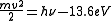Photoelectrochemical processes
Encyclopedia
Photoelectrochemical processes usually involve transforming light into other forms of energy. These processes apply to photochemistry, optically pumped lasers, sensitized solar cells, luminescence, and the effect of reversible change of color upon exposure to light.
 Electron excitation is the movement of an electron
Electron excitation is the movement of an electron
to a higher energy state. This can either be done by photoexcitation (PE), where the original electron absorbs the photon and gains all the photon's energy or by electrical excitation
(EE), where the original electron absorbs the energy of another, energetic electron. Within a semiconductor crystal lattice, thermal excitation is a process where lattice vibrations provide enough energy to move electrons to a higher energy band. When an excited electron falls back to a lower energy state again, it is called electron relaxation. This can be done by radiation of a photon or giving the energy to a third spectator particle as well.
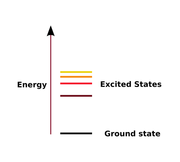
In physics there is a specific technical definition for energy level
which is often associated with an atom being excited to an excited state
. The excited state, in general, is in relation to the ground state
, where the excited state is at a higher energy level
than the ground state.
by photon
absorption, when the energy of the photon is too low to cause photoionization. The absorption of photon takes place in accordance to the Planck's Quantum Theory.
Photoexcitation plays role in photoisomerization. Photoexcitation is exploited in dye-sensitized solar cells, photochemistry
, luminescence
, optically pumped
lasers, and in some photochromic applications.
, photoisomerization is molecular
behavior in which structural change between isomer
s is caused by photoexcitation. Both reversible and irreversible photoisomerization reactions exist. However, the word "photoisomerization" usually indicates a reversible process. Photoisomerizable molecules are already put to practical use, for instance, in pigment
s for rewritable CDs
, DVDs
, and 3D optical data storage
solutions. In addition, recent interest in photoisomerizable molecules has been aimed at molecular devices, such as molecular switches, molecular motors, and molecular electronics.
Photoisomerization behavior can be roughly categorized into several classes: trans (or E) and cis (or Z) conversion, and open ring and closed ring transition. Instances of the former include stilbene
and azobenzene
. This class of compounds has a double bond
, and rotation or inversion around the double bond affords isomerization between the two states. Examples of the latter include fulgide and diarylethene
. These types of compounds undergo bond cleavage and bond creation upon irradiation with particular wavelengths of light. Sill another type is the Di-pi-methane rearrangement
.
ejects one or more electron
s from an atom
, ion
or molecule
. This is essentially the same process that occurs with the photoelectric effect with metals. In the case of a gas, the term photoionization is more common.
The ejected electrons, known as photoelectrons, carry information about their pre-ionized states. For example, a single electron can have a kinetic energy
equal to the energy of the incident photon minus the electron binding energy of the state it left. Photons with energies less than the electron binding energy may be absorbed or scattered
but will not photoionize the atom or ion.
For example, to ionize hydrogen
, photons need an energy greater than 13.6 electronvolt
s, which corresponds to a wavelength of 91.2 nm. For photons with greater energy than this, the energy of the emitted photoelectron is given by:
where h is Planck's constant and ν is the frequency
of the photon.
This formula defines the photoelectric effect
.
Not every photon which encounters an atom or ion will photoionize it. The probability of photoionization is related to the photoionization cross-section
, which depends on the energy of the photon and the target being considered. For photon energies below the ionization threshold, the photoionization cross-section is near zero. But with the development of pulsed lasers it has become possible to create extremely intense, coherent light where multi-photon ionization may occur. At even higher intensities (around 1015 - 1016 W/cm2 of infrared or visible light), non-perturbative phenomena such as barrier suppression ionization and rescattering ionization are observed.
Above-threshold ionization (ATI) is an extension of multi-photon ionization where even more photons are absorbed than actually would be necessary to ionize the atom. The excess energy gives the released electron higher kinetic energy
than the usual case of just-above threshold ionization. More precisely, The system will have multiple peaks in its photoelectron spectrum which are separated by the photon energies, this indicates that the emitted electron has more kinetic energy than in the normal (lowest possible number of photons) ionization case. The electrons released from the target will have approximately an integer number of photon-energies more kinetic energy.
effect (named after its discoverer H. Dember) consists in the formation of a charge dipole
in the vicinity of a semiconductor
surface after ultra-fast photo-generation of charge carriers. The dipole forms owing to the difference of mobilities (or diffusion constants) for holes and electrons which combined with the break of symmetry provided by the surface lead to an effective charge separation in the direction perpendicular to the surface.
s and phosphor
s must be able to absorb "light" at optical frequencies. This law provides a basis for fluorescence
and phosphorescence
. The law was first proposed in 1817 by Theodor Grotthuss and in 1842, independently, by John William Draper
.
