energy of ΔpH (in A. thaliana by AtMHX). Transport of Mg2+ into cells may use either the negative ΔΨ or the ΔpH. The transport of Mg2+ into mitochondria probably uses ΔΨ as in the mitochondria of yeast, and it is likely that chloroplasts take Mg2+ by a similar system. The mechanism and the molecular basis for the release of Mg2+ from vacuoles and from the cell is not known. Likewise the light-regulated Mg2+ concentration changes in chloroplasts are not fully understood, but do require the transport of H+ ions across the thylakoid membrane.

Magnesium, chloroplasts and photosynthesis
Mg2+ is the coordinating metal ion in the chlorophyll molecule, and in plants where the ion is in high supply about 6% of the total Mg2+ is bound to chlorophyll. Thylakoid stacking is stabilised by Mg2+ and is important for the efficiency of photosynthesis, allowing phase transitions to occur.Mg2+ is probably taken up into chloroplasts to the greatest extent during the light induced development from proplastid to chloroplast or etioplast to chloroplast. At these times the synthesis of chlorophyll and the biogenesis of the thylakoid membrane stacks absolutely require the divalent cation.
Whether Mg2+ is able to move into and out of chloroplasts after this initial developmental phase has been the subject of several conflicting reports. Deshaies et al. (1984) found that Mg2+ did move in and out of isolated chloroplasts from young pea plants, but Gupta and Berkowitz (1989) were unable to reproduce the result using older spinach chloroplasts. Deshaies et al. had stated in their paper that older pea chloroplasts showed less significant changes in Mg2+ content than those used to form their conclusions. Perhaps the relative proportion of immature chloroplasts present in the preparations might explain these observations.
The metabolic state of the chloroplast changes considerably between night and day. During the day the chloroplast is actively harvesting the energy of light and converting it into chemical energy. The activation of the metabolic pathways involved comes from the changes in the chemical nature of the stroma on the addition of light. H+ is pumped out of the stroma (into both the cytoplasm and the lumen) leading to an alkaline pH. Mg2+ (along with K+) is released from the lumen into the stroma, in an electroneutralisation process to balance the flow of H+. Finally, thiol groups on enzymes are reduced by a change in the redox state of the stroma. Examples of enzymes activated in response to these changes are fructose 1,6-bisphosphatase, sedoheptulose bisphosphatase and ribulose-1,5-bisphosphate carboxylase. During the dark period, if these enzymes were active a wasteful cycling of products and substrates would occur.
Two major classes of the enzymes that interact with Mg2+ in the stroma during the light phase can be identified. Firstly, enzymes in the glycolytic pathway most often interact with two atoms of Mg2+. The first atom is as an allosteric modulator of the enzymes’ activity, while the second forms part of the active site and is directly involved in the catalytic reaction. The second class of enzymes include those where the Mg2+ is complexed to nucleotide di- and tri-phosphates (ADP and ATP) and the chemical change involves phosphoryl transfer. Mg2+ may also serve in a structural maintenance role in these enzymes (e.g. enolase).
Magnesium stress
Plant stress responses can be observed in plants that are under or over supplied with Mg2+. The first observable signs of Mg2+ stress in plants for both starvation and toxicity is a depression of the rate of photosynthesis, presumably because of the strong relationships between Mg2+ and chloroplasts/chlorophyll. In pine trees, even before the visible appearance of yellowing and necrotic spots, the photosynthetic efficiency of the needles drops markedly. In Mg2+ deficiency, reported secondary effects include carbohydrate immobility, loss of RNA transcription and loss of protein synthesis. However, due to the mobility of Mg2+ within the plant, the deficiency phenotype may be present only in the older parts of the plant. For example, in Pinus radiata starved of Mg2+ one of the earliest identifying signs is the chlorosis in the needles on the lower branches of the tree. This is because Mg2+ has been recovered from these tissues and moved to growing (green) needles higher in the tree.A Mg2+ deficit can be caused by the lack of the ion in the media (soil), but more commonly comes from inhibition of its uptake. Mg2+ binds quite weakly to the negatively charged groups in the root cell walls, so that excesses of other cations such as K+, NH4+, Ca2+ and Mn2+ can all impede uptake.(Kurvits and Kirkby, 1980; In acid soils Al3+ is a particularly strong inhibitor of Mg2+ uptake. The inhibition by Al3+ and Mn2+ is more severe than can be explained by simple displacement, hence it is possible that these ions bind to the Mg2+ uptake system directly. In bacteria and yeast, such binding by Mn2+ has already been observed. Stress responses in the plant develop as cellular processes halt due to a lack of Mg2+ (e.g. maintenance of ΔpH across the plasma and vacuole membranes). Interestingly, in Mg2+-starved plants under low light conditions the percentage of Mg2+ bound to chlorophyll has been recorded at 50%. Presumably, this imbalance has detrimental effects on other cellular processes.
Mg2+ toxicity stress is more difficult to develop. When Mg2+ is plentiful the plants generally take up the ion and store it (Stelzer et al., 1990). However, if this is followed by drought then ionic concentrations within the cell can increase dramatically. High cytoplasmic Mg2+ concentrations block a K+ channel in the inner envelope membrane of the chloroplast, in turn inhibiting the removal of H+ ions from the chloroplast stroma. This leads to an acidification of the stroma that inactivates key enzymes in carbon fixation, which all leads to the production of oxygen free radicals in the chloroplast that then cause oxidative damage.
External links
- Magnesium Deficiency
- List of foods rich in Magnesium
- The Magnesium Website- Includes full text papers and textbook chapters by leading magnesium authorities Mildred Seelig, Jean Durlach, Burton M. Altura and Bella T. Altura. Links to over 300 articles discussing magnesium and magnesium deficiency.
- Dietary Reference Intake
- Healing Thresholds - description of research studies regarding supplementation with magnesium and other therapies for autism
See also
- Ion channels
- Myers' cocktailMyers' cocktailThe "Myers' cocktail" is the colloquial name for a nutrient cocktail invented by John Myers, a physician from Baltimore, Maryland, and developed by Alan R. Gaby, administered intravenously and promoted as an alternative treatment for a broad range of conditions including asthma, fibromyalgia and...
- Magnesium deficiency (medicine)Magnesium deficiency (medicine)Magnesium deficiency refers to an intake of dietary magnesium below minimal levels, which can result in numerous symptoms and diseases. These can generally be remedied by an increase of magnesium in diet or oral supplements...
- Magnesium deficiency (agriculture)

