
Liquid
Encyclopedia
Liquid is one of the three classical states of matter
(the others being gas
and solid
). Like a gas, a liquid is able to flow
and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly constant density. A distinctive property of the liquid state is surface tension
, leading to wetting
phenomena.
The density of a liquid is usually close to that of a solid, and much higher than in a gas. Therefore, liquid and solid are both termed condensed matter
. On the other hand, as liquids and gases share the ability to flow, they are both called fluid
s.
, with the others being solid
and gas
. A liquid is a fluid
. Unlike a solid, the molecule
s in a liquid have a much greater freedom to move. The forces that bind the molecules together in a solid are only temporary in a liquid, allowing a liquid to flow while a solid remains rigid.
A liquid, like a gas, displays the properties of a fluid. A liquid can flow, assume the shape of a container, and, if placed in a sealed container, will distribute applied pressure evenly to every surface in the container. Unlike a gas, a liquid may not always mix readily with another liquid, will not always fill every space in the container, forming its own surface, and will not compress significantly, except under extremely high pressures. These properties make a liquid suitable for applications such as hydraulics
.
Liquid particles are bound firmly but not rigidly. They are able to move around one another freely, resulting in a limited degree of particle mobility. As the temperature
increases, the increased vibrations of the molecules causes distances between the molecules to increase. When a liquid reaches its boiling point
, the cohesive forces that bind the molecules closely together break, and the liquid changes to its gaseous state (unless superheating
occurs). If the temperature is decreased, the distances between the molecules become smaller. When the liquid reaches its freezing point
the molecules will usually lock into a very specific order, called crystallizing, and the bonds between them become more rigid, changing the liquid into its solid state (unless supercooling
occurs).
are liquid at room temperature and pressure: mercury
and bromine
. Five more elements have melting points slightly above room temperature: francium
, caesium
, gallium
and rubidium
. Metal alloys that are liquid at room temperature include NaK
, a sodium-potassium metal alloy, galinstan
, a fusible alloy liquid, and some amalgams
(alloys involving mercury).
Pure substances that are liquid under normal conditions include water
, ethanol
and many other organic solvents. Liquid water is of vital importance in chemistry and biology; it is believed to be a necessity for the existence of life
.
Important everyday liquids include aqueous solution
s like household bleach
, other mixture
s of different substances such as mineral oil
and gasoline
, emulsion
s like vinaigrette
or mayonnaise
, suspensions
like blood
, and colloid
s like paint
and milk
.
Many gases can be liquefied
by cooling, producing liquids such as liquid oxygen
, liquid nitrogen
, liquid hydrogen
and liquid helium
. Not all gases can be liquified at atmospheric pressure, for example carbon dioxide
can only be liquified at pressures above 5.1 atm
.
Some materials cannot be classified within the classical three states of matter; they possess solid-like and liquid-like properties. Examples include liquid crystal
s, used in LCD displays, and biological membrane
s.
In tribology
, liquids are studied for their properties as lubricants. Lubricants such as oil
are chosen for viscosity
and flow characteristics that are suitable throughout the operating temperature
range of the component. Oils are often used in engine
s, gear boxes, metalworking
, and hydraulic systems for their good lubrication properties.
Many liquids are used as solvents, to dissolve other liquids or solids. Solution
s are found in a wide variety of applications, including paint
s, sealant
s, and adhesive
s. Naptha and acetone
are used frequently in industry to clean oil, grease, and tar from parts and machinery. Body fluids are water based solutions.
Surfactant
s are commonly found in soap
s and detergent
s. Solvents like alcohol
are often used as antimicrobial
s. They are found in cosmetics
, ink
s, and liquid dye laser
s. They are used in the food industry, in processes such as the extraction of vegetable oil.
Liquids tend to have better thermal conductivity
than gases, and the ability to flow makes a liquid suitable for removing excess heat from mechanical components. The heat can be removed by channeling the liquid through a heat exchanger
, such as a radiator
, or the heat can be removed with the liquid during evaporation
. Water or glycol coolants are used to keep engine
s from overheating. The coolants used in nuclear reactor
s include water or liquid metals, such as sodium
or bismuth
. Liquid propellant films are used to cool the thrust chambers of rocket
s. In machining
, water and oils are used to remove the excess heat generated, which can quickly ruin both the work piece and the tooling. During perspiration, sweat removes heat from the human body by evaporating. In the heating, ventilation, and air-conditioning industry (HVAC), liquids such as water are used to transfer heat from one area to another.
Liquid is the primary component of hydraulic systems, which take advantage of Pascal's law
to provide fluid power
. Devices such as pump
s and waterwheels have been used to change liquid motion into mechanical work
since ancient times. Oil
s are forced through hydraulic pump
s, which transmit this force to hydraulic cylinder
s. Hydraulics can be found in many applications, such as automotive brakes and transmissions, heavy equipment, and airplane control systems. Various hydraulic press
es are used extensively in repair and manufacturing, for lifting, pressing, clamping and forming.
Liquids are sometimes used in measuring devices. A thermometer
often uses the thermal expansion
of liquids, such as mercury
, combined with their ability to flow to indicate temperature. A manometer uses the weight of the liquid to indicate air pressure.
. These include the SI
unit cubic metre
(m3) and its divisions, in particular the cubic decimetre, more commonly called the litre
(1 dm3 = 1 L = 0.001 m3), and the cubic centimetre, also called millilitre (1 cm3 = 1 mL = 0.001 L = 10−6 m3).
The volume
of a quantity of liquid is fixed by its temperature
and pressure
. Liquids generally expand when heated, and contract when cooled. Water
between 0 °C and 4 °C is a notable exception.
Liquids have little compressibility: water, for example, requires a pressure of the order of 200 bar to increase its density
by 1/1000. In the study of fluid dynamics
, liquids are often treated as incompressible, especially when studying incompressible flow
.
, liquids exert pressure
on the sides of a container as well as on anything within the liquid itself. This pressure is transmitted in all directions and increases with depth. If a liquid is at rest in a uniform gravitational field, the pressure, p, at any depth, z, is given by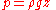
where: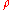 is the density
is the density
of the liquid (assumed constant)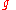 is the gravitational acceleration.
is the gravitational acceleration.
Note that this formula assumes that the pressure at the free surface is zero, and that surface tension
effects may be neglected.
Objects immersed in liquids are subject to the phenomenon of buoyancy
. (Buoyancy is also observed in other fluids, but is especially strong in liquids due to their high density.)
 Unless the volume of a liquid exactly matches the volume of its container, one or more surfaces are observed. The surface of a liquid behaves like an elastic membrane in which surface tension
Unless the volume of a liquid exactly matches the volume of its container, one or more surfaces are observed. The surface of a liquid behaves like an elastic membrane in which surface tension
appears, allowing the formation of drops
and bubble
s. Surface wave
s, capillary action
, wetting
, and ripples are other consequences of surface tension
.
measures the resistance of a liquid which is being deformed by either shear stress or extensional stress.
When a liquid is supercooled
towards the glass transition
, the viscosity increases dramatically. The liquid then becomes a viscoelastic
medium that shows both the elasticity
of a solid and the fluidity of a liquid, depending on the time scale of observation or on the frequency of perturbation.
is to volumetric deformation (a fluid does not sustain shear forces). Hence the speed of sound in a fluid is given by
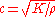 where K is the bulk modulus
where K is the bulk modulus
of the fluid, and ρ the density. To give a typical value, in fresh water c=1497 m/s at 25 °C.
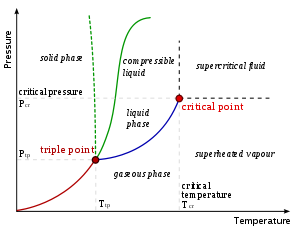 At a temperature below the boiling point
At a temperature below the boiling point
, any matter in liquid form will evaporate until the condensation of gas above reach an equilibrium. At this point the gas will condense at the same rate as the liquid evaporates. Thus, a liquid cannot exist permanently if the evaporated liquid is continually removed. A liquid at its boiling point
will evaporate more quickly than the gas can condense at the current pressure. A liquid at or above its boiling point will normally boil, though superheating
can prevent this in certain circumstances.
At a temperature below the freezing point, a liquid will tend to crystallize
, changing to its solid form. Unlike the transition to gas, there is no equilibrium at this transition under constant pressure, so unless supercooling
occurs, the liquid will eventually completely crystallize. Note that this is only true under constant pressure, so e.g. water and ice in a closed, strong container might reach an equilibrium where both phases coexist. For the opposite transition from solid to liquid, see melting
.
in Italian salad dressing
. A familiar set of miscible liquids is water and alcohol. Liquid components in a mixture can often be separated from one another via fractional distillation
.
In a liquid, atoms do not form a crystalline lattice, nor do they show any other form of long-range order. This is evidenced by the absence of Bragg peak
s in X-ray and neutron diffraction
. Under normal conditions, the diffraction pattern has circular symmetry, expressing the isotropy
of the liquid. In radial direction, the diffraction intensity smoothly oscillates. This is usually described by the static structure factor S(q), with wavenumber q=(4π/λ)sinθ given by the wavelength λ of the probe (photon or neutron) and the Bragg angle θ. The oscillations of S(q) express the near order of the liquid, i.e. the correlations between an atom and a few shells of nearest, second nearest, ... neighbors.
A more intuitive description of these correlations is given by the radial distribution function
g(r), which is basically the Fourier transform
of S(q). It represents a spatial average of a temporal snapshot of pair correlations in the liquid.
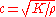 contains the bulk modulus
contains the bulk modulus
K. If K is frequency independent then the liquid behaves as a linear medium
, so that sound propagates without dissipation
and without mode coupling
. In reality, any liquid shows some dispersion
: with increasing frequency, K crosses over from the low-frequency, liquid-like limit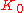 to the high-frequency, solid-like limit
to the high-frequency, solid-like limit 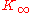 . In normal liquids, most of this cross over takes place at frequencies between GHz and THz, sometimes called hypersound.
. In normal liquids, most of this cross over takes place at frequencies between GHz and THz, sometimes called hypersound.
At sub-GHz frequencies, a normal liquid cannot sustain shear waves: the zero-frequency limit of the shear modulus is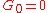 . This is sometimes seen as the defining property of a liquid.
. This is sometimes seen as the defining property of a liquid.
However, just as the bulk modulus K, the shear modulus G is frequency dependent,
and at hypersound frequencies it shows a similar cross over from the liquid-like limit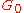 to a solid-like, non-zero limit
to a solid-like, non-zero limit 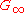 .
.
According to the Kramers-Kronig relation
, the dispersion in the sound velocity (given by the real part of K or G) goes along with a maximum in the sound attenuation (dissipation, given by the imaginary part of K or G). According to linear response theory, the Fourier transform of K or G describes how the system returns to equilibrium after an external perturbation; for this reason, the dispersion step in the GHz..THz region is also called structural relaxation. According the fluctuation-dissipation theorem, relaxation towards equilibrium is intimately connected to fluctuations in equilibrium. The density fluctuations associated with sound waves can be experimentally observed by Brillouin scattering
.
On supercooling a liquid towards the glass transition, the crossover from liquid-like to solid-like response moves from GHz to MHz, kHz, Hz, ...; equivalently, the characteristic time of structural relaxation increases from ns to μs, ms, s, ... This is the microscopic explanation for the above mentioned viscoelastic behaviour of glass-forming liquids.
(or particle displacement
) in solids are closely related to the mechanisms of viscous flow and solidification in liquid materials. Descriptions of viscosity
in terms of molecular "free space" within the liquid
were modified as needed in order to account for liquids whose molecules are known to be "associated" in the liquid state at ordinary temperatures. When various molecules combine together to form an associated molecule, they enclose within a semi-rigid system a certain amount of space which before was available as free space for mobile molecules. Thus, increase in viscosity upon cooling due to the tendency of most substances to become associated on cooling.
Similar arguments could be used to describe the effects of pressure
on viscosity, where it may be assumed that the viscosity is chiefly a function of the volume for liquids with a finite compressibility. An increasing viscosity with rise of pressure is therefore expected. In addition, if the volume is expanded by heat but reduced again by pressure, the viscosity remains the same.
The local tendency to orientation of molecules in small groups lends the liquid (as referred to previously) a certain degree of association. This association results in a considerable "internal pressure" within a liquid, which is due almost entirely to those molecules which, on account of their temporary low velocities (following the Maxwell distribution) have coalesced with other molecules. The internal pressure between several such molecules might correspond to that between a group of molecules in the solid form.
State of matter
States of matter are the distinct forms that different phases of matter take on. Solid, liquid and gas are the most common states of matter on Earth. However, much of the baryonic matter of the universe is in the form of hot plasma, both as rarefied interstellar medium and as dense...
(the others being gas
Gas
Gas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
and solid
Solid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
). Like a gas, a liquid is able to flow
Fluid
In physics, a fluid is a substance that continually deforms under an applied shear stress. Fluids are a subset of the phases of matter and include liquids, gases, plasmas and, to some extent, plastic solids....
and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly constant density. A distinctive property of the liquid state is surface tension
Surface tension
Surface tension is a property of the surface of a liquid that allows it to resist an external force. It is revealed, for example, in floating of some objects on the surface of water, even though they are denser than water, and in the ability of some insects to run on the water surface...
, leading to wetting
Wetting
Wetting is the ability of a liquid to maintain contact with a solid surface, resulting from intermolecular interactions when the two are brought together. The degree of wetting is determined by a force balance between adhesive and cohesive forces.Wetting is important in the bonding or adherence of...
phenomena.
The density of a liquid is usually close to that of a solid, and much higher than in a gas. Therefore, liquid and solid are both termed condensed matter
Condensed matter physics
Condensed matter physics deals with the physical properties of condensed phases of matter. These properties appear when a number of atoms at the supramolecular and macromolecular scale interact strongly and adhere to each other or are otherwise highly concentrated in a system. The most familiar...
. On the other hand, as liquids and gases share the ability to flow, they are both called fluid
Fluid
In physics, a fluid is a substance that continually deforms under an applied shear stress. Fluids are a subset of the phases of matter and include liquids, gases, plasmas and, to some extent, plastic solids....
s.
Introduction
Liquid is one of the three primary states of matterState of matter
States of matter are the distinct forms that different phases of matter take on. Solid, liquid and gas are the most common states of matter on Earth. However, much of the baryonic matter of the universe is in the form of hot plasma, both as rarefied interstellar medium and as dense...
, with the others being solid
Solid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
and gas
Gas
Gas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
. A liquid is a fluid
Fluid
In physics, a fluid is a substance that continually deforms under an applied shear stress. Fluids are a subset of the phases of matter and include liquids, gases, plasmas and, to some extent, plastic solids....
. Unlike a solid, the molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
s in a liquid have a much greater freedom to move. The forces that bind the molecules together in a solid are only temporary in a liquid, allowing a liquid to flow while a solid remains rigid.
A liquid, like a gas, displays the properties of a fluid. A liquid can flow, assume the shape of a container, and, if placed in a sealed container, will distribute applied pressure evenly to every surface in the container. Unlike a gas, a liquid may not always mix readily with another liquid, will not always fill every space in the container, forming its own surface, and will not compress significantly, except under extremely high pressures. These properties make a liquid suitable for applications such as hydraulics
Hydraulics
Hydraulics is a topic in applied science and engineering dealing with the mechanical properties of liquids. Fluid mechanics provides the theoretical foundation for hydraulics, which focuses on the engineering uses of fluid properties. In fluid power, hydraulics is used for the generation, control,...
.
Liquid particles are bound firmly but not rigidly. They are able to move around one another freely, resulting in a limited degree of particle mobility. As the temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
increases, the increased vibrations of the molecules causes distances between the molecules to increase. When a liquid reaches its boiling point
Boiling point
The boiling point of an element or a substance is the temperature at which the vapor pressure of the liquid equals the environmental pressure surrounding the liquid....
, the cohesive forces that bind the molecules closely together break, and the liquid changes to its gaseous state (unless superheating
Superheating
In physics, superheating is the phenomenon in which a liquid is heated to a temperature higher than its boiling point, without boiling...
occurs). If the temperature is decreased, the distances between the molecules become smaller. When the liquid reaches its freezing point
Melting point
The melting point of a solid is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at standard atmospheric pressure...
the molecules will usually lock into a very specific order, called crystallizing, and the bonds between them become more rigid, changing the liquid into its solid state (unless supercooling
Supercooling
Supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point without it becoming a solid....
occurs).
Examples
Only two elementsChemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
are liquid at room temperature and pressure: mercury
Mercury (element)
Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum...
and bromine
Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
. Five more elements have melting points slightly above room temperature: francium
Francium
Francium is a chemical element with symbol Fr and atomic number 87. It was formerly known as eka-caesium and actinium K.Actually the least unstable isotope, francium-223 It has the lowest electronegativity of all known elements, and is the second rarest naturally occurring element...
, caesium
Caesium
Caesium or cesium is the chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-gold alkali metal with a melting point of 28 °C , which makes it one of only five elemental metals that are liquid at room temperature...
, gallium
Gallium
Gallium is a chemical element that has the symbol Ga and atomic number 31. Elemental gallium does not occur in nature, but as the gallium salt in trace amounts in bauxite and zinc ores. A soft silvery metallic poor metal, elemental gallium is a brittle solid at low temperatures. As it liquefies...
and rubidium
Rubidium
Rubidium is a chemical element with the symbol Rb and atomic number 37. Rubidium is a soft, silvery-white metallic element of the alkali metal group. Its atomic mass is 85.4678. Elemental rubidium is highly reactive, with properties similar to those of other elements in group 1, such as very rapid...
. Metal alloys that are liquid at room temperature include NaK
NaK
NaK, or sodium-potassium alloy, an alloy, of potassium , and sodium , is usually liquid at room temperature. Various commercial grades are available. NaK is highly reactive with water and may catch fire when exposed to air, so must be handled with special precautions...
, a sodium-potassium metal alloy, galinstan
Galinstan
Galinstan is a family of eutectic alloys mainly consisting of gallium, indium, and tin, which are liquids at room temperature, typically freezing at . Due to the low toxicity and low reactivity of its component metals, it finds use as a replacement for many applications that previously employed...
, a fusible alloy liquid, and some amalgams
Amalgam (chemistry)
An amalgam is a substance formed by the reaction of mercury with another metal. Almost all metals can form amalgams with mercury, notable exceptions being iron and platinum. Silver-mercury amalgams are important in dentistry, and gold-mercury amalgam is used in the extraction of gold from ore.The...
(alloys involving mercury).
Pure substances that are liquid under normal conditions include water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
, ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
and many other organic solvents. Liquid water is of vital importance in chemistry and biology; it is believed to be a necessity for the existence of life
Life
Life is a characteristic that distinguishes objects that have signaling and self-sustaining processes from those that do not, either because such functions have ceased , or else because they lack such functions and are classified as inanimate...
.
Important everyday liquids include aqueous solution
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
s like household bleach
Bleach
Bleach refers to a number of chemicals that remove color, whiten, or disinfect, often via oxidation. Common chemical bleaches include household chlorine bleach , lye, oxygen bleach , and bleaching powder...
, other mixture
Mixture
In chemistry, a mixture is a material system made up by two or more different substances which are mixed together but are not combined chemically...
s of different substances such as mineral oil
Mineral oil
A mineral oil is any of various colorless, odorless, light mixtures of alkanes in the C15 to C40 range from a non-vegetable source, particularly a distillate of petroleum....
and gasoline
Gasoline
Gasoline , or petrol , is a toxic, translucent, petroleum-derived liquid that is primarily used as a fuel in internal combustion engines. It consists mostly of organic compounds obtained by the fractional distillation of petroleum, enhanced with a variety of additives. Some gasolines also contain...
, emulsion
Emulsion
An emulsion is a mixture of two or more liquids that are normally immiscible . Emulsions are part of a more general class of two-phase systems of matter called colloids. Although the terms colloid and emulsion are sometimes used interchangeably, emulsion is used when both the dispersed and the...
s like vinaigrette
Vinaigrette
The word vinaigrette or vinegarette can refer to:*Vinaigrette, the salad dressing or sauce...
or mayonnaise
Mayonnaise
Mayonnaise, , often abbreviated as mayo, is a sauce. It is a stable emulsion of oil, egg yolk and either vinegar or lemon juice, with many options for embellishment with other herbs and spices. Lecithin in the egg yolk is the emulsifier. Mayonnaise varies in color but is often white, cream, or pale...
, suspensions
Suspension (chemistry)
In chemistry, a suspension is a heterogeneous fluid containing solid particles that are sufficiently large for sedimentation. Usually they must be larger than 1 micrometer. The internal phase is dispersed throughout the external phase through mechanical agitation, with the use of certain...
like blood
Blood
Blood is a specialized bodily fluid in animals that delivers necessary substances such as nutrients and oxygen to the cells and transports metabolic waste products away from those same cells....
, and colloid
Colloid
A colloid is a substance microscopically dispersed evenly throughout another substance.A colloidal system consists of two separate phases: a dispersed phase and a continuous phase . A colloidal system may be solid, liquid, or gaseous.Many familiar substances are colloids, as shown in the chart below...
s like paint
Paint
Paint is any liquid, liquefiable, or mastic composition which after application to a substrate in a thin layer is converted to an opaque solid film. One may also consider the digital mimicry thereof...
and milk
Milk
Milk is a white liquid produced by the mammary glands of mammals. It is the primary source of nutrition for young mammals before they are able to digest other types of food. Early-lactation milk contains colostrum, which carries the mother's antibodies to the baby and can reduce the risk of many...
.
Many gases can be liquefied
Liquefaction of gases
Liquefaction of gases includes a number of phases used to convert a gas into a liquid state. The processes are used for scientific, industrial and commercial purposes. Many gases can be put into a liquid state at normal atmospheric pressure by simple cooling; a few, such as carbon dioxide, require...
by cooling, producing liquids such as liquid oxygen
Liquid oxygen
Liquid oxygen — abbreviated LOx, LOX or Lox in the aerospace, submarine and gas industries — is one of the physical forms of elemental oxygen.-Physical properties:...
, liquid nitrogen
Liquid nitrogen
Liquid nitrogen is nitrogen in a liquid state at a very low temperature. It is produced industrially by fractional distillation of liquid air. Liquid nitrogen is a colourless clear liquid with density of 0.807 g/mL at its boiling point and a dielectric constant of 1.4...
, liquid hydrogen
Liquid hydrogen
Liquid hydrogen is the liquid state of the element hydrogen. Hydrogen is found naturally in the molecular H2 form.To exist as a liquid, H2 must be pressurized above and cooled below hydrogen's Critical point. However, for hydrogen to be in a full liquid state without boiling off, it needs to be...
and liquid helium
Liquid helium
Helium exists in liquid form only at extremely low temperatures. The boiling point and critical point depend on the isotope of the helium; see the table below for values. The density of liquid helium-4 at its boiling point and 1 atmosphere is approximately 0.125 g/mL Helium-4 was first liquefied...
. Not all gases can be liquified at atmospheric pressure, for example carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
can only be liquified at pressures above 5.1 atm
Atmosphere (unit)
The standard atmosphere is an international reference pressure defined as 101325 Pa and formerly used as unit of pressure. For practical purposes it has been replaced by the bar which is 105 Pa...
.
Some materials cannot be classified within the classical three states of matter; they possess solid-like and liquid-like properties. Examples include liquid crystal
Liquid crystal
Liquid crystals are a state of matter that have properties between those of a conventional liquid and those of a solid crystal. For instance, an LC may flow like a liquid, but its molecules may be oriented in a crystal-like way. There are many different types of LC phases, which can be...
s, used in LCD displays, and biological membrane
Biological membrane
A biological membrane or biomembrane is an enclosing or separatingmembrane that acts as a selective barrier, within or around a cell. It consists of a lipid bilayer with embedded proteins that may constitute close to 50% of membrane content...
s.
Applications
Liquids have a variety of uses, as lubricants, solvents, and coolants. In hydraulic systems, liquid is used to transmit power.In tribology
Tribology
Tribology is the science and engineering of interacting surfaces in relative motion. It includes the study and application of the principles of friction, lubrication and wear...
, liquids are studied for their properties as lubricants. Lubricants such as oil
Oil
An oil is any substance that is liquid at ambient temperatures and does not mix with water but may mix with other oils and organic solvents. This general definition includes vegetable oils, volatile essential oils, petrochemical oils, and synthetic oils....
are chosen for viscosity
Viscosity
Viscosity is a measure of the resistance of a fluid which is being deformed by either shear or tensile stress. In everyday terms , viscosity is "thickness" or "internal friction". Thus, water is "thin", having a lower viscosity, while honey is "thick", having a higher viscosity...
and flow characteristics that are suitable throughout the operating temperature
Operating temperature
An operating temperature is the temperature at which an electrical or mechanical device operates. The device will operate effectively within a specified temperature range which varies based on the device function and application context, and ranges from the minimum operating temperature to the...
range of the component. Oils are often used in engine
Engine
An engine or motor is a machine designed to convert energy into useful mechanical motion. Heat engines, including internal combustion engines and external combustion engines burn a fuel to create heat which is then used to create motion...
s, gear boxes, metalworking
Metalworking
Metalworking is the process of working with metals to create individual parts, assemblies, or large scale structures. The term covers a wide range of work from large ships and bridges to precise engine parts and delicate jewelry. It therefore includes a correspondingly wide range of skills,...
, and hydraulic systems for their good lubrication properties.
Many liquids are used as solvents, to dissolve other liquids or solids. Solution
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
s are found in a wide variety of applications, including paint
Paint
Paint is any liquid, liquefiable, or mastic composition which after application to a substrate in a thin layer is converted to an opaque solid film. One may also consider the digital mimicry thereof...
s, sealant
Sealant
A sealant may be viscous material that has little or no flow characteristics and stay where they are applied or thin and runny so as to allow it to penetrate the substrate by means of capillary reaction...
s, and adhesive
Adhesive
An adhesive, or glue, is a mixture in a liquid or semi-liquid state that adheres or bonds items together. Adhesives may come from either natural or synthetic sources. The types of materials that can be bonded are vast but they are especially useful for bonding thin materials...
s. Naptha and acetone
Acetone
Acetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
are used frequently in industry to clean oil, grease, and tar from parts and machinery. Body fluids are water based solutions.
Surfactant
Surfactant
Surfactants are compounds that lower the surface tension of a liquid, the interfacial tension between two liquids, or that between a liquid and a solid...
s are commonly found in soap
Soap
In chemistry, soap is a salt of a fatty acid.IUPAC. "" Compendium of Chemical Terminology, 2nd ed. . Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford . XML on-line corrected version: created by M. Nic, J. Jirat, B. Kosata; updates compiled by A. Jenkins. ISBN...
s and detergent
Detergent
A detergent is a surfactant or a mixture of surfactants with "cleaning properties in dilute solutions." In common usage, "detergent" refers to alkylbenzenesulfonates, a family of compounds that are similar to soap but are less affected by hard water...
s. Solvents like alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
are often used as antimicrobial
Antimicrobial
An anti-microbial is a substance that kills or inhibits the growth of microorganisms such as bacteria, fungi, or protozoans. Antimicrobial drugs either kill microbes or prevent the growth of microbes...
s. They are found in cosmetics
Cosmetics
Cosmetics are substances used to enhance the appearance or odor of the human body. Cosmetics include skin-care creams, lotions, powders, perfumes, lipsticks, fingernail and toe nail polish, eye and facial makeup, towelettes, permanent waves, colored contact lenses, hair colors, hair sprays and...
, ink
Ink
Ink is a liquid or paste that contains pigments and/or dyes and is used to color a surface to produce an image, text, or design. Ink is used for drawing and/or writing with a pen, brush, or quill...
s, and liquid dye laser
Dye laser
A dye laser is a laser which uses an organic dye as the lasing medium, usually as a liquid solution. Compared to gases and most solid state lasing media, a dye can usually be used for a much wider range of wavelengths. The wide bandwidth makes them particularly suitable for tunable lasers and...
s. They are used in the food industry, in processes such as the extraction of vegetable oil.
Liquids tend to have better thermal conductivity
Thermal conductivity
In physics, thermal conductivity, k, is the property of a material's ability to conduct heat. It appears primarily in Fourier's Law for heat conduction....
than gases, and the ability to flow makes a liquid suitable for removing excess heat from mechanical components. The heat can be removed by channeling the liquid through a heat exchanger
Heat exchanger
A heat exchanger is a piece of equipment built for efficient heat transfer from one medium to another. The media may be separated by a solid wall, so that they never mix, or they may be in direct contact...
, such as a radiator
Radiator
Radiators are heat exchangers used to transfer thermal energy from one medium to another for the purpose of cooling and heating. The majority of radiators are constructed to function in automobiles, buildings, and electronics...
, or the heat can be removed with the liquid during evaporation
Evaporation
Evaporation is a type of vaporization of a liquid that occurs only on the surface of a liquid. The other type of vaporization is boiling, which, instead, occurs on the entire mass of the liquid....
. Water or glycol coolants are used to keep engine
Engine
An engine or motor is a machine designed to convert energy into useful mechanical motion. Heat engines, including internal combustion engines and external combustion engines burn a fuel to create heat which is then used to create motion...
s from overheating. The coolants used in nuclear reactor
Nuclear reactor
A nuclear reactor is a device to initiate and control a sustained nuclear chain reaction. Most commonly they are used for generating electricity and for the propulsion of ships. Usually heat from nuclear fission is passed to a working fluid , which runs through turbines that power either ship's...
s include water or liquid metals, such as sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
or bismuth
Bismuth
Bismuth is a chemical element with symbol Bi and atomic number 83. Bismuth, a trivalent poor metal, chemically resembles arsenic and antimony. Elemental bismuth may occur naturally uncombined, although its sulfide and oxide form important commercial ores. The free element is 86% as dense as lead...
. Liquid propellant films are used to cool the thrust chambers of rocket
Rocket
A rocket is a missile, spacecraft, aircraft or other vehicle which obtains thrust from a rocket engine. In all rockets, the exhaust is formed entirely from propellants carried within the rocket before use. Rocket engines work by action and reaction...
s. In machining
Machining
Conventional machining is a form of subtractive manufacturing, in which a collection of material-working processes utilizing power-driven machine tools, such as saws, lathes, milling machines, and drill presses, are used with a sharp cutting tool to physical remove material to achieve a desired...
, water and oils are used to remove the excess heat generated, which can quickly ruin both the work piece and the tooling. During perspiration, sweat removes heat from the human body by evaporating. In the heating, ventilation, and air-conditioning industry (HVAC), liquids such as water are used to transfer heat from one area to another.
Liquid is the primary component of hydraulic systems, which take advantage of Pascal's law
Pascal's law
In the physical sciences, Pascal's law or the Principle of transmission of fluid-pressure states that "pressure exerted anywhere in a confined incompressible fluid is transmitted equally in all directions throughout the fluid such that the pressure ratio remains the same." The law was established...
to provide fluid power
Fluid power
Fluid power is the use of fluids under pressure to generate, control, and transmit power. Fluid power is subdivided into hydraulics using a liquid such as mineral oil or water, and pneumatics using a gas such as air or other gases...
. Devices such as pump
Pump
A pump is a device used to move fluids, such as liquids, gases or slurries.A pump displaces a volume by physical or mechanical action. Pumps fall into three major groups: direct lift, displacement, and gravity pumps...
s and waterwheels have been used to change liquid motion into mechanical work
Mechanical work
In physics, work is a scalar quantity that can be described as the product of a force times the distance through which it acts, and it is called the work of the force. Only the component of a force in the direction of the movement of its point of application does work...
since ancient times. Oil
Oil
An oil is any substance that is liquid at ambient temperatures and does not mix with water but may mix with other oils and organic solvents. This general definition includes vegetable oils, volatile essential oils, petrochemical oils, and synthetic oils....
s are forced through hydraulic pump
Hydraulic pump
Hydraulic pumps are used in hydraulic drive systems and can be hydrostatic or hydrodynamic.Hydrostatic pumps are positive displacement pumps while hydrodynamic pumps can be fixed displacement pumps, in which the displacement cannot be adjusted, or variable displacement pumps, which have a more...
s, which transmit this force to hydraulic cylinder
Hydraulic cylinder
A Hydraulic cylinder is a mechanical actuator that is used to give a unidirectional force through a unidirectional stroke. It has many applications, notably in engineering vehicles.- Operation :...
s. Hydraulics can be found in many applications, such as automotive brakes and transmissions, heavy equipment, and airplane control systems. Various hydraulic press
Hydraulic press
A hydraulic is a machine using a hydraulic cylinder to generate a compressive force. It uses the hydraulic equivalenta mechanical lever, and was also known as a Bramah press after the inventor, Joseph Bramah, of England. He invented and was issued a patent on this press in 1795...
es are used extensively in repair and manufacturing, for lifting, pressing, clamping and forming.
Liquids are sometimes used in measuring devices. A thermometer
Thermometer
Developed during the 16th and 17th centuries, a thermometer is a device that measures temperature or temperature gradient using a variety of different principles. A thermometer has two important elements: the temperature sensor Developed during the 16th and 17th centuries, a thermometer (from the...
often uses the thermal expansion
Thermal expansion
Thermal expansion is the tendency of matter to change in volume in response to a change in temperature.When a substance is heated, its particles begin moving more and thus usually maintain a greater average separation. Materials which contract with increasing temperature are rare; this effect is...
of liquids, such as mercury
Mercury (element)
Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum...
, combined with their ability to flow to indicate temperature. A manometer uses the weight of the liquid to indicate air pressure.
Volume
Quantities of liquids are commonly measured in units of volumeVolume
Volume is the quantity of three-dimensional space enclosed by some closed boundary, for example, the space that a substance or shape occupies or contains....
. These include the SI
International System of Units
The International System of Units is the modern form of the metric system and is generally a system of units of measurement devised around seven base units and the convenience of the number ten. The older metric system included several groups of units...
unit cubic metre
Cubic metre
The cubic metre is the SI derived unit of volume. It is the volume of a cube with edges one metre in length. An alternative name, which allowed a different usage with metric prefixes, was the stère...
(m3) and its divisions, in particular the cubic decimetre, more commonly called the litre
Litre
pic|200px|right|thumb|One litre is equivalent to this cubeEach side is 10 cm1 litre water = 1 kilogram water The litre is a metric system unit of volume equal to 1 cubic decimetre , to 1,000 cubic centimetres , and to 1/1,000 cubic metre...
(1 dm3 = 1 L = 0.001 m3), and the cubic centimetre, also called millilitre (1 cm3 = 1 mL = 0.001 L = 10−6 m3).
The volume
Volume
Volume is the quantity of three-dimensional space enclosed by some closed boundary, for example, the space that a substance or shape occupies or contains....
of a quantity of liquid is fixed by its temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
and pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
. Liquids generally expand when heated, and contract when cooled. Water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
between 0 °C and 4 °C is a notable exception.
Liquids have little compressibility: water, for example, requires a pressure of the order of 200 bar to increase its density
Density
The mass density or density of a material is defined as its mass per unit volume. The symbol most often used for density is ρ . In some cases , density is also defined as its weight per unit volume; although, this quantity is more properly called specific weight...
by 1/1000. In the study of fluid dynamics
Fluid dynamics
In physics, fluid dynamics is a sub-discipline of fluid mechanics that deals with fluid flow—the natural science of fluids in motion. It has several subdisciplines itself, including aerodynamics and hydrodynamics...
, liquids are often treated as incompressible, especially when studying incompressible flow
Incompressible flow
In fluid mechanics or more generally continuum mechanics, incompressible flow refers to flow in which the material density is constant within an infinitesimal volume that moves with the velocity of the fluid...
.
Pressure and buoyancy
In a gravitational fieldGravitational field
The gravitational field is a model used in physics to explain the existence of gravity. In its original concept, gravity was a force between point masses...
, liquids exert pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
on the sides of a container as well as on anything within the liquid itself. This pressure is transmitted in all directions and increases with depth. If a liquid is at rest in a uniform gravitational field, the pressure, p, at any depth, z, is given by
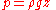
where:
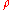 is the density
is the densityDensity
The mass density or density of a material is defined as its mass per unit volume. The symbol most often used for density is ρ . In some cases , density is also defined as its weight per unit volume; although, this quantity is more properly called specific weight...
of the liquid (assumed constant)
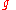 is the gravitational acceleration.
is the gravitational acceleration.Note that this formula assumes that the pressure at the free surface is zero, and that surface tension
Surface tension
Surface tension is a property of the surface of a liquid that allows it to resist an external force. It is revealed, for example, in floating of some objects on the surface of water, even though they are denser than water, and in the ability of some insects to run on the water surface...
effects may be neglected.
Objects immersed in liquids are subject to the phenomenon of buoyancy
Buoyancy
In physics, buoyancy is a force exerted by a fluid that opposes an object's weight. In a column of fluid, pressure increases with depth as a result of the weight of the overlying fluid. Thus a column of fluid, or an object submerged in the fluid, experiences greater pressure at the bottom of the...
. (Buoyancy is also observed in other fluids, but is especially strong in liquids due to their high density.)
Surfaces

Surface tension
Surface tension is a property of the surface of a liquid that allows it to resist an external force. It is revealed, for example, in floating of some objects on the surface of water, even though they are denser than water, and in the ability of some insects to run on the water surface...
appears, allowing the formation of drops
Drop (liquid)
A drop or droplet is a small column of liquid, bounded completely or almost completely by free surfaces. A drop may form when liquid accumulates at the lower end of a tube or other surface boundary, producing a hanging drop called a pendant drop...
and bubble
Liquid bubble
A bubble is a globule of one substance in another, usually gas in a liquid.Due to the Marangoni effect, bubbles may remain intact when they reach the surface of the immersive substance.-Common examples:...
s. Surface wave
Surface wave
In physics, a surface wave is a mechanical wave that propagates along the interface between differing media, usually two fluids with different densities. A surface wave can also be an electromagnetic wave guided by a refractive index gradient...
s, capillary action
Capillary action
Capillary action, or capilarity, is the ability of a liquid to flow against gravity where liquid spontanously rise in a narrow space such as between the hair of a paint-brush, in a thin tube, or in porous material such as paper or in some non-porous material such as liquified carbon fiber, or in a...
, wetting
Wetting
Wetting is the ability of a liquid to maintain contact with a solid surface, resulting from intermolecular interactions when the two are brought together. The degree of wetting is determined by a force balance between adhesive and cohesive forces.Wetting is important in the bonding or adherence of...
, and ripples are other consequences of surface tension
Surface tension
Surface tension is a property of the surface of a liquid that allows it to resist an external force. It is revealed, for example, in floating of some objects on the surface of water, even though they are denser than water, and in the ability of some insects to run on the water surface...
.
Flow
ViscosityViscosity
Viscosity is a measure of the resistance of a fluid which is being deformed by either shear or tensile stress. In everyday terms , viscosity is "thickness" or "internal friction". Thus, water is "thin", having a lower viscosity, while honey is "thick", having a higher viscosity...
measures the resistance of a liquid which is being deformed by either shear stress or extensional stress.
When a liquid is supercooled
Supercooling
Supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point without it becoming a solid....
towards the glass transition
Glass transition
The liquid-glass transition is the reversible transition in amorphous materials from a hard and relatively brittle state into a molten or rubber-like state. An amorphous solid that exhibits a glass transition is called a glass...
, the viscosity increases dramatically. The liquid then becomes a viscoelastic
Viscoelasticity
Viscoelasticity is the property of materials that exhibit both viscous and elastic characteristics when undergoing deformation. Viscous materials, like honey, resist shear flow and strain linearly with time when a stress is applied. Elastic materials strain instantaneously when stretched and just...
medium that shows both the elasticity
Elasticity
Elasticity may refer to:*Elasticity , continuum mechanics of bodies that deform reversibly under stressNumerous uses are derived from this physical sense of the term, which is inherently mathematical, such as used in Engineering, Chemistry, Construction and variously in Economics:*Elasticity , the...
of a solid and the fluidity of a liquid, depending on the time scale of observation or on the frequency of perturbation.
Sound propagation
In a fluid the only non-zero stiffnessStiffness
Stiffness is the resistance of an elastic body to deformation by an applied force along a given degree of freedom when a set of loading points and boundary conditions are prescribed on the elastic body.-Calculations:...
is to volumetric deformation (a fluid does not sustain shear forces). Hence the speed of sound in a fluid is given by
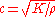 where K is the bulk modulus
where K is the bulk modulusBulk modulus
The bulk modulus of a substance measures the substance's resistance to uniform compression. It is defined as the pressure increase needed to decrease the volume by a factor of 1/e...
of the fluid, and ρ the density. To give a typical value, in fresh water c=1497 m/s at 25 °C.
Phase transitions

Boiling point
The boiling point of an element or a substance is the temperature at which the vapor pressure of the liquid equals the environmental pressure surrounding the liquid....
, any matter in liquid form will evaporate until the condensation of gas above reach an equilibrium. At this point the gas will condense at the same rate as the liquid evaporates. Thus, a liquid cannot exist permanently if the evaporated liquid is continually removed. A liquid at its boiling point
Boiling point
The boiling point of an element or a substance is the temperature at which the vapor pressure of the liquid equals the environmental pressure surrounding the liquid....
will evaporate more quickly than the gas can condense at the current pressure. A liquid at or above its boiling point will normally boil, though superheating
Superheating
In physics, superheating is the phenomenon in which a liquid is heated to a temperature higher than its boiling point, without boiling...
can prevent this in certain circumstances.
At a temperature below the freezing point, a liquid will tend to crystallize
Crystallization
Crystallization is the process of formation of solid crystals precipitating from a solution, melt or more rarely deposited directly from a gas. Crystallization is also a chemical solid–liquid separation technique, in which mass transfer of a solute from the liquid solution to a pure solid...
, changing to its solid form. Unlike the transition to gas, there is no equilibrium at this transition under constant pressure, so unless supercooling
Supercooling
Supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point without it becoming a solid....
occurs, the liquid will eventually completely crystallize. Note that this is only true under constant pressure, so e.g. water and ice in a closed, strong container might reach an equilibrium where both phases coexist. For the opposite transition from solid to liquid, see melting
Melting
Melting, or fusion, is a physical process that results in the phase change of a substance from a solid to a liquid. The internal energy of a substance is increased, typically by the application of heat or pressure, resulting in a rise of its temperature to the melting point, at which the rigid...
.
Solutions
Liquids can display immiscibility. The most familiar mixture of two immiscible liquids in everyday life is the vegetable oil and waterWater
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
in Italian salad dressing
Italian dressing
Italian dressing is a vinaigrette-type salad dressing in United States and Canadian cuisine, consisting of water, vinegar or lemon juice, vegetable oil, chopped bell peppers, usually sugar or corn syrup, and a various herbs and spices including oregano, garlic, fennel, dill and salt. Onion and...
. A familiar set of miscible liquids is water and alcohol. Liquid components in a mixture can often be separated from one another via fractional distillation
Fractional distillation
Fractional distillation is the separation of a mixture into its component parts, or fractions, such as in separating chemical compounds by their boiling point by heating them to a temperature at which several fractions of the compound will evaporate. It is a special type of distillation...
.
Static structure factor
In a liquid, atoms do not form a crystalline lattice, nor do they show any other form of long-range order. This is evidenced by the absence of Bragg peak
Bragg Peak
The Bragg peak is a pronounced peak on the Bragg curve which plots the energy loss of ionizing radiation during its travel through matter. For protons, α-rays, and other ion rays, the peak occurs immediately before the particles come to rest...
s in X-ray and neutron diffraction
Neutron diffraction
Neutron diffraction or elastic neutron scattering is the application of neutron scattering to the determination of the atomic and/or magnetic structure of a material: A sample to be examined is placed in a beam of thermal or cold neutrons to obtain a diffraction pattern that provides information of...
. Under normal conditions, the diffraction pattern has circular symmetry, expressing the isotropy
Isotropy
Isotropy is uniformity in all orientations; it is derived from the Greek iso and tropos . Precise definitions depend on the subject area. Exceptions, or inequalities, are frequently indicated by the prefix an, hence anisotropy. Anisotropy is also used to describe situations where properties vary...
of the liquid. In radial direction, the diffraction intensity smoothly oscillates. This is usually described by the static structure factor S(q), with wavenumber q=(4π/λ)sinθ given by the wavelength λ of the probe (photon or neutron) and the Bragg angle θ. The oscillations of S(q) express the near order of the liquid, i.e. the correlations between an atom and a few shells of nearest, second nearest, ... neighbors.
A more intuitive description of these correlations is given by the radial distribution function
Radial distribution function
In statistical mechanics, a radial distribution function , g, describes how the atomic density varies as a function of the distance from one particular atom....
g(r), which is basically the Fourier transform
Fourier transform
In mathematics, Fourier analysis is a subject area which grew from the study of Fourier series. The subject began with the study of the way general functions may be represented by sums of simpler trigonometric functions...
of S(q). It represents a spatial average of a temporal snapshot of pair correlations in the liquid.
Sound dispersion and structural relaxation
The above expression for the sound velocity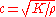 contains the bulk modulus
contains the bulk modulusBulk modulus
The bulk modulus of a substance measures the substance's resistance to uniform compression. It is defined as the pressure increase needed to decrease the volume by a factor of 1/e...
K. If K is frequency independent then the liquid behaves as a linear medium
Linear medium
A linear medium is any medium which is intended to be written to or accessed in a linear fashion, literally meaning in a line.This means that the information is written to or read from the medium in a given order, so for example a book containing a novel is intended to be read from front to back,...
, so that sound propagates without dissipation
Dissipation
In physics, dissipation embodies the concept of a dynamical system where important mechanical models, such as waves or oscillations, lose energy over time, typically from friction or turbulence. The lost energy converts into heat, which raises the temperature of the system. Such systems are called...
and without mode coupling
Mode coupling
In the term mode coupling, as used in physics and electrical engineering, the word "mode" refers to eigenmodes of an idealized, "unperturbed", linear system. Most often, these eigenmodes are plane waves...
. In reality, any liquid shows some dispersion
Dispersion
Dispersion may refer to:In physics:*The dependence of wave velocity on frequency or wavelength:**Dispersion , for light waves**Dispersion **Acoustic dispersion, for sound waves...
: with increasing frequency, K crosses over from the low-frequency, liquid-like limit
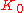 to the high-frequency, solid-like limit
to the high-frequency, solid-like limit 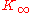 . In normal liquids, most of this cross over takes place at frequencies between GHz and THz, sometimes called hypersound.
. In normal liquids, most of this cross over takes place at frequencies between GHz and THz, sometimes called hypersound.At sub-GHz frequencies, a normal liquid cannot sustain shear waves: the zero-frequency limit of the shear modulus is
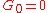 . This is sometimes seen as the defining property of a liquid.
. This is sometimes seen as the defining property of a liquid.However, just as the bulk modulus K, the shear modulus G is frequency dependent,
and at hypersound frequencies it shows a similar cross over from the liquid-like limit
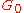 to a solid-like, non-zero limit
to a solid-like, non-zero limit 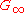 .
.According to the Kramers-Kronig relation
Kramers-Kronig relation
The Kramers–Kronig relations are bidirectional mathematical relations, connecting the real and imaginary parts of any complex function that is analytic in the upper half-plane...
, the dispersion in the sound velocity (given by the real part of K or G) goes along with a maximum in the sound attenuation (dissipation, given by the imaginary part of K or G). According to linear response theory, the Fourier transform of K or G describes how the system returns to equilibrium after an external perturbation; for this reason, the dispersion step in the GHz..THz region is also called structural relaxation. According the fluctuation-dissipation theorem, relaxation towards equilibrium is intimately connected to fluctuations in equilibrium. The density fluctuations associated with sound waves can be experimentally observed by Brillouin scattering
Brillouin scattering
Brillouin scattering, named after Léon Brillouin, occurs when light in a medium interacts with time dependent optical density variations and changes its energy and path. The density variations may be due to acoustic modes, such as phonons, magnetic modes, such as magnons, or temperature gradients...
.
On supercooling a liquid towards the glass transition, the crossover from liquid-like to solid-like response moves from GHz to MHz, kHz, Hz, ...; equivalently, the characteristic time of structural relaxation increases from ns to μs, ms, s, ... This is the microscopic explanation for the above mentioned viscoelastic behaviour of glass-forming liquids.
Effects of association
The mechanisms of atomic/molecular diffusionDiffusion
Molecular diffusion, often called simply diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size of the particles...
(or particle displacement
Particle displacement
Particle displacement or particle amplitude is a measurement of distance of the movement of a particle from its equilibrium position in a medium as it transmits a wave....
) in solids are closely related to the mechanisms of viscous flow and solidification in liquid materials. Descriptions of viscosity
Viscosity
Viscosity is a measure of the resistance of a fluid which is being deformed by either shear or tensile stress. In everyday terms , viscosity is "thickness" or "internal friction". Thus, water is "thin", having a lower viscosity, while honey is "thick", having a higher viscosity...
in terms of molecular "free space" within the liquid
were modified as needed in order to account for liquids whose molecules are known to be "associated" in the liquid state at ordinary temperatures. When various molecules combine together to form an associated molecule, they enclose within a semi-rigid system a certain amount of space which before was available as free space for mobile molecules. Thus, increase in viscosity upon cooling due to the tendency of most substances to become associated on cooling.
Similar arguments could be used to describe the effects of pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
on viscosity, where it may be assumed that the viscosity is chiefly a function of the volume for liquids with a finite compressibility. An increasing viscosity with rise of pressure is therefore expected. In addition, if the volume is expanded by heat but reduced again by pressure, the viscosity remains the same.
The local tendency to orientation of molecules in small groups lends the liquid (as referred to previously) a certain degree of association. This association results in a considerable "internal pressure" within a liquid, which is due almost entirely to those molecules which, on account of their temporary low velocities (following the Maxwell distribution) have coalesced with other molecules. The internal pressure between several such molecules might correspond to that between a group of molecules in the solid form.