This is considered to be one of the two basic laws of photochemistry
. The second law is the Stark–Einstein law, which says that primary chemical or physical reactions occur with each photon
absorbed.
and Albert Einstein
, who independently formulated the law between 1908 and 1913. It is also known as the photochemical equivalence law or photoequivalence law. In essence it says that every photon that is absorbed will cause a (primary) chemical or physical reaction.
The photon is a quantum of radiation, or one unit of radiation. Therefore, this is a single unit of EM radiation that is equal to Planck's constant (h) times the frequency of light. This quantity is symbolized by γ, hν, or ħω.
The photochemical equivalence law is also restated as follows: for every mole
of a substance that reacts, an equivalent mole of quanta of light are absorbed. The formula is:
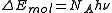
where NA is Avogadro's number
.
The photochemical equivalence law applies to the part of a light-induced reaction that is referred to as the primary process (i.e. absorption
or fluorescence
).
In most photochemical reactions the primary process is usually followed by so-called secondary photochemical processes that are normal interactions between reactants not requiring absorption of light. As a result such reactions do not appear to obey the one quantum–one molecule reactant relationship.
The law is further restricted to conventional photochemical processes using light sources with moderate intensities; high-intensity light sources such as those used in flash photolysis
and in laser experiments are known to cause so-called biphotonic processes; i.e., the absorption by a molecule of a substance of two photons of light.
, absorption of electromagnetic radiation is the way by which the energy
of a photon
is taken up by matter, typically the electrons of an atom. Thus, the electromagnetic energy is transformed to other forms of energy, for example, to heat. The absorption of light during wave propagation
is often called attenuation. Usually, the absorption of waves does not depend on their intensity (linear absorption), although in certain conditions (usually, in optics
), the medium changes its transparency dependently on the intensity of waves going through, and the Saturable absorption
(or nonlinear absorption) occurs.
of absorbed light. After absorption, the energy is transferred to the (chosen) reactants. This is part of the work of photochemistry
in general. In particular this process is commonly employed where reactions require light sources of certain wavelength
s that are not readily available.
For example, mercury
absorbs radiation at 1849 and 2537 angstrom
s, and the source is often high-intensity mercury lamps
. It is a commonly used sensitizer. When mercury vapor is mixed with ethylene
, and the compound is irradiated with a mercury lamp, this results in the photodecomposition of ethylene to acetylene. This occurs on absorption of light to yield excited state mercury atoms, which are able to transfer this energy to the ethylene molecules, and are in turn deactivated to their initial energy state.
Cadmium
; some of the noble gas
es, for example xenon
; zinc
; benzophenone
; and a large number of organic dyes, are also used as sensitizers.
Photosensitisers are a key component of photodynamic therapy
used to treat cancers.
is a chemical compound, capable of light emission after it has received energy from a molecule, which became excited previously in the chemical reaction. A good example is this:
When an alkaline solution of sodium hypochlorite and a concentrated solution of hydrogen peroxide
are mixed, a reaction occurs:
O2*is excited oxygen - meaning, one or more electrons in the O2 molecule have been promoted to higher-energy molecular orbital
s. Hence, oxygen produced by this chemical reaction somehow 'absorbed' the energy released by the reaction and became excited. This energy state is unstable, therefore it will return to the ground state
by lowering its energy. It can do that in more than one way:
The intensity, duration and color of emitted light depend on quantum
and kinetical
factors. However, excited molecules are frequently less capable of light emission in terms of brightness and duration when compared to sensitizers. This is because sensitizers can store energy (that is, be excited) for longer periods of time than other excited molecules. The energy is stored through means of quantum vibration, so sensitizers are usually compounds which either include systems of aromatic
rings or many conjugated double and triple bonds
in their structure. Hence, if an excited molecule transfers its energy to a sensitizer thus exciting it, longer and easier to quantify light emission is often observed.
The color (that is, the wavelength
), brightness and duration of emission depend upon the sensitizer used. Usually, for a certain chemical reaction, many different sensitizers can be used.
from a sample. It involves using a beam of light, usually ultraviolet light, that excites the electrons in molecules of certain compounds and causes them to emit light of a lower energy, typically, but not necessarily, visible light. A complementary technique is absorption spectroscopy
.
Devices that measure fluorescence
are called fluorometer
s or fluorimeters.
techniques that measure the absorption of radiation, as a function of frequency or wavelength, due to its interaction with a sample. The sample absorbs energy, i.e., photons, from the radiating field. The intensity of the absorption varies as a function of frequency, and this variation is the absorption spectrum. Absorption spectroscopy is performed across the electromagnetic spectrum
.
Electron excitation

Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
to a higher energy state. This can either be done by photoexcitation (PE), where the original electron absorbs the photon and gains all the photon's energy or by electrical excitation
Excited state
Excitation is an elevation in energy level above an arbitrary baseline energy state. In physics there is a specific technical definition for energy level which is often associated with an atom being excited to an excited state....
(EE), where the original electron absorbs the energy of another, energetic electron. Within a semiconductor crystal lattice, thermal excitation is a process where lattice vibrations provide enough energy to move electrons to a higher energy band. When an excited electron falls back to a lower energy state again, it is called electron relaxation. This can be done by radiation of a photon or giving the energy to a third spectator particle as well.
In physics there is a specific technical definition for energy level
Energy level
A quantum mechanical system or particle that is bound -- that is, confined spatially—can only take on certain discrete values of energy. This contrasts with classical particles, which can have any energy. These discrete values are called energy levels...
which is often associated with an atom being excited to an excited state
Excited state
Excitation is an elevation in energy level above an arbitrary baseline energy state. In physics there is a specific technical definition for energy level which is often associated with an atom being excited to an excited state....
. The excited state, in general, is in relation to the ground state
Ground state
The ground state of a quantum mechanical system is its lowest-energy state; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state...
, where the excited state is at a higher energy level
Energy level
A quantum mechanical system or particle that is bound -- that is, confined spatially—can only take on certain discrete values of energy. This contrasts with classical particles, which can have any energy. These discrete values are called energy levels...
than the ground state.
Photoexcitation
Photoexcitation is the mechanism of electron excitationElectron excitation
Electron excitation is the movement of an electron to a higher energy state. This can either be done by photoexcitation , where the original electron absorbs the photon and gains all the photon's energy or by electrical excitation , where the original electron absorbs the energy of another,...
by photon
Photon
In physics, a photon is an elementary particle, the quantum of the electromagnetic interaction and the basic unit of light and all other forms of electromagnetic radiation. It is also the force carrier for the electromagnetic force...
absorption, when the energy of the photon is too low to cause photoionization. The absorption of photon takes place in accordance to the Planck's Quantum Theory.
Photoexcitation plays role in photoisomerization. Photoexcitation is exploited in dye-sensitized solar cells, photochemistry
Photochemistry
Photochemistry, a sub-discipline of chemistry, is the study of chemical reactions that proceed with the absorption of light by atoms or molecules.. Everyday examples include photosynthesis, the degradation of plastics and the formation of vitamin D with sunlight.-Principles:Light is a type of...
, luminescence
Luminescence
Luminescence is emission of light by a substance not resulting from heat; it is thus a form of cold body radiation. It can be caused by chemical reactions, electrical energy, subatomic motions, or stress on a crystal. This distinguishes luminescence from incandescence, which is light emitted by a...
, optically pumped
Laser pumping
Laser pumping is the act of energy transfer from an external source into the gain medium of a laser. The energy is absorbed in the medium, producing excited states in its atoms. When the number of particles in one excited state exceeds the number of particles in the ground state or a less-excited...
lasers, and in some photochromic applications.
Photoisomerization
In chemistryChemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
, photoisomerization is molecular
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
behavior in which structural change between isomer
Isomer
In chemistry, isomers are compounds with the same molecular formula but different structural formulas. Isomers do not necessarily share similar properties, unless they also have the same functional groups. There are many different classes of isomers, like stereoisomers, enantiomers, geometrical...
s is caused by photoexcitation. Both reversible and irreversible photoisomerization reactions exist. However, the word "photoisomerization" usually indicates a reversible process. Photoisomerizable molecules are already put to practical use, for instance, in pigment
Pigment
A pigment is a material that changes the color of reflected or transmitted light as the result of wavelength-selective absorption. This physical process differs from fluorescence, phosphorescence, and other forms of luminescence, in which a material emits light.Many materials selectively absorb...
s for rewritable CDs
CD-RW
A CD-RW is a rewritable optical disc. It was introduced in 1997, and was known as "CD-Writable" during development. It was preceded by the CD-MO, which was never commercially released....
, DVDs
DVD-RW
A DVD-RW disc is a rewritable optical disc with equal storage capacity to a DVD-R, typically 4.7 GB. The format was developed by Pioneer in November 1999 and has been approved by the DVD Forum. The smaller Mini DVD-RW holds 1.46 GB, with a diameter of 8 cm.The primary advantage of DVD-RW over...
, and 3D optical data storage
3D optical data storage
3D optical data storage is the term given to any form of optical data storage in which information can be recorded and/or read with three dimensional resolution ....
solutions. In addition, recent interest in photoisomerizable molecules has been aimed at molecular devices, such as molecular switches, molecular motors, and molecular electronics.
Photoisomerization behavior can be roughly categorized into several classes: trans (or E) and cis (or Z) conversion, and open ring and closed ring transition. Instances of the former include stilbene
Stilbene
-Stilbene, is a diarylethene, i.e., a hydrocarbon consisting of a trans ethene double bond substituted with a phenyl group on both carbon atoms of the double bond. The name stilbene is derived from the Greek word stilbos, which means shining....
and azobenzene
Azobenzene
Azobenzene is a chemical compound composed of two phenyl rings linked by a N=N double bond. It is the best known example of an azo compound. The term 'azobenzene' or simply 'azo' is often used to refer to a wide class of molecules that share the core azobenzene structure, with different chemical...
. This class of compounds has a double bond
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
, and rotation or inversion around the double bond affords isomerization between the two states. Examples of the latter include fulgide and diarylethene
Diarylethene
In chemistry, diarylethene is the general name of a class of compounds that have aromatic groups bonded to each end of a carbon-carbon double bond...
. These types of compounds undergo bond cleavage and bond creation upon irradiation with particular wavelengths of light. Sill another type is the Di-pi-methane rearrangement
Di-pi-methane rearrangement
The di-pi-methane rearrangement is a photochemical reaction of a molecular entity comprising two π-systems, separated by a saturated carbon atom , to form an ene- substituted cyclopropane...
.
Photoionization
Photoionization is the physical process in which an incident photonPhoton
In physics, a photon is an elementary particle, the quantum of the electromagnetic interaction and the basic unit of light and all other forms of electromagnetic radiation. It is also the force carrier for the electromagnetic force...
ejects one or more electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s from an atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
, ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
or molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
. This is essentially the same process that occurs with the photoelectric effect with metals. In the case of a gas, the term photoionization is more common.
The ejected electrons, known as photoelectrons, carry information about their pre-ionized states. For example, a single electron can have a kinetic energy
Kinetic energy
The kinetic energy of an object is the energy which it possesses due to its motion.It is defined as the work needed to accelerate a body of a given mass from rest to its stated velocity. Having gained this energy during its acceleration, the body maintains this kinetic energy unless its speed changes...
equal to the energy of the incident photon minus the electron binding energy of the state it left. Photons with energies less than the electron binding energy may be absorbed or scattered
Scattering
Scattering is a general physical process where some forms of radiation, such as light, sound, or moving particles, are forced to deviate from a straight trajectory by one or more localized non-uniformities in the medium through which they pass. In conventional use, this also includes deviation of...
but will not photoionize the atom or ion.
For example, to ionize hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
, photons need an energy greater than 13.6 electronvolt
Electronvolt
In physics, the electron volt is a unit of energy equal to approximately joule . By definition, it is equal to the amount of kinetic energy gained by a single unbound electron when it accelerates through an electric potential difference of one volt...
s, which corresponds to a wavelength of 91.2 nm. For photons with greater energy than this, the energy of the emitted photoelectron is given by:
where h is Planck's constant and ν is the frequency
Frequency
Frequency is the number of occurrences of a repeating event per unit time. It is also referred to as temporal frequency.The period is the duration of one cycle in a repeating event, so the period is the reciprocal of the frequency...
of the photon.
This formula defines the photoelectric effect
Photoelectric effect
In the photoelectric effect, electrons are emitted from matter as a consequence of their absorption of energy from electromagnetic radiation of very short wavelength, such as visible or ultraviolet light. Electrons emitted in this manner may be referred to as photoelectrons...
.
Not every photon which encounters an atom or ion will photoionize it. The probability of photoionization is related to the photoionization cross-section
Photoionisation cross section
Photoionisation cross section in the context of condensed matter physics refers to the probability of a particle being emitted from its electronic state.- Cross section in photoemission :...
, which depends on the energy of the photon and the target being considered. For photon energies below the ionization threshold, the photoionization cross-section is near zero. But with the development of pulsed lasers it has become possible to create extremely intense, coherent light where multi-photon ionization may occur. At even higher intensities (around 1015 - 1016 W/cm2 of infrared or visible light), non-perturbative phenomena such as barrier suppression ionization and rescattering ionization are observed.
Multi-photon ionization
Several photons of energy below the ionization threshold may actually combine their energies to ionize an atom. This probability decreases rapidly with the number of photons required, but the development of very intense, pulsed lasers still makes it possible. In the perturbative regime (below about 1014 W/cm2 at optical frequencies), the probability of absorbing N photons depends on the laser-light intensity I as IN .Above-threshold ionization (ATI) is an extension of multi-photon ionization where even more photons are absorbed than actually would be necessary to ionize the atom. The excess energy gives the released electron higher kinetic energy
Kinetic energy
The kinetic energy of an object is the energy which it possesses due to its motion.It is defined as the work needed to accelerate a body of a given mass from rest to its stated velocity. Having gained this energy during its acceleration, the body maintains this kinetic energy unless its speed changes...
than the usual case of just-above threshold ionization. More precisely, The system will have multiple peaks in its photoelectron spectrum which are separated by the photon energies, this indicates that the emitted electron has more kinetic energy than in the normal (lowest possible number of photons) ionization case. The electrons released from the target will have approximately an integer number of photon-energies more kinetic energy.
Photo-Dember
In semiconductor physics the Photo-DemberPhoto-dember
In semiconductor physics, the photo-Dember effect consists in the formation of a charge dipole in the vicinity of a semiconductor surface after ultra-fast photo-generation of charge carriers...
effect (named after its discoverer H. Dember) consists in the formation of a charge dipole
Dipole
In physics, there are several kinds of dipoles:*An electric dipole is a separation of positive and negative charges. The simplest example of this is a pair of electric charges of equal magnitude but opposite sign, separated by some distance. A permanent electric dipole is called an electret.*A...
in the vicinity of a semiconductor
Semiconductor
A semiconductor is a material with electrical conductivity due to electron flow intermediate in magnitude between that of a conductor and an insulator. This means a conductivity roughly in the range of 103 to 10−8 siemens per centimeter...
surface after ultra-fast photo-generation of charge carriers. The dipole forms owing to the difference of mobilities (or diffusion constants) for holes and electrons which combined with the break of symmetry provided by the surface lead to an effective charge separation in the direction perpendicular to the surface.
Grotthuss–Draper law
The Grotthuss–Draper law (also called the Principle of Photochemical Activation) states that only that light which is absorbed by a system can bring about a photochemical change. Materials such as dyeDye
A dye is a colored substance that has an affinity to the substrate to which it is being applied. The dye is generally applied in an aqueous solution, and requires a mordant to improve the fastness of the dye on the fiber....
s and phosphor
Phosphor
A phosphor, most generally, is a substance that exhibits the phenomenon of luminescence. Somewhat confusingly, this includes both phosphorescent materials, which show a slow decay in brightness , and fluorescent materials, where the emission decay takes place over tens of nanoseconds...
s must be able to absorb "light" at optical frequencies. This law provides a basis for fluorescence
Fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation of a different wavelength. It is a form of luminescence. In most cases, emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation...
and phosphorescence
Phosphorescence
Phosphorescence is a specific type of photoluminescence related to fluorescence. Unlike fluorescence, a phosphorescent material does not immediately re-emit the radiation it absorbs. The slower time scales of the re-emission are associated with "forbidden" energy state transitions in quantum...
. The law was first proposed in 1817 by Theodor Grotthuss and in 1842, independently, by John William Draper
John William Draper
John William Draper was an American scientist, philosopher, physician, chemist, historian, and photographer. He is credited with producing the first clear photograph of a female face and the first detailed photograph of the Moon...
.
This is considered to be one of the two basic laws of photochemistry
Photochemistry
Photochemistry, a sub-discipline of chemistry, is the study of chemical reactions that proceed with the absorption of light by atoms or molecules.. Everyday examples include photosynthesis, the degradation of plastics and the formation of vitamin D with sunlight.-Principles:Light is a type of...
. The second law is the Stark–Einstein law, which says that primary chemical or physical reactions occur with each photon
Photon
In physics, a photon is an elementary particle, the quantum of the electromagnetic interaction and the basic unit of light and all other forms of electromagnetic radiation. It is also the force carrier for the electromagnetic force...
absorbed.
Stark–Einstein law
The Stark–Einstein law is named after German-born physicists Johannes StarkJohannes Stark
Johannes Stark was a German physicist, and Physics Nobel Prize laureate who was closely involved with the Deutsche Physik movement under the Nazi regime.-Early years:...
and Albert Einstein
Albert Einstein
Albert Einstein was a German-born theoretical physicist who developed the theory of general relativity, effecting a revolution in physics. For this achievement, Einstein is often regarded as the father of modern physics and one of the most prolific intellects in human history...
, who independently formulated the law between 1908 and 1913. It is also known as the photochemical equivalence law or photoequivalence law. In essence it says that every photon that is absorbed will cause a (primary) chemical or physical reaction.
The photon is a quantum of radiation, or one unit of radiation. Therefore, this is a single unit of EM radiation that is equal to Planck's constant (h) times the frequency of light. This quantity is symbolized by γ, hν, or ħω.
The photochemical equivalence law is also restated as follows: for every mole
Mole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
of a substance that reacts, an equivalent mole of quanta of light are absorbed. The formula is:
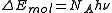
where NA is Avogadro's number
Avogadro's number
In chemistry and physics, the Avogadro constant is defined as the ratio of the number of constituent particles N in a sample to the amount of substance n through the relationship NA = N/n. Thus, it is the proportionality factor that relates the molar mass of an entity, i.e...
.
The photochemical equivalence law applies to the part of a light-induced reaction that is referred to as the primary process (i.e. absorption
Absorption (electromagnetic radiation)
In physics, absorption of electromagnetic radiation is the way by which the energy of a photon is taken up by matter, typically the electrons of an atom. Thus, the electromagnetic energy is transformed to other forms of energy for example, to heat. The absorption of light during wave propagation is...
or fluorescence
Fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation of a different wavelength. It is a form of luminescence. In most cases, emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation...
).
In most photochemical reactions the primary process is usually followed by so-called secondary photochemical processes that are normal interactions between reactants not requiring absorption of light. As a result such reactions do not appear to obey the one quantum–one molecule reactant relationship.
The law is further restricted to conventional photochemical processes using light sources with moderate intensities; high-intensity light sources such as those used in flash photolysis
Flash photolysis
Flash photolysis is a pump-probe laboratory technique, in which a sample is firstly excited by a strong pulse of light from a laser of nanosecond, picosecond, or femtosecond pulse width or by a short-pulse light source such as a flash lamp...
and in laser experiments are known to cause so-called biphotonic processes; i.e., the absorption by a molecule of a substance of two photons of light.
Absorption
In physicsPhysics
Physics is a natural science that involves the study of matter and its motion through spacetime, along with related concepts such as energy and force. More broadly, it is the general analysis of nature, conducted in order to understand how the universe behaves.Physics is one of the oldest academic...
, absorption of electromagnetic radiation is the way by which the energy
Energy
In physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
of a photon
Photon
In physics, a photon is an elementary particle, the quantum of the electromagnetic interaction and the basic unit of light and all other forms of electromagnetic radiation. It is also the force carrier for the electromagnetic force...
is taken up by matter, typically the electrons of an atom. Thus, the electromagnetic energy is transformed to other forms of energy, for example, to heat. The absorption of light during wave propagation
Wave propagation
Wave propagation is any of the ways in which waves travel.With respect to the direction of the oscillation relative to the propagation direction, we can distinguish between longitudinal wave and transverse waves....
is often called attenuation. Usually, the absorption of waves does not depend on their intensity (linear absorption), although in certain conditions (usually, in optics
Optics
Optics is the branch of physics which involves the behavior and properties of light, including its interactions with matter and the construction of instruments that use or detect it. Optics usually describes the behavior of visible, ultraviolet, and infrared light...
), the medium changes its transparency dependently on the intensity of waves going through, and the Saturable absorption
Saturable absorption
Saturable absorption is a property of materials where the absorption of light decreases with increasing light intensity. Most materials show some saturable absorption, but often only at very high optical intensities ....
(or nonlinear absorption) occurs.
Photosensitization
Photosensitization is a process of transferring the energyEnergy
In physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
of absorbed light. After absorption, the energy is transferred to the (chosen) reactants. This is part of the work of photochemistry
Photochemistry
Photochemistry, a sub-discipline of chemistry, is the study of chemical reactions that proceed with the absorption of light by atoms or molecules.. Everyday examples include photosynthesis, the degradation of plastics and the formation of vitamin D with sunlight.-Principles:Light is a type of...
in general. In particular this process is commonly employed where reactions require light sources of certain wavelength
Wavelength
In physics, the wavelength of a sinusoidal wave is the spatial period of the wave—the distance over which the wave's shape repeats.It is usually determined by considering the distance between consecutive corresponding points of the same phase, such as crests, troughs, or zero crossings, and is a...
s that are not readily available.
For example, mercury
Mercury (element)
Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum...
absorbs radiation at 1849 and 2537 angstrom
Ångström
The angstrom or ångström, is a unit of length equal to 1/10,000,000,000 of a meter . Its symbol is the Swedish letter Å....
s, and the source is often high-intensity mercury lamps
Arc lamp
"Arc lamp" or "arc light" is the general term for a class of lamps that produce light by an electric arc . The lamp consists of two electrodes, first made from carbon but typically made today of tungsten, which are separated by a gas...
. It is a commonly used sensitizer. When mercury vapor is mixed with ethylene
Ethylene
Ethylene is a gaseous organic compound with the formula . It is the simplest alkene . Because it contains a carbon-carbon double bond, ethylene is classified as an unsaturated hydrocarbon. Ethylene is widely used in industry and is also a plant hormone...
, and the compound is irradiated with a mercury lamp, this results in the photodecomposition of ethylene to acetylene. This occurs on absorption of light to yield excited state mercury atoms, which are able to transfer this energy to the ethylene molecules, and are in turn deactivated to their initial energy state.
Cadmium
Cadmium
Cadmium is a chemical element with the symbol Cd and atomic number 48. This soft, bluish-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Similar to zinc, it prefers oxidation state +2 in most of its compounds and similar to mercury it shows a low...
; some of the noble gas
Noble gas
The noble gases are a group of chemical elements with very similar properties: under standard conditions, they are all odorless, colorless, monatomic gases, with very low chemical reactivity...
es, for example xenon
Xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. The element name is pronounced or . A colorless, heavy, odorless noble gas, xenon occurs in the Earth's atmosphere in trace amounts...
; zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
; benzophenone
Benzophenone
Benzophenone is the organic compound with the formula 2CO, generally abbreviated Ph2CO. Benzophenone is a widely used building block in organic chemistry, being the parent diarylketone.-Uses:...
; and a large number of organic dyes, are also used as sensitizers.
Photosensitisers are a key component of photodynamic therapy
Photodynamic therapy
Photodynamic therapy is used clinically to treat a wide range of medical conditions, including malignant cancers, and is recognised as a treatment strategy which is both minimally invasive and minimally toxic...
used to treat cancers.
Sensitizer
A sensitizer in chemoluminescenceChemoluminescence
Chemiluminescence is the emission of light with limited emission of heat , as the result of a chemical reaction...
is a chemical compound, capable of light emission after it has received energy from a molecule, which became excited previously in the chemical reaction. A good example is this:
When an alkaline solution of sodium hypochlorite and a concentrated solution of hydrogen peroxide
Hydrogen peroxide
Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
are mixed, a reaction occurs:
- ClO-(aq) + H2O2(aq) → O2*(g) + H+(aq) + Cl-(aq) + OH-(aq)
O2*is excited oxygen - meaning, one or more electrons in the O2 molecule have been promoted to higher-energy molecular orbital
Molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term "orbital" was first...
s. Hence, oxygen produced by this chemical reaction somehow 'absorbed' the energy released by the reaction and became excited. This energy state is unstable, therefore it will return to the ground state
Ground state
The ground state of a quantum mechanical system is its lowest-energy state; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state...
by lowering its energy. It can do that in more than one way:
- it can react further, without any light emission
- it can lose energy without emission, for example, giving off heat to the surroundings or transferring energy to another molecule
- it can emit light
The intensity, duration and color of emitted light depend on quantum
Quantum mechanics
Quantum mechanics, also known as quantum physics or quantum theory, is a branch of physics providing a mathematical description of much of the dual particle-like and wave-like behavior and interactions of energy and matter. It departs from classical mechanics primarily at the atomic and subatomic...
and kinetical
Chemical kinetics
Chemical kinetics, also known as reaction kinetics, is the study of rates of chemical processes. Chemical kinetics includes investigations of how different experimental conditions can influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition...
factors. However, excited molecules are frequently less capable of light emission in terms of brightness and duration when compared to sensitizers. This is because sensitizers can store energy (that is, be excited) for longer periods of time than other excited molecules. The energy is stored through means of quantum vibration, so sensitizers are usually compounds which either include systems of aromatic
Aromaticity
In organic chemistry, Aromaticity is a chemical property in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. The earliest use of the term was in an article by August...
rings or many conjugated double and triple bonds
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
in their structure. Hence, if an excited molecule transfers its energy to a sensitizer thus exciting it, longer and easier to quantify light emission is often observed.
The color (that is, the wavelength
Wavelength
In physics, the wavelength of a sinusoidal wave is the spatial period of the wave—the distance over which the wave's shape repeats.It is usually determined by considering the distance between consecutive corresponding points of the same phase, such as crests, troughs, or zero crossings, and is a...
), brightness and duration of emission depend upon the sensitizer used. Usually, for a certain chemical reaction, many different sensitizers can be used.
List of some common sensitizers
- Violanthrone
- Isoviolanthrone
- FluoresceinFluoresceinFluorescein is a synthetic organic compound available as a dark orange/red powder soluble in water and alcohol. It is widely used as a fluorescent tracer for many applications....
e - RubreneRubreneRubrene is a red colored polycyclic aromatic hydrocarbon. Rubrene is used as a sensitiser in chemoluminescence and as a yellow light source in lightsticks....
- 9,10-Diphenylanthracene9,10-Diphenylanthracene9,10-Diphenylanthracene is a polycyclic aromatic hydrocarbon. It has the appearance of a slightly yellow powder. 9,10-Diphenylanthracene is used as a sensitiser in chemiluminescence. In lightsticks it is used to produce blue light. It is a molecular organic semiconductor, used in blue OLEDs and...
- TetraceneTetraceneTetracene, also called naphthacene, is a polycyclic aromatic hydrocarbon. It has the appearance of a pale orange powder. Tetracene is the four-ringed member of the series of acenes, the previous one being anthracene and the next one being pentacene.Tetracene is a molecular organic semiconductor,...
- 13,13'-Dibenzantronile
- Levulinic AcidLevulinic acidLevulinic acid, or 4-oxopentanoic acid, is an organic compound with the formula CH3CCH2CH2CO2H. It is a keto acid. This white crystalline is soluble in water, ethanol, and diethyl ether.-Synthesis and uses:...
Fluorescence spectroscopy
Fluorescence spectroscopy aka fluorometry or spectrofluorometry, is a type of electromagnetic spectroscopy which analyzes fluorescenceFluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation of a different wavelength. It is a form of luminescence. In most cases, emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation...
from a sample. It involves using a beam of light, usually ultraviolet light, that excites the electrons in molecules of certain compounds and causes them to emit light of a lower energy, typically, but not necessarily, visible light. A complementary technique is absorption spectroscopy
Absorption spectroscopy
Absorption spectroscopy refers to spectroscopic techniques that measure the absorption of radiation, as a function of frequency or wavelength, due to its interaction with a sample. The sample absorbs energy, i.e., photons, from the radiating field. The intensity of the absorption varies as a...
.
Devices that measure fluorescence
Fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation of a different wavelength. It is a form of luminescence. In most cases, emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation...
are called fluorometer
Fluorometer
A fluorometer or fluorimeter is a device used to measure parameters of fluorescence: its intensity and wavelength distribution of emission spectrum after excitation by a certain spectrum of light. These parameters are used to identify the presence and the amount of specific molecules in a medium...
s or fluorimeters.
Absorption spectroscopy
Absorption spectroscopy refers to spectroscopicSpectroscopy
Spectroscopy is the study of the interaction between matter and radiated energy. Historically, spectroscopy originated through the study of visible light dispersed according to its wavelength, e.g., by a prism. Later the concept was expanded greatly to comprise any interaction with radiative...
techniques that measure the absorption of radiation, as a function of frequency or wavelength, due to its interaction with a sample. The sample absorbs energy, i.e., photons, from the radiating field. The intensity of the absorption varies as a function of frequency, and this variation is the absorption spectrum. Absorption spectroscopy is performed across the electromagnetic spectrum
Electromagnetic spectrum
The electromagnetic spectrum is the range of all possible frequencies of electromagnetic radiation. The "electromagnetic spectrum" of an object is the characteristic distribution of electromagnetic radiation emitted or absorbed by that particular object....
.
See also
- Ionization energyIonization energyThe ionization energy of a chemical species, i.e. an atom or molecule, is the energy required to remove an electron from the species to a practically infinite distance. Large atoms or molecules have a low ionization energy, while small molecules tend to have higher ionization energies.The property...
- Isomerization
- Photoionization modePhotoionization modeA photoionization mode is a mode of interaction between a laser beam and matter, giving rise to a photoionization pattern, having very specific characteristics in terms of photoionization spatial distribution and density, as well as relative yields of photolytic species...
- PhotochromismPhotochromismPhotochromism is the reversible transformation of a chemical species between two forms by the absorption of electromagnetic radiation, where the two forms have different absorption spectra. Trivially, this can be described as a reversible change of colour upon exposure to light...
- Photoelectric effectPhotoelectric effectIn the photoelectric effect, electrons are emitted from matter as a consequence of their absorption of energy from electromagnetic radiation of very short wavelength, such as visible or ultraviolet light. Electrons emitted in this manner may be referred to as photoelectrons...
- Photoionization detectorPhotoionization detectorA photoionization detector or PID is a type of gas detector.Typical photoionization detectors measure volatile organic compounds and other gases in concentrations from sub parts per billion to 10 000 parts per million . The photoionizaton detector is an efficient and inexpensive detector for many...