
Absorption spectroscopy
Encyclopedia
Absorption spectroscopy refers to spectroscopic
techniques that measure the absorption
of radiation
, as a function of frequency
or wavelength
, due to its interaction with a sample. The sample absorbs energy, i.e., photons, from the radiating field. The intensity of the absorption varies as a function of frequency, and this variation is the absorption spectrum. Absorption spectroscopy is performed across the electromagnetic spectrum
.
Absorption spectroscopy is employed as an analytical chemistry tool to determine the presence of a particular substance in a sample and, in many cases, to quantify the amount of the substance present. Infrared
and ultraviolet-visible spectroscopy
are particularly common in analytical applications. Absorption spectroscopy is also employed in studies of molecular and atomic physics, astronomical spectroscopy and remote sensing.
There are a wide range of experimental approaches to measuring absorption spectra. The most common arrangement is to direct a generated beam of radiation at a sample and detect the intensity of the radiation that passes through it. The transmitted energy can be used to calculate the absorption. The source, sample arrangement and detection technique vary significantly depending on the frequency range and the purpose of the experiment.
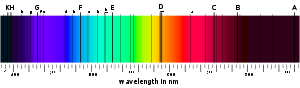 A material's absorption spectrum is the fraction of incident radiation absorbed by the material over a range of frequencies. The absorption spectrum is primarily determined by the atom
A material's absorption spectrum is the fraction of incident radiation absorbed by the material over a range of frequencies. The absorption spectrum is primarily determined by the atom
ic and molecular
composition of the material. Radiation is more likely to be absorbed at frequencies that match the energy difference between two quantum mechanical states of the molecules. The absorption that occurs due to a transition between two states is referred to as an absorption line
and a spectrum is typically composed of many lines.
The frequencies where absorption lines occur, as well as their relative intensities, primarily depend on the electronic and molecular structure
of the molecule. The frequencies will also depend on the interactions between molecules in the sample, the crystal structure
in solids, and on several environmental factors (e.g., temperature
, pressure
, electromagnetic field
). The lines will also have a width
and shape that are primarily determined by the spectral density
or the density of states
of the system.
, for instance, occur when the rotational state of a molecule is changed. Rotational lines are typically found in the microwave spectral region. Vibrational lines correspond to changes in the vibrational state of the molecule and are typically found in the infrared region. Electronic lines correspond to a change in the electronic state of an atom or molecule and are typically found in the visible and ultraviolet region. X-ray absorptions are associated with the excitation of inner shell electrons in atoms. These changes can also be combined (e.g. rotation-vibration transitions
), leading to new absorption lines at the combined energy of the two changes.
The energy associated with the quantum mechanical change primarily determines the frequency of the absorption line but the frequency can be shifted by several types of interactions. Electric and magnetic fields can cause a shift. Interactions with neighboring molecules can cause shifts. For instance, absorption lines of the gas phase molecule can shift significantly when that molecule is in a liquid or solid phase and interacting more strongly with neighboring molecules.
Absorption lines are often depicted as infinitesimally thin lines, i.e., delta function
s, but observed lines always have a shape that is determined by the instrument used for the observation, the material absorbing the radiation and the physical environment of that material. It is common for lines to have the shape of a Gaussian or Lorentzian distribution. It is also common for a line to be characterized solely by its intensity and width
instead of the entire shape being characterized.
The integrated intensity—obtained by integrating
the area under the absorption line—is proportional to the amount of the absorbing substance present. The intensity is also related to the temperature of the substance and the quantum mechanical interaction between the radiation and the absorber. This interaction is quantified by the transition moment and depends on the particular lower state the transition starts from and the upper state it is connected to.
The width of absorption lines may be determined by the spectrometer
used to record it. A spectrometer has an inherent limit on how narrow a line it can resolve
and so the observed width may be at this limit. If the width is larger than the resolution limit, then it is primarily determined by the environment of the absorber. A liquid or solid absorber, in which neighboring molecules strongly interact with one another, tends to have broader absorption lines than a gas. Increasing the temperature or pressure of the absorbing material will also tend to increase the line width. It is also common for several neighboring transitions to be close enough to one another that their lines overlap and the resulting overall line is therefore broader yet.
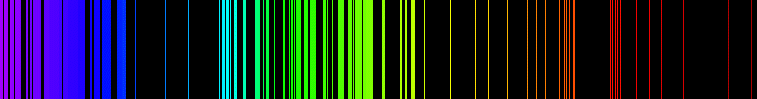 Emission is a process by which a substance releases energy in the form of electromagnetic radiation. Emission can occur at any frequency at which absorption can occur, and this allows the absorption lines to be determined from an emission spectrum. The emission spectrum
Emission is a process by which a substance releases energy in the form of electromagnetic radiation. Emission can occur at any frequency at which absorption can occur, and this allows the absorption lines to be determined from an emission spectrum. The emission spectrum
will typically have a quite different intensity pattern from the absorption spectrum, though, so the two are not equivalent. The absorption spectrum can be calculated from the emission spectrum using appropriate theoretical models and additional information about the quantum mechanical states of the substance.
. Therefore, the absorption spectrum can be derived from a scattering or reflection spectrum. This typically requires simplifying assumptions or models, and so the derived absorption spectrum is an approximation.
s can be used to identify the presence of pollutants in the air, distinguishing the pollutant from nitrogen, oxygen, water and other expected constituents.
The specificity also allows unknown samples to be identified by comparing a measured spectrum with a library of reference spectra. In many cases, it is possible to determine qualitative information about a sample even if it is not in a library. Infrared spectