
Bromine
Encyclopedia
Bromine is a chemical element
with the symbol Br, an atomic number
of 35, and an atomic mass
of 79.904. It is in the halogen
element group. The element was isolated independently by two chemists, Carl Jacob Löwig
and Antoine Jerome Balard
, in 1825–1826. Elemental bromine is a fuming red-brown liquid at room temperature, corrosive and toxic, with properties between those of chlorine
and iodine
. Free bromine does not occur in nature, but occurs as colorless soluble crystalline mineral halide salts, analogous to table salt.
Bromine is rarer than about three-quarters of elements in the Earth's crust, however the high solubility of bromide ion has caused its accumulation in the oceans, and commercially the element is easily extracted from brine pools, mostly in the United States, Israel and China. About 556,000 tonne
s were produced in 2007, an amount similar to the far more abundant element magnesium
.
At high temperatures, organobromine compound
s are easily converted to free bromine atoms, a process which acts to terminate free radical chemical chain reaction
s. This makes such compounds useful fire retardant
s and this is bromine's primary industrial use, consuming more than half of world production of the element. The same property allows volatile organobromine compounds, under the action of sunlight, to form free bromine atoms in the atmosphere which are highly effective in ozone depletion
. This unwanted side-effect has caused many common volatile brominated organics like methyl bromide, a pesticide that was formerly a large industrial bromine consumer, to be abandoned. Remaining uses of bromine compounds are in well-drilling fluids, as an intermediate in manufacture of organic chemicals, and in film photography.
Bromine has no essential function in mammals, though it is preferentially used over chloride by one antiparasitic enzyme in the human immune system. Organobromides are needed and produced enzymatically from bromide by some lower life forms in the sea, particularly algae
, and the ash of seaweed was one source of bromine's discovery. As a pharmaceutical, simple bromide ion, Br–, has inhibitory effects on the central nervous system, and bromide salts were once a major medical sedative, before being replaced by shorter-acting drugs. They retain niche uses as antiepileptics.
 Elemental bromine exists as a diatomic molecule, Br2. It is a dense, mobile, slightly transparent reddish-brown liquid, that evaporates easily at standard temperature and pressures to give an orange vapor (its color resembles nitrogen dioxide
Elemental bromine exists as a diatomic molecule, Br2. It is a dense, mobile, slightly transparent reddish-brown liquid, that evaporates easily at standard temperature and pressures to give an orange vapor (its color resembles nitrogen dioxide
) that has a strongly disagreeable odor resembling that of chlorine
. It is one of only two elements on the periodic table that are liquids at room temperature (mercury
is the other, although caesium
, gallium
, and rubidium
melt just above room temperature).
At a pressure of 55 GPa bromine converts to a metal. At 75 GPa it converts to a face centered orthorhombic structure. At 100 GPa it converts to a body centered orthorhombic monoatomic form.
but more reactive than iodine
, bromine reacts vigorously with metals, especially in the presence of water, to give bromide salts. It is also reactive toward most organic compounds, especially upon illumination
, conditions that favor the dissociation of the diatomic molecule into bromine radicals:
It bonds
easily with many elements and has a strong bleaching action.
Bromine is slightly soluble
in water, but it is highly soluble in organic solvents such as carbon disulfide
, carbon tetrachloride
, aliphatic alcohol
s, and acetic acid
.
s, 79Br (50.69 %) and 81Br (49.31%). At least 23 other radioisotopes are known. Many of the bromine isotopes are fission products. Several of the heavier bromine isotopes from fission are delayed neutron emitters. All of the radioactive bromine isotopes are relatively short lived. The longest half-life is the neutron deficient 77Br at 2.376 days. The longest half-life on the neutron rich side is 82Br at 1.471 days. A number of the bromine isotopes exhibit metastable isomers. Stable 79Br exhibits a radioactive isomer
, with a half-life of 4.86 seconds. It decays by isomeric transition to the stable ground state.
and Antoine Balard
, in 1825 and 1826, respectively.
Balard found bromide chemicals in the ash of seaweed
from the salt marsh
es of Montpellier
. The seaweed was used to produce iodine, but also contained bromine. Balard distilled the bromine from a solution of seaweed ash saturated with chlorine. The properties of the resulting substance resembled that of an intermediate of chlorine and iodine; with those results he tried to prove that the substance was iodine monochloride
(ICl), but after failing to do so he was sure that he had found a new element and named it muride, derived from the Latin
word muria for brine.
Löwig isolated bromine from a mineral water spring from his hometown Bad Kreuznach
in 1825. Löwig used a solution of the mineral salt saturated with chlorine and extracted the bromine with diethyl ether
. After evaporation of the ether a brown liquid remained. With this liquid as a sample for his work he applied for a position in the laboratory of Leopold Gmelin
in Heidelberg
. The publication of the results was delayed and Balard published his results first.
After the French chemists Louis Nicolas Vauquelin
, Louis Jacques Thénard
, and Joseph-Louis Gay-Lussac approved the experiments of the young pharmacist Balard, the results were presented at a lecture of the Académie des Sciences and published in Annales de Chimie et Physique. In his publication Balard states that he changed the name from muride to brôme on the proposal of M. Anglada. (Brôme (bromine) derives from the Greek βρωμος (stench).) Other sources claim that the French chemist and physicist Joseph-Louis Gay-Lussac suggested the name brôme for the characteristic smell of the vapors. Bromine was not produced in large quantities until 1860.
The first commercial use, besides some minor medical applications, was the use of bromine for the daguerreotype
. In 1840 it was discovered that bromine had some advantages over the previously used iodine vapor to create the light sensitive silver halide
layer used for daguerreotypy.
Potassium bromide
and sodium bromide
were used as anticonvulsant
s and sedative
s in the late 19th and early 20th centuries, until they were gradually superseded by chloral hydrate
and then the barbiturate
s.
 The diatomic element Br2 does not occur naturally. Instead, bromine exists exclusively as bromide salts
The diatomic element Br2 does not occur naturally. Instead, bromine exists exclusively as bromide salts
in diffuse amounts in crustal
rock. Owing to leaching
, bromide salts have accumulated in sea water at 65 part per million (ppm), which is less than chloride. Bromine may be economically recovered from bromide-rich brine wells and from the Dead Sea
waters (up to 50,000 ppm). It exists in the Earth's crust at an average concentration of 0.4 ppm, making it the 62nd most abundant element
. The bromine concentration in soils varies normally between 5 and 40 ppm, but some volcanic soils can contain up to 500 ppm. The concentration of bromine in the atmosphere is extremely low, at only a few ppt. A large number of organobromine compounds are found in small amounts in nature.
China's bromine reserves are located in the Shandong Province and Israel's bromine reserves are contained in the waters of the Dead Sea
. The largest bromine reserve in the United States is located in Columbia County
and Union County, Arkansas, U.S.
(226,000 t) and Israel
(210,000 t). US production was excluded from the United States Geological Survey
after 2007, and from the 380,000 tonnes mined by other countries in 2010, 140,000 t were produced by China, 130,000 t by Israel and 80,000 t by Jordan.
Bromide-rich brines are treated with chlorine gas, flushing through with air. In this treatment, bromide anions are oxidized to bromine by the chlorine gas.
with concentrated sulfuric acid
(H2SO4). The first stage is formation of hydrogen bromide
(HBr), which is a gas, but under the reaction conditions some of the HBr is oxidized further by the sulfuric acid to form bromine (Br2) and sulfur dioxide
(SO2).
Non oxidizing acid alternatives, such as the use of dilute hydrobromic acid
with sodium hypobromite
, are also available, as the hypobromous acid
formed from them is unstable in the presence of bromide, being reduced by it according to the reaction:
The reactions are the reverse of disproportionation
reactions of elemental bromine in base, and are called comproportionation. A similar reaction happens with sodium hypochlorite, acid, and chloride, leading to elemental chlorine.
Reactions involving an oxidizing agent, such as potassium permanganate or manganese dioxide, on bromide ions in the presence of an acid, also give bromine in the reactions analogous to the formation of elemental chlorine and iodine from an acid and oxidant.
Like iodine, bromine is soluble in chloroform but only slightly soluble in water. In water, the solubility can be increased by the presence of bromide ions. Concentrated solutions of bromine are rarely prepared in the lab because of hazards. As is the case with chlorine solutions or iodine solutions, sodium thiosulphate (or any soluble thiosulphate) is an effective reagent for reducing bromine to colorless odorless bromide, thus dealing with stains and odor from the element in unwanted places. For the same reason, thiosulfate ("fixer's hypo") is used in photography to deal with free bromine in silver bromide film emulsions.
 Organic compounds are brominated by either addition
Organic compounds are brominated by either addition
or substitution reactions. Bromine undergoes electrophilic addition to the double-bonds of alkene
s, via a cyclic bromonium intermediate. In non-aqueous solvents such as carbon disulfide
, this yields the di-bromo product. For example, reaction with ethylene
will produce 1,2-dibromoethane
. Bromine also undergoes electrophilic addition to phenol
s and aniline
s. When used as bromine water, a small amount of the corresponding bromohydrin is formed
as well as the dibromo compound. So reliable is the reactivity of bromine that bromine water is employed as a reagent to test for the presence of alkenes, phenols, and anilines. Like the other halogens, bromine participates in free radical reaction
s. For example, hydrocarbons are brominated upon treatment with bromine in the presence of light.
Bromine, sometimes with a catalytic amount of phosphorus
, easily brominates carboxylic acid
s at the α-position. This method, the Hell-Volhard-Zelinsky reaction, is the basis of the commercial route to bromoacetic acid
. N-Bromosuccinimide
is commonly used as a substitute for elemental bromine, being easier to handle, and reacting more mildly and thus more selectively. Organic bromides are often preferable relative to the less reactive chlorides and more expensive iodide-containing reagents. Thus, Grignard
and organolithium compound are most often generated from the corresponding bromides.
Certain bromine-related compounds have been evaluated to have an ozone depletion potential
or bioaccumulate in living organisms. As a result, many industrial bromine compounds are no longer manufactured, are being restricted, or scheduled for phasing out. The Montreal Protocol
mentions several organobromine compounds for this phase out.
of bromine
|-
| −1 ||
|-
| 0 ||
|-
| +1 ||
|-
| +3 ||
|-
| +5 ||
|-
| +5 ||
|-
| +7 ||
|}
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
with the symbol Br, an atomic number
Atomic number
In chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element...
of 35, and an atomic mass
Atomic mass
The atomic mass is the mass of a specific isotope, most often expressed in unified atomic mass units. The atomic mass is the total mass of protons, neutrons and electrons in a single atom....
of 79.904. It is in the halogen
Halogen
The halogens or halogen elements are a series of nonmetal elements from Group 17 IUPAC Style of the periodic table, comprising fluorine , chlorine , bromine , iodine , and astatine...
element group. The element was isolated independently by two chemists, Carl Jacob Löwig
Carl Jacob Löwig
Carl Jacob Löwig was a German chemist and discovered bromine independently of Antoine Jérôme Balard.He received his PhD at the University of Heidelberg for his work with Leopold Gmelin....
and Antoine Jerome Balard
Antoine Jérôme Balard
-External links:* , PasteurBrewing.com...
, in 1825–1826. Elemental bromine is a fuming red-brown liquid at room temperature, corrosive and toxic, with properties between those of chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
and iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
. Free bromine does not occur in nature, but occurs as colorless soluble crystalline mineral halide salts, analogous to table salt.
Bromine is rarer than about three-quarters of elements in the Earth's crust, however the high solubility of bromide ion has caused its accumulation in the oceans, and commercially the element is easily extracted from brine pools, mostly in the United States, Israel and China. About 556,000 tonne
Tonne
The tonne, known as the metric ton in the US , often put pleonastically as "metric tonne" to avoid confusion with ton, is a metric system unit of mass equal to 1000 kilograms. The tonne is not an International System of Units unit, but is accepted for use with the SI...
s were produced in 2007, an amount similar to the far more abundant element magnesium
Magnesium
Magnesium is a chemical element with the symbol Mg, atomic number 12, and common oxidation number +2. It is an alkaline earth metal and the eighth most abundant element in the Earth's crust and ninth in the known universe as a whole...
.
At high temperatures, organobromine compound
Organobromine compound
Organobromine compounds are organic compounds that contain carbon bonded to bromine. The most pervasive is the naturally produced bromomethane. One prominent application is the use of polybrominated diphenyl ethers as fire-retardants. A variety of minor organobromine compounds are found in...
s are easily converted to free bromine atoms, a process which acts to terminate free radical chemical chain reaction
Chain reaction
A chain reaction is a sequence of reactions where a reactive product or by-product causes additional reactions to take place. In a chain reaction, positive feedback leads to a self-amplifying chain of events....
s. This makes such compounds useful fire retardant
Fire retardant
A fire retardant is a substance other than water that reduces flammability of fuels or delays their combustion. This typically refers to chemical retardants but may also include substances that work by physical action, such as cooling the fuels; examples of these include fire-fighting foams and...
s and this is bromine's primary industrial use, consuming more than half of world production of the element. The same property allows volatile organobromine compounds, under the action of sunlight, to form free bromine atoms in the atmosphere which are highly effective in ozone depletion
Ozone depletion
Ozone depletion describes two distinct but related phenomena observed since the late 1970s: a steady decline of about 4% per decade in the total volume of ozone in Earth's stratosphere , and a much larger springtime decrease in stratospheric ozone over Earth's polar regions. The latter phenomenon...
. This unwanted side-effect has caused many common volatile brominated organics like methyl bromide, a pesticide that was formerly a large industrial bromine consumer, to be abandoned. Remaining uses of bromine compounds are in well-drilling fluids, as an intermediate in manufacture of organic chemicals, and in film photography.
Bromine has no essential function in mammals, though it is preferentially used over chloride by one antiparasitic enzyme in the human immune system. Organobromides are needed and produced enzymatically from bromide by some lower life forms in the sea, particularly algae
Algae
Algae are a large and diverse group of simple, typically autotrophic organisms, ranging from unicellular to multicellular forms, such as the giant kelps that grow to 65 meters in length. They are photosynthetic like plants, and "simple" because their tissues are not organized into the many...
, and the ash of seaweed was one source of bromine's discovery. As a pharmaceutical, simple bromide ion, Br–, has inhibitory effects on the central nervous system, and bromide salts were once a major medical sedative, before being replaced by shorter-acting drugs. They retain niche uses as antiepileptics.
Physical

Nitrogen dioxide
Nitrogen dioxide is the chemical compound with the formula it is one of several nitrogen oxides. is an intermediate in the industrial synthesis of nitric acid, millions of tons of which are produced each year. This reddish-brown toxic gas has a characteristic sharp, biting odor and is a prominent...
) that has a strongly disagreeable odor resembling that of chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
. It is one of only two elements on the periodic table that are liquids at room temperature (mercury
Mercury (element)
Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum...
is the other, although caesium
Caesium
Caesium or cesium is the chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-gold alkali metal with a melting point of 28 °C , which makes it one of only five elemental metals that are liquid at room temperature...
, gallium
Gallium
Gallium is a chemical element that has the symbol Ga and atomic number 31. Elemental gallium does not occur in nature, but as the gallium salt in trace amounts in bauxite and zinc ores. A soft silvery metallic poor metal, elemental gallium is a brittle solid at low temperatures. As it liquefies...
, and rubidium
Rubidium
Rubidium is a chemical element with the symbol Rb and atomic number 37. Rubidium is a soft, silvery-white metallic element of the alkali metal group. Its atomic mass is 85.4678. Elemental rubidium is highly reactive, with properties similar to those of other elements in group 1, such as very rapid...
melt just above room temperature).
At a pressure of 55 GPa bromine converts to a metal. At 75 GPa it converts to a face centered orthorhombic structure. At 100 GPa it converts to a body centered orthorhombic monoatomic form.
Chemical
Being less reactive than chlorineChlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
but more reactive than iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
, bromine reacts vigorously with metals, especially in the presence of water, to give bromide salts. It is also reactive toward most organic compounds, especially upon illumination
Photochemistry
Photochemistry, a sub-discipline of chemistry, is the study of chemical reactions that proceed with the absorption of light by atoms or molecules.. Everyday examples include photosynthesis, the degradation of plastics and the formation of vitamin D with sunlight.-Principles:Light is a type of...
, conditions that favor the dissociation of the diatomic molecule into bromine radicals:
- Br2 2 Br·
It bonds
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
easily with many elements and has a strong bleaching action.
Bromine is slightly soluble
Solubility
Solubility is the property of a solid, liquid, or gaseous chemical substance called solute to dissolve in a solid, liquid, or gaseous solvent to form a homogeneous solution of the solute in the solvent. The solubility of a substance fundamentally depends on the used solvent as well as on...
in water, but it is highly soluble in organic solvents such as carbon disulfide
Carbon disulfide
Carbon disulfide is a colorless volatile liquid with the formula CS2. The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical non-polar solvent...
, carbon tetrachloride
Carbon tetrachloride
Carbon tetrachloride, also known by many other names is the organic compound with the formula CCl4. It was formerly widely used in fire extinguishers, as a precursor to refrigerants, and as a cleaning agent...
, aliphatic alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s, and acetic acid
Acetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
.
Isotopes
Bromine has two stable isotopeIsotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
s, 79Br (50.69 %) and 81Br (49.31%). At least 23 other radioisotopes are known. Many of the bromine isotopes are fission products. Several of the heavier bromine isotopes from fission are delayed neutron emitters. All of the radioactive bromine isotopes are relatively short lived. The longest half-life is the neutron deficient 77Br at 2.376 days. The longest half-life on the neutron rich side is 82Br at 1.471 days. A number of the bromine isotopes exhibit metastable isomers. Stable 79Br exhibits a radioactive isomer
Nuclear isomer
A nuclear isomer is a metastable state of an atomic nucleus caused by the excitation of one or more of its nucleons . "Metastable" refers to the fact that these excited states have half-lives more than 100 to 1000 times the half-lives of the other possible excited nuclear states...
, with a half-life of 4.86 seconds. It decays by isomeric transition to the stable ground state.
History
Bromine was discovered independently by two chemists, Carl Jacob LöwigCarl Jacob Löwig
Carl Jacob Löwig was a German chemist and discovered bromine independently of Antoine Jérôme Balard.He received his PhD at the University of Heidelberg for his work with Leopold Gmelin....
and Antoine Balard
Antoine Jérôme Balard
-External links:* , PasteurBrewing.com...
, in 1825 and 1826, respectively.
Balard found bromide chemicals in the ash of seaweed
Seaweed
Seaweed is a loose, colloquial term encompassing macroscopic, multicellular, benthic marine algae. The term includes some members of the red, brown and green algae...
from the salt marsh
Salt marsh
A salt marsh is an environment in the upper coastal intertidal zone between land and salt water or brackish water, it is dominated by dense stands of halophytic plants such as herbs, grasses, or low shrubs. These plants are terrestrial in origin and are essential to the stability of the salt marsh...
es of Montpellier
Montpellier
-Neighbourhoods:Since 2001, Montpellier has been divided into seven official neighbourhoods, themselves divided into sub-neighbourhoods. Each of them possesses a neighbourhood council....
. The seaweed was used to produce iodine, but also contained bromine. Balard distilled the bromine from a solution of seaweed ash saturated with chlorine. The properties of the resulting substance resembled that of an intermediate of chlorine and iodine; with those results he tried to prove that the substance was iodine monochloride
Iodine monochloride
Iodine monochloride is an interhalogen compound with the formula ICl. It is a red-brown compound that melts near room temperature. Because of the difference in the electronegativity of iodine and chlorine, ICl is highly polar and behaves as a source of I+....
(ICl), but after failing to do so he was sure that he had found a new element and named it muride, derived from the Latin
Latin
Latin is an Italic language originally spoken in Latium and Ancient Rome. It, along with most European languages, is a descendant of the ancient Proto-Indo-European language. Although it is considered a dead language, a number of scholars and members of the Christian clergy speak it fluently, and...
word muria for brine.
Löwig isolated bromine from a mineral water spring from his hometown Bad Kreuznach
Bad Kreuznach
Bad Kreuznach is the capital of the district of Bad Kreuznach, Rhineland-Palatinate, Germany. It is located on the Nahe river, a tributary of the Rhine...
in 1825. Löwig used a solution of the mineral salt saturated with chlorine and extracted the bromine with diethyl ether
Diethyl ether
Diethyl ether, also known as ethyl ether, simply ether, or ethoxyethane, is an organic compound in the ether class with the formula . It is a colorless, highly volatile flammable liquid with a characteristic odor...
. After evaporation of the ether a brown liquid remained. With this liquid as a sample for his work he applied for a position in the laboratory of Leopold Gmelin
Leopold Gmelin
Leopold Gmelin was a German chemist.Gmelin was the son of Johann Friedrich Gmelin. He studied medicine and chemistry at Göttingen, Tübingen and Vienna, and in 1813 began to lecture on chemistry at Heidelberg, where in 1814 he was appointed extraordinary-, and in 1817 ordinary-, professor of...
in Heidelberg
Heidelberg
-Early history:Between 600,000 and 200,000 years ago, "Heidelberg Man" died at nearby Mauer. His jaw bone was discovered in 1907; with scientific dating, his remains were determined to be the earliest evidence of human life in Europe. In the 5th century BC, a Celtic fortress of refuge and place of...
. The publication of the results was delayed and Balard published his results first.
After the French chemists Louis Nicolas Vauquelin
Louis Nicolas Vauquelin
Nicolas Louis Vauquelin , was a French pharmacist and chemist.-Early life:Vauquelin was born at Saint-André-d'Hébertot in Normandy, France. His first acquaintance with chemistry was gained as laboratory assistant to an apothecary in Rouen , and after various vicissitudes he obtained an introduction...
, Louis Jacques Thénard
Louis Jacques Thénard
Louis Jacques Thénard , was a French chemist.His father, a poor peasant, managed to have him educated at the academy of Sens, and sent him at the age of sixteen to study pharmacy in Paris. There he attended the lectures of Antoine François Fourcroy and Louis Nicolas Vauquelin...
, and Joseph-Louis Gay-Lussac approved the experiments of the young pharmacist Balard, the results were presented at a lecture of the Académie des Sciences and published in Annales de Chimie et Physique. In his publication Balard states that he changed the name from muride to brôme on the proposal of M. Anglada. (Brôme (bromine) derives from the Greek βρωμος (stench).) Other sources claim that the French chemist and physicist Joseph-Louis Gay-Lussac suggested the name brôme for the characteristic smell of the vapors. Bromine was not produced in large quantities until 1860.
The first commercial use, besides some minor medical applications, was the use of bromine for the daguerreotype
Daguerreotype
The daguerreotype was the first commercially successful photographic process. The image is a direct positive made in the camera on a silvered copper plate....
. In 1840 it was discovered that bromine had some advantages over the previously used iodine vapor to create the light sensitive silver halide
Silver halide
A silver halide is one of the compounds formed between silver and one of the halogens — silver bromide , chloride , iodide , and three forms of silver fluorides. As a group, they are often referred to as the silver halides, and are often given the pseudo-chemical notation AgX...
layer used for daguerreotypy.
Potassium bromide
Potassium bromide
Potassium bromide is a salt, widely used as an anticonvulsant and a sedative in the late 19th and early 20th centuries, with over-the-counter use extending to 1975 in the United States. Its action is due to the bromide ion...
and sodium bromide
Sodium bromide
Sodium bromide is an inorganic compound with the formula NaBr. It is a high-melting white, crystalline solid that resembles sodium chloride. It is a widely used source of the bromide ion and has many applications.-Synthesis, structure, reactions:...
were used as anticonvulsant
Anticonvulsant
The anticonvulsants are a diverse group of pharmaceuticals used in the treatment of epileptic seizures. Anticonvulsants are also increasingly being used in the treatment of bipolar disorder, since many seem to act as mood stabilizers, and in the treatment of neuropathic pain. The goal of an...
s and sedative
Sedative
A sedative or tranquilizer is a substance that induces sedation by reducing irritability or excitement....
s in the late 19th and early 20th centuries, until they were gradually superseded by chloral hydrate
Chloral hydrate
Chloral hydrate is a sedative and hypnotic drug as well as a chemical reagent and precursor. The name chloral hydrate indicates that it is formed from chloral by the addition of one molecule of water. Its chemical formula is C2H3Cl3O2....
and then the barbiturate
Barbiturate
Barbiturates are drugs that act as central nervous system depressants, and can therefore produce a wide spectrum of effects, from mild sedation to total anesthesia. They are also effective as anxiolytics, as hypnotics, and as anticonvulsants...
s.
Occurrence


Bromide
A bromide is a chemical compound containing bromide ion, that is bromine atom with effective charge of −1. The class name can include ionic compounds such as caesium bromide or covalent compounds such as sulfur dibromide.-Natural occurrence:...
in diffuse amounts in crustal
Crust (geology)
In geology, the crust is the outermost solid shell of a rocky planet or natural satellite, which is chemically distinct from the underlying mantle...
rock. Owing to leaching
Leaching (chemical science)
Leaching is the process of extracting minerals from a solid by dissolving them in a liquid, either in nature or through an industrial process. In the chemical processing industry, leaching has a variety of commercial applications, including separation of metal from ore using acid, and sugar from...
, bromide salts have accumulated in sea water at 65 part per million (ppm), which is less than chloride. Bromine may be economically recovered from bromide-rich brine wells and from the Dead Sea
Dead Sea
The Dead Sea , also called the Salt Sea, is a salt lake bordering Jordan to the east and Israel and the West Bank to the west. Its surface and shores are below sea level, the lowest elevation on the Earth's surface. The Dead Sea is deep, the deepest hypersaline lake in the world...
waters (up to 50,000 ppm). It exists in the Earth's crust at an average concentration of 0.4 ppm, making it the 62nd most abundant element
Abundance of the chemical elements
The abundance of a chemical element measures how relatively common the element is, or how much of the element is present in a given environment by comparison to all other elements...
. The bromine concentration in soils varies normally between 5 and 40 ppm, but some volcanic soils can contain up to 500 ppm. The concentration of bromine in the atmosphere is extremely low, at only a few ppt. A large number of organobromine compounds are found in small amounts in nature.
China's bromine reserves are located in the Shandong Province and Israel's bromine reserves are contained in the waters of the Dead Sea
Dead Sea
The Dead Sea , also called the Salt Sea, is a salt lake bordering Jordan to the east and Israel and the West Bank to the west. Its surface and shores are below sea level, the lowest elevation on the Earth's surface. The Dead Sea is deep, the deepest hypersaline lake in the world...
. The largest bromine reserve in the United States is located in Columbia County
Columbia County, Arkansas
Columbia County is a county located in the U.S. state of Arkansas. As of 2010, the population was 24,552. The county seat is Magnolia. Columbia County was formed on December 17, 1852, and was named for Christopher Columbus...
and Union County, Arkansas, U.S.
Production
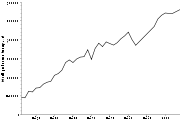
Bromine production is rather dynamic and has increased sixfold since the 1960s. Approximately 556,000 tonnes (worth around US $2.5 billion) was produced in 2007 worldwide, with the predominant contribution from the United StatesUnited States
The United States of America is a federal constitutional republic comprising fifty states and a federal district...
(226,000 t) and Israel
Israel
The State of Israel is a parliamentary republic located in the Middle East, along the eastern shore of the Mediterranean Sea...
(210,000 t). US production was excluded from the United States Geological Survey
United States Geological Survey
The United States Geological Survey is a scientific agency of the United States government. The scientists of the USGS study the landscape of the United States, its natural resources, and the natural hazards that threaten it. The organization has four major science disciplines, concerning biology,...
after 2007, and from the 380,000 tonnes mined by other countries in 2010, 140,000 t were produced by China, 130,000 t by Israel and 80,000 t by Jordan.
Bromide-rich brines are treated with chlorine gas, flushing through with air. In this treatment, bromide anions are oxidized to bromine by the chlorine gas.
- 2 Br− + Cl2 → 2 Cl− + Br2
Laboratory methods of production
In the laboratory, because of its commercial availability and long shelf-life, bromine is not typically prepared. Small amounts of bromine can however be generated through the reaction of solid sodium bromideSodium bromide
Sodium bromide is an inorganic compound with the formula NaBr. It is a high-melting white, crystalline solid that resembles sodium chloride. It is a widely used source of the bromide ion and has many applications.-Synthesis, structure, reactions:...
with concentrated sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
(H2SO4). The first stage is formation of hydrogen bromide
Hydrogen bromide
Hydrogen bromide is the diatomic molecule HBr. HBr is a gas at standard conditions. Hydrobromic acid forms upon dissolving HBr in water. Conversely, HBr can be liberated from hydrobromic acid solutions with the addition of a dehydration agent, but not by distillation. Hydrogen bromide and...
(HBr), which is a gas, but under the reaction conditions some of the HBr is oxidized further by the sulfuric acid to form bromine (Br2) and sulfur dioxide
Sulfur dioxide
Sulfur dioxide is the chemical compound with the formula . It is released by volcanoes and in various industrial processes. Since coal and petroleum often contain sulfur compounds, their combustion generates sulfur dioxide unless the sulfur compounds are removed before burning the fuel...
(SO2).
- NaBr (s) + H2SO4 (aq) → HBr (aq) + NaHSO4 (aq)
- 2 HBr (aq) + H2SO4 (aq) → Br2 (g) + SO2 (g) + 2 H2O (l)
Non oxidizing acid alternatives, such as the use of dilute hydrobromic acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
with sodium hypobromite
Hypobromite
The hypobromite ion, also called alkaline bromine water, is BrO−. Bromine is in the +1 oxidation state. Hypobromite is the bromine compound analogous to hypochlorites found in common bleaches, and in immune cells...
, are also available, as the hypobromous acid
Hypobromous acid
Hypobromous acid is a weak, unstable acid with chemical formula HBrO. It is also called bromic acid, bromanol or hydroxidobromine. It occurs only in solution and has chemical and physical properties that are very similar to those of hypochlorous acid.In aqueous solution, hypobromous acid partially...
formed from them is unstable in the presence of bromide, being reduced by it according to the reaction:
- 2 OBr- (aq) + 4 HBr (aq) → 2Br2 + 2H2O + 2Br-
The reactions are the reverse of disproportionation
Disproportionation
Disproportionation, also known as dismutation is used to describe a specific type of redox reaction in which a species is simultaneously reduced and oxidized so as to form two different products....
reactions of elemental bromine in base, and are called comproportionation. A similar reaction happens with sodium hypochlorite, acid, and chloride, leading to elemental chlorine.
Reactions involving an oxidizing agent, such as potassium permanganate or manganese dioxide, on bromide ions in the presence of an acid, also give bromine in the reactions analogous to the formation of elemental chlorine and iodine from an acid and oxidant.
Like iodine, bromine is soluble in chloroform but only slightly soluble in water. In water, the solubility can be increased by the presence of bromide ions. Concentrated solutions of bromine are rarely prepared in the lab because of hazards. As is the case with chlorine solutions or iodine solutions, sodium thiosulphate (or any soluble thiosulphate) is an effective reagent for reducing bromine to colorless odorless bromide, thus dealing with stains and odor from the element in unwanted places. For the same reason, thiosulfate ("fixer's hypo") is used in photography to deal with free bromine in silver bromide film emulsions.
Organic chemistry

Addition reaction
An addition reaction, in organic chemistry, is in its simplest terms an organic reaction where two or more molecules combine to form a larger one....
or substitution reactions. Bromine undergoes electrophilic addition to the double-bonds of alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s, via a cyclic bromonium intermediate. In non-aqueous solvents such as carbon disulfide
Carbon disulfide
Carbon disulfide is a colorless volatile liquid with the formula CS2. The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical non-polar solvent...
, this yields the di-bromo product. For example, reaction with ethylene
Ethylene
Ethylene is a gaseous organic compound with the formula . It is the simplest alkene . Because it contains a carbon-carbon double bond, ethylene is classified as an unsaturated hydrocarbon. Ethylene is widely used in industry and is also a plant hormone...
will produce 1,2-dibromoethane
1,2-Dibromoethane
1,2-Dibromoethane, also known as ethylene dibromide , is the chemical compound with the formula BrCH2CH2Br. Although trace amounts occur naturally in the ocean, where it is formed probably by algae and kelp, it is mainly a synthetic...
. Bromine also undergoes electrophilic addition to phenol
Phenol
Phenol, also known as carbolic acid, phenic acid, is an organic compound with the chemical formula C6H5OH. It is a white crystalline solid. The molecule consists of a phenyl , bonded to a hydroxyl group. It is produced on a large scale as a precursor to many materials and useful compounds...
s and aniline
Aniline
Aniline, phenylamine or aminobenzene is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the prototypical aromatic amine. Being a precursor to many industrial chemicals, its main use is in the manufacture of precursors to polyurethane...
s. When used as bromine water, a small amount of the corresponding bromohydrin is formed
Halohydrin formation reaction
The halohydrin formation reaction is a chemical reaction in which a halogen is added to an alkene in aqueous solution to form a halohydrin. The reaction is a form of electrophilic addition; it is similar to the halogen addition reaction....
as well as the dibromo compound. So reliable is the reactivity of bromine that bromine water is employed as a reagent to test for the presence of alkenes, phenols, and anilines. Like the other halogens, bromine participates in free radical reaction
Free radical reaction
A free radical reaction is any chemical reaction involving free radicals. This reaction type is abundant in organic reactions.Two pioneering studies into free radical reactions have been the discovery of the triphenylmethyl radical by Moses Gomberg and the lead-mirror experiment described by...
s. For example, hydrocarbons are brominated upon treatment with bromine in the presence of light.
Bromine, sometimes with a catalytic amount of phosphorus
Phosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
, easily brominates carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
s at the α-position. This method, the Hell-Volhard-Zelinsky reaction, is the basis of the commercial route to bromoacetic acid
Bromoacetic acid
Bromoacetic acid is the chemical compound with the formula CH2BrCO2H. This colorless solid is a relatively strong alkylating agent. Bromoacetic acid and its esters are widely used building blocks in organic synthesis, for example in pharmaceutical chemistry....
. N-Bromosuccinimide
N-Bromosuccinimide
N-Bromosuccinimide or NBS is a chemical reagent which is used in radical substitution and electrophilic addition reactions in organic chemistry. NBS can be considered a convenient source of cationic bromine.-Preparation:...
is commonly used as a substitute for elemental bromine, being easier to handle, and reacting more mildly and thus more selectively. Organic bromides are often preferable relative to the less reactive chlorides and more expensive iodide-containing reagents. Thus, Grignard
Grignard reaction
The Grignard reaction is an organometallic chemical reaction in which alkyl- or aryl-magnesium halides add to a carbonyl group in an aldehyde or ketone. This reaction is an important tool for the formation of carbon–carbon bonds...
and organolithium compound are most often generated from the corresponding bromides.
Certain bromine-related compounds have been evaluated to have an ozone depletion potential
Ozone depletion potential
The ozone depletion potential of a chemical compound is the relative amount of degradation to the ozone layer it can cause, with trichlorofluoromethane being fixed at an ODP of 1.0. Chlorodifluoromethane , for example, has an ODP of 0.055...
or bioaccumulate in living organisms. As a result, many industrial bromine compounds are no longer manufactured, are being restricted, or scheduled for phasing out. The Montreal Protocol
Montreal Protocol
The Montreal Protocol on Substances That Deplete the Ozone Layer is an international treaty designed to protect the ozone layer by phasing out the production of numerous substances believed to be responsible for ozone depletion...
mentions several organobromine compounds for this phase out.
Inorganic chemistry
Inorganic bromine compounds adopt a variety of oxidation states from −1 to +7.of bromine
|-
| −1 ||
|-
| 0 ||
|-
| +1 ||
|-
| +3 ||
|-
| +5 ||
|-
| +5 ||
|-
| +7 ||
|}
Bromine is an oxidizer, and it will oxidize iodide
Iodide
An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. This page is for the iodide ion and its salts. For information on organoiodides, see organohalides. In everyday life, iodide is most commonly encountered as a component of iodized salt,...
ions to iodine, being itself reduced to bromide
Bromide
A bromide is a chemical compound containing bromide ion, that is bromine atom with effective charge of −1. The class name can include ionic compounds such as caesium bromide or covalent compounds such as sulfur dibromide.-Natural occurrence:...
:
- Br2 + 2 I− → 2 Br− + I2
Bromine will also oxidize metals and metalloids to the corresponding bromides. Anhydrous
Anhydrous
As a general term, a substance is said to be anhydrous if it contains no water. The way of achieving the anhydrous form differs from one substance to another...
bromine is less reactive toward many metals than hydrated bromine, however. Dry bromine reacts vigorously with aluminium
Aluminium
Aluminium or aluminum is a silvery white member of the boron group of chemical elements. It has the symbol Al, and its atomic number is 13. It is not soluble in water under normal circumstances....
, titanium
Titanium
Titanium is a chemical element with the symbol Ti and atomic number 22. It has a low density and is a strong, lustrous, corrosion-resistant transition metal with a silver color....
, mercury
Mercury (element)
Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum...
as well as alkaline earth
Alkaline earth metal
The alkaline earth metals are a group in the periodic table. In the modern IUPAC nomenclature, the alkaline earth metals are called the group 2 elements. Previously, they were called the Group IIA elements . The alkaline earth metals contain beryllium , magnesium , calcium , strontium , barium and...
s and alkali metal
Alkali metal
The alkali metals are a series of chemical elements in the periodic table. In the modern IUPAC nomenclature, the alkali metals comprise the group 1 elements, along with hydrogen. The alkali metals are lithium , sodium , potassium , rubidium , caesium , and francium...
s.
Dissolving bromine in alkaline solution gives a mixture of bromide and hypobromite
Hypobromite
The hypobromite ion, also called alkaline bromine water, is BrO−. Bromine is in the +1 oxidation state. Hypobromite is the bromine compound analogous to hypochlorites found in common bleaches, and in immune cells...
:
- Br2 + 2 OH− → Br− + OBr− + H2O
This hypobromite is responsible for the bleaching abilities of bromide solutions. Warming of these solutions causes the disproportion reaction of the hypobromite to give bromate
Bromate
The bromate anion, BrO, is a bromine-based oxoanion. A bromate is a chemical compound that contains this ion. Examples of bromates include sodium bromate, , and potassium bromate, .Bromates are formed many different ways in municipal drinking water...
, a strong oxidising agent very similar to chlorate.
- 3 → + 2
In contrast to the route to perchlorate
Perchlorate
Perchlorates are the salts derived from perchloric acid . They occur both naturally and through manufacturing. They have been used as a medicine for more than 50 years to treat thyroid gland disorders. They are used extensively within the pyrotechnics industry, and ammonium perchlorate is also a...
s, perbromates are not accessible through electrolysis but only by reacting bromate solutions with fluorine
Fluorine
Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
or ozone
Ozone
Ozone , or trioxygen, is a triatomic molecule, consisting of three oxygen atoms. It is an allotrope of oxygen that is much less stable than the diatomic allotrope...
.
- BrO3− + H2O + F2 → + 2 HF
- BrO3− + O3 → + O2
Bromine reacts violently and explosively with aluminium metal, forming aluminium bromide:
- 2 Al + 3 Br2 → 2 AlBr3Aluminium bromideAluminium bromide is any chemical compound with the empirical formula AlBrx. The species called "aluminium tribromide," is the most common aluminium bromide. The species aluminium monobromide forms from the reaction of HBr with Al metal at high temperature...
Bromine reacts with hydrogen in gaseous form and gives hydrogen bromide
Hydrogen bromide
Hydrogen bromide is the diatomic molecule HBr. HBr is a gas at standard conditions. Hydrobromic acid forms upon dissolving HBr in water. Conversely, HBr can be liberated from hydrobromic acid solutions with the addition of a dehydration agent, but not by distillation. Hydrogen bromide and...
:
- H2HydrogenHydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
+ Br2 → 2HBrHydrogen bromideHydrogen bromide is the diatomic molecule HBr. HBr is a gas at standard conditions. Hydrobromic acid forms upon dissolving HBr in water. Conversely, HBr can be liberated from hydrobromic acid solutions with the addition of a dehydration agent, but not by distillation. Hydrogen bromide and...
Bromine reacts with alkali metal
Alkali metal
The alkali metals are a series of chemical elements in the periodic table. In the modern IUPAC nomenclature, the alkali metals comprise the group 1 elements, along with hydrogen. The alkali metals are lithium , sodium , potassium , rubidium , caesium , and francium...
iodides in a displacement reaction. This reaction forms alkali metal bromides and produces elemental iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
:
- 2 NaISodium iodideSodium iodide is a white, crystalline salt with chemical formula NaI used in radiation detection, treatment of iodine deficiency, and as a reactant in the Finkelstein reaction.-Uses:Sodium iodide is commonly used to treat and prevent iodine deficiency....
+ Br2 → 2 NaBrSodium bromideSodium bromide is an inorganic compound with the formula NaBr. It is a high-melting white, crystalline solid that resembles sodium chloride. It is a widely used source of the bromide ion and has many applications.-Synthesis, structure, reactions:...
+ I2 - 2 KIPotassium iodidePotassium iodide is an inorganic compound with the chemical formula KI. This white salt is the most commercially significant iodide compound, with approximately 37,000 tons produced in 1985. It is less hygroscopic than sodium iodide, making it easier to work with...
+ Br2 → 2 KBrPotassium bromidePotassium bromide is a salt, widely used as an anticonvulsant and a sedative in the late 19th and early 20th centuries, with over-the-counter use extending to 1975 in the United States. Its action is due to the bromide ion...
+ I2
Applications
A wide variety of organobromine compounds are used in industryIndustry
Industry refers to the production of an economic good or service within an economy.-Industrial sectors:There are four key industrial economic sectors: the primary sector, largely raw material extraction industries such as mining and farming; the secondary sector, involving refining, construction,...
. Some are prepared from bromine and others are prepared from hydrogen bromide
Hydrogen bromide
Hydrogen bromide is the diatomic molecule HBr. HBr is a gas at standard conditions. Hydrobromic acid forms upon dissolving HBr in water. Conversely, HBr can be liberated from hydrobromic acid solutions with the addition of a dehydration agent, but not by distillation. Hydrogen bromide and...
, which is obtained by burning hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
in bromine.
Illustrative of the addition reaction is the preparation of 1,2-dibromoethane
1,2-Dibromoethane
1,2-Dibromoethane, also known as ethylene dibromide , is the chemical compound with the formula BrCH2CH2Br. Although trace amounts occur naturally in the ocean, where it is formed probably by algae and kelp, it is mainly a synthetic...
, the organobromine compound produced in the largest amounts:
- C2H4 + Br2 → CH2BrCH2Br
Flame retardant

Hydrobromic acid
Hydrobromic acid is a strong acid formed by dissolving the diatomic molecule hydrogen bromide in water. "Constant boiling" hydrobromic acid is an aqueous solution that distills at 124.3 °C and contains 47.6% HBr by weight, which is 8.89 mol/L. Hydrobromic acid has a pKa of −9, making it a...
which interferes in the radical chain reaction
Chain reaction
A chain reaction is a sequence of reactions where a reactive product or by-product causes additional reactions to take place. In a chain reaction, positive feedback leads to a self-amplifying chain of events....
of the oxidation reaction of the fire. The mechanism is that the highly reactive hydrogen oxygen and hydroxy radicals react with hydrobromic acid and form less reactive bromine radicals (free bromine atoms). These also react with radicals in the first to help terminate the reaction.
The bromine-containing compounds can be placed in the polymers either during polymerization
Polymerization
In polymer chemistry, polymerization is a process of reacting monomer molecules together in a chemical reaction to form three-dimensional networks or polymer chains...
if a small amount of brominated monomer is added or the bromine containing compound is added after polymerization. Tetrabromobisphenol A
Tetrabromobisphenol A
Tetrabromobisphenol A is a brominated flame retardant.- Synthesis :TBBPA is a derivative of bisphenol A and is synthesized from this substance. Most commercial TBBPA products are of a relatively low purity, in fact containing a mixture of products brominated to varying extents. This is not...
can be added to produce polyester
Polyester
Polyester is a category of polymers which contain the ester functional group in their main chain. Although there are many polyesters, the term "polyester" as a specific material most commonly refers to polyethylene terephthalate...
s or epoxy resins. Epoxy used in printed circuit board
Printed circuit board
A printed circuit board, or PCB, is used to mechanically support and electrically connect electronic components using conductive pathways, tracks or signal traces etched from copper sheets laminated onto a non-conductive substrate. It is also referred to as printed wiring board or etched wiring...
s (PCB) are normally made from flame retardant resins, indicated by the FR in the abbreviation of the products (FR-4
FR-4
FR-4 is a grade designation assigned to glass-reinforced epoxy laminate sheets, tubes, rods and printed circuit boards . FR-4 is a composite material composed of woven fiberglass cloth with an epoxy resin binder that is flame resistant .FR-4 glass epoxy is a popular and versatile high-pressure...
and FR-2
FR-2
FR-2 is an abbreviation for Flame Resistant 2. It is a NEMA designation for synthetic resin bonded paper, a composite material made of paper impregnated with a plasticized phenol formaldehyde resin, used in the manufacture of printed circuit boards...
. Vinyl bromide
Vinyl bromide
Vinyl bromide is a simple vinyl halide. It is soluble in chloroform, ethanol, diethyl ether, acetone and benzene.- Uses :Vinyl bromide is used to manufacture bromopolymers and mainly polyvinyl bromide...
can be used in the production of polyethylene
Polyethylene
Polyethylene or polythene is the most widely used plastic, with an annual production of approximately 80 million metric tons...
, polyvinylchloride or polypropylene
Polypropylene
Polypropylene , also known as polypropene, is a thermoplastic polymer used in a wide variety of applications including packaging, textiles , stationery, plastic parts and reusable containers of various types, laboratory equipment, loudspeakers, automotive components, and polymer banknotes...
. Decabromodiphenyl ether
Decabromodiphenyl ether
Decabromodiphenyl ether is a brominated flame retardant which belongs to the group of polybrominated diphenyl ethers ....
can be added to the final polymers.
Gasoline additive
Ethylene bromide1,2-Dibromoethane
1,2-Dibromoethane, also known as ethylene dibromide , is the chemical compound with the formula BrCH2CH2Br. Although trace amounts occur naturally in the ocean, where it is formed probably by algae and kelp, it is mainly a synthetic...
was an additive in gasolines
Gasoline additive
Gasoline additives increase gasoline's octane rating or act as corrosion inhibitors or lubricants, thus allowing the use of higher compression ratios for greater efficiency and power, however some carry heavy environmental risks...
containing lead anti-engine knocking
Engine knocking
Knocking in spark-ignition internal combustion engines occurs when combustion of the air/fuel mixture in the cylinder starts off correctly in response to ignition by the spark plug, but one or more pockets of air/fuel mixture explode outside the envelope of the normal combustion front.The...
agents. It scavenges lead by forming volatile lead bromide, which is exhausted from the engine. This application accounted for 77% of the bromine use in 1966 in the US. This application has declined since the 1970s due to environmental regulations (see below).
Pesticide

Pesticide
Pesticides are substances or mixture of substances intended for preventing, destroying, repelling or mitigating any pest.A pesticide may be a chemical unicycle, biological agent , antimicrobial, disinfectant or device used against any pest...
to fumigate
Fumigation
Fumigation is a method of pest control that completely fills an area with gaseous pesticides—or fumigants—to suffocate or poison the pests within. It is utilized for control of pests in buildings , soil, grain, and produce, and is also used during processing of goods to be imported or...
soil and to fumagate housing, by the tenting method. Ethylene bromide was similarly used. These volatile organobromine compounds are all now regulated as ozone depletion
Ozone depletion
Ozone depletion describes two distinct but related phenomena observed since the late 1970s: a steady decline of about 4% per decade in the total volume of ozone in Earth's stratosphere , and a much larger springtime decrease in stratospheric ozone over Earth's polar regions. The latter phenomenon...
agents. The Montreal Protocol on Substances that Deplete the Ozone
Montreal Protocol
The Montreal Protocol on Substances That Deplete the Ozone Layer is an international treaty designed to protect the ozone layer by phasing out the production of numerous substances believed to be responsible for ozone depletion...
scheduled the phase out for the ozone depleting chemical by 2005, and organobromide pesticides are no longer used (in housing fumagation they have been replaced by such compounds as sulfuryl fluoride
Sulfuryl fluoride
Sulfuryl fluoride is the inorganic compound with the formula SO2F2. This easily condensed gas has properties more similar to sulfur hexafluoride than sulfuryl chloride, being resistant to hydrolysis even up to 150 °C...
, which contain neither the chlorine or bromine organics which harm ozone). Prior to the Montreal protocol in 1991 (for example) an estimated 35,000 tonnes of the chemical were used to control nematodes, fungi, weed
Weed
A weed in a general sense is a plant that is considered by the user of the term to be a nuisance, and normally applied to unwanted plants in human-controlled settings, especially farm fields and gardens, but also lawns, parks, woods, and other areas. More specifically, the term is often used to...
s and other soil-borne diseases.
Medical and veterinary
Use. BromideBromide
A bromide is a chemical compound containing bromide ion, that is bromine atom with effective charge of −1. The class name can include ionic compounds such as caesium bromide or covalent compounds such as sulfur dibromide.-Natural occurrence:...
compounds, especially potassium bromide
Potassium bromide
Potassium bromide is a salt, widely used as an anticonvulsant and a sedative in the late 19th and early 20th centuries, with over-the-counter use extending to 1975 in the United States. Its action is due to the bromide ion...
, were frequently used as general sedatives in the 19th and early 20th century. Bromides in the form of simple salts are still used as anticonvulsants in both veterinary and human medicine, although the latter use varies from country to country. For example, the U.S. Food and Drug Administration
Food and Drug Administration
The Food and Drug Administration is an agency of the United States Department of Health and Human Services, one of the United States federal executive departments...
(FDA) does not approve bromide for the treatment of any disease, and it was removed from over-the-counter sedative products like Bromo-Seltzer
Bromo-Seltzer
Bromo-Seltzer , is an antacid used to relieve pain occurring together with heartburn, upset stomach, or acid indigestion. Originally produced by inventor Isaac E...
, in 1975. Thus, bromide levels are not routinely measured by medical laboratories in the U.S. However, U.S. veterinary medical diagnostic testing laboratories will measure blood bromide levels on request, as an aid to treatment of epilepsy in dogs.
Toxicity. Long-term use of potassium bromide (or any bromide salt) can lead to bromism
Bromism
Bromism is the syndrome which results from the long-term use of the potassium bromide based sedatives. Bromism was once a very common disorder being responsible for 5-10% of psychiatric hospital admissions. It is now an uncommon disorder due to bromide being withdrawn from clinical use in many...
. This state of central nervous system depression causes the moderate toxicity of bromide in multi-gram doses for humans and other mammals. The very long half-life of bromide ion in the body (~12 days) also contributes to toxicity from bromide build-up in body fluids. Bromide ingestion may also cause a skin eruption resembling acne
Acne
Acne is a general term used for acneiform eruptions. It is usually used as a synonym for acne vulgaris, but may also refer to:*Acne aestivalis*Acne conglobata*Acne cosmetica*Acne fulminans*Acne keloidalis nuchae*Acne mechanica...
.
Other uses

- The bromides of calcium, sodium, and zinc account for a sizable part of the bromine market. These salts form dense solutions in water that are used as drilling fluidDrilling fluidIn geotechnical engineering, drilling fluid is a fluid used to aid the drilling of boreholes into the earth. Often used while drilling oil and natural gas wells and on exploration drilling rigs, drilling fluids are also used for much simpler boreholes, such as water wells. Liquid drilling fluid...
s sometimes called clear brine fluids. - Bromine is also used in the production of brominated vegetable oilBrominated vegetable oilBrominated vegetable oil is vegetable oil that has had atoms of the element bromine bonded to it. Brominated vegetable oil is used as an emulsifier in citrus-flavored soft drinks to help natural fat-soluble citrus flavors stay suspended in the drink and to produce a cloudy appearance...
, which is used as an emulsifier in many citrusCitrusCitrus is a common term and genus of flowering plants in the rue family, Rutaceae. Citrus is believed to have originated in the part of Southeast Asia bordered by Northeastern India, Myanmar and the Yunnan province of China...
-flavored soft drinks (for example, Mountain Dew). After the introduction in the 1940s the compound was extensively used until the UK and the US limited its use in the mid 1970s and alternative emulsifiers were developed.
Soft drinks containing brominated vegetable oil are still sold in the US (2011).

- Several dyeDyeA dye is a colored substance that has an affinity to the substrate to which it is being applied. The dye is generally applied in an aqueous solution, and requires a mordant to improve the fastness of the dye on the fiber....
s, agrichemicals, and pharmaceuticals are organobromine compounds. 1-Bromo-3-chloropropane, 1-bromoethylbenzene, and 1-bromoalkanes are prepared by the antimarkovnikov addition of HBr to alkenes. Ethidium bromideEthidium bromideEthidium bromide is an intercalating agent commonly used as a fluorescent tag in molecular biology laboratories for techniques such as agarose gel electrophoresis. It is commonly abbreviated as "EtBr", which is also an abbreviation for bromoethane...
, EtBr, is used as a DNADNADeoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
stain in gel electrophoresisGel electrophoresisGel electrophoresis is a method used in clinical chemistry to separate proteins by charge and or size and in biochemistry and molecular biology to separate a mixed population of DNA and RNA fragments by length, to estimate the size of DNA and RNA fragments or to separate proteins by charge...
. - High refractive indexRefractive indexIn optics the refractive index or index of refraction of a substance or medium is a measure of the speed of light in that medium. It is expressed as a ratio of the speed of light in vacuum relative to that in the considered medium....
compounds - Bromine, like chlorine, is used in maintenance of swimming pools, especially spas (hot tubs), where it is generated in situ from a bromide plus hydrogen peroxide. In spas, the high water temperatures render chlorinated water purification and buffering compounds unstable, and bromine compounds may improve the life of the free-halogen antimicrobial.
- Water purificationWater purificationWater purification is the process of removing undesirable chemicals, materials, and biological contaminants from contaminated water. The goal is to produce water fit for a specific purpose...
compounds, disinfectants and insecticideInsecticideAn insecticide is a pesticide used against insects. They include ovicides and larvicides used against the eggs and larvae of insects respectively. Insecticides are used in agriculture, medicine, industry and the household. The use of insecticides is believed to be one of the major factors behind...
s, such as tralomethrinTralomethrinTralomethrin is a pyrethroid insecticide.Tralomethrin has potent insecticidal properties; it kills by modifying the gating kinetics of the sodium channels in neurons, increasing the length of time the channel remains open after a stimulus, thereby depolarizing the neuron for a longer period of...
(C22H19Br4NO3). - Potassium bromide is used in some photographic developers to inhibit the formation of fog (undesired reduction of silver).
- Bromine vapor is used as the second step in sensitizing daguerreotypeDaguerreotypeThe daguerreotype was the first commercially successful photographic process. The image is a direct positive made in the camera on a silvered copper plate....
plates to be developed under mercury vapor. Bromine acts as an accelerator to the light sensitivity of the previously iodized plate. - Bromine is also used to reduce mercury pollution from coal-fired power plants. This can be achieved either by treating activated carbonActivated carbonActivated carbon, also called activated charcoal, activated coal or carbo activatus, is a form of carbon that has been processed to make it extremely porous and thus to have a very large surface area available for adsorption or chemical reactions.The word activated in the name is sometimes replaced...
with bromine or by injecting bromine compounds onto the coal prior to combustion. - Bromine can also be artificially substituted for the methyl substituent in the nitrogenous base thymine of DNA, creating the base analog 5-bromouracil5-Bromouracil5-Bromouracil is a brominated derivative of uracil that acts as an antimetabolite or base analog, substituting for thymine in DNA, and can induce DNA mutation in the same way as 2-aminopurine...
. When this base is incorporated into DNA its different hydrogen bonding properties may cause mutation at the site of that base pair. The compound 5-bromouracil is thus an artificial mutagen.
Biological role

Organobromine compound
Organobromine compounds are organic compounds that contain carbon bonded to bromine. The most pervasive is the naturally produced bromomethane. One prominent application is the use of polybrominated diphenyl ethers as fire-retardants. A variety of minor organobromine compounds are found in...
s do occur naturally, and some may be of use to higher organisms in dealing with parasites. For example, in the presence of H2O2 formed by the eosinophil, and either chloride or bromide ions, eosinophil peroxidase provides a potent mechanism by which eosinophils kill multicellular parasites
Parasitism
Parasitism is a type of symbiotic relationship between organisms of different species where one organism, the parasite, benefits at the expense of the other, the host. Traditionally parasite referred to organisms with lifestages that needed more than one host . These are now called macroparasites...
(such as, for example, the nematode worms involved in filariasis
Filariasis
Filariasis is a parasitic disease and is considered an infectious tropical disease, that is caused by thread-like nematodes belonging to the superfamily Filarioidea, also known as "filariae"....
); and also certain bacteria
Bacteria
Bacteria are a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria have a wide range of shapes, ranging from spheres to rods and spirals...
(such as tuberculosis
Tuberculosis
Tuberculosis, MTB, or TB is a common, and in many cases lethal, infectious disease caused by various strains of mycobacteria, usually Mycobacterium tuberculosis. Tuberculosis usually attacks the lungs but can also affect other parts of the body...
bacteria). Eosinophil peroxidase is a haloperoxidase
Haloperoxidase
Haloperoxidases are peroxidases that are able to mediate the oxidation of halides by hydrogen peroxide. Both halides and hydrogen peroxide are widely available in the environment....
that preferentially uses bromide over chloride for this purpose, generating hypobromite
Hypobromite
The hypobromite ion, also called alkaline bromine water, is BrO−. Bromine is in the +1 oxidation state. Hypobromite is the bromine compound analogous to hypochlorites found in common bleaches, and in immune cells...
(hypobromous acid
Hypobromous acid
Hypobromous acid is a weak, unstable acid with chemical formula HBrO. It is also called bromic acid, bromanol or hydroxidobromine. It occurs only in solution and has chemical and physical properties that are very similar to those of hypochlorous acid.In aqueous solution, hypobromous acid partially...
).
Marine organisms are the main source of organobromine compounds. Over 1600 compounds were identified by 1999. The most abundant one is methyl bromide (CH3Br) with an estimated 56,000 tonnes produced by marine algae each year. The essential oil of the Hawaiian alga Asparagopsis taxiformis consists of 80% methyl bromide. Most of such organobromine compounds in the sea are made via the action of a unique algal enzyme, vanadium bromoperoxidase
Vanadium bromoperoxidase
Vanadium bromoperoxidase is a haloperoxidase, used to synthesize halogenated organic compounds associated with defense and pigmentation in seaweeds and marine algae. It is the source of the bulk of organobromine compounds in the ocean...
. Though this enzyme is the most prolific creator of organic bromides by living organisms, other bromoperoxidase
Bromoperoxidase
Bromoperoxidases are enzymes that catalyse the bromination of hydrocarbons. The enzymes accomplish this reaction via the following reaction:Related chloroperoxidase enzymes effect chlorination....
s exist in nature that do not use vanadium.
A famous example of a bromine-containing organic compound that has been used by humans since ancient times is the fabric dye Tyrian purple
Tyrian purple
Tyrian purple , also known as royal purple, imperial purple or imperial dye, is a purple-red natural dye, which is extracted from sea snails, and which was possibly first produced by the ancient Phoenicians...
. The brominated indole
Indole
Indole is an aromatic heterocyclic organic compound. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing pyrrole ring. Indole is a popular component of fragrances and the precursor to many pharmaceuticals. Compounds that contain an...
indigo dye is produced by a medium-sized predatory sea snail
Snail
Snail is a common name applied to most of the members of the molluscan class Gastropoda that have coiled shells in the adult stage. When the word is used in its most general sense, it includes sea snails, land snails and freshwater snails. The word snail without any qualifier is however more often...
, the marine
Marine (ocean)
Marine is an umbrella term. As an adjective it is usually applicable to things relating to the sea or ocean, such as marine biology, marine ecology and marine geology...
gastropod Murex brandaris. The organobromine nature of the compound was not discovered until 1909 (see Paul Friedländer
Paul Friedländer (chemist)
Paul Friedländer was a German chemist best known for his research on derivates of indigo and isolation of Tyrian purple from Murex brandaris.-Life and work:...
).
Safety
Elemental bromine is toxic and causes burns. As an oxidizing agent, it is incompatible with most organic and inorganic compounds. Care needs to be taken when transporting bromine; it is commonly carried in steel tanks lined with lead, supported by strong metal frames.When certain ionic compounds containing bromine are mixed with potassium permanganate
Potassium permanganate
Potassium permanganate is an inorganic chemical compound with the formula KMnO4. It is a salt consisting of K+ and MnO4− ions. Formerly known as permanganate of potash or Condy's crystals, it is a strong oxidizing agent. It dissolves in water to give intensely purple solutions, the...
(KMnO4) and an acidic substance, they will form a pale brown cloud of bromine gas.
- 6 Br– + 2 MnO4– + 8 H+ → 3 Br2 + 2 MnO2 + 4 H2O
This gas smells like bleach and is very irritating to the mucous membranes. Upon exposure, one should move to fresh air immediately. If symptoms of bromine poisoning arise, medical attention is needed.
See also
External links
- Theodoregray.com – Bromine
- Bromine Science and Environmental Forum (BSEF)
- Chemistry in its element podcast (MP3) from the Royal Society of ChemistryRoyal Society of ChemistryThe Royal Society of Chemistry is a learned society in the United Kingdom with the goal of "advancing the chemical sciences." It was formed in 1980 from the merger of the Chemical Society, the Royal Institute of Chemistry, the Faraday Society and the Society for Analytical Chemistry with a new...
's Chemistry WorldChemistry WorldChemistry World is a monthly chemistry news magazine published by the Royal Society of Chemistry. The magazine addresses current events in world of chemistry including research, international business news and government policy as it affects the chemical science community, plus the best product...
: Bromine

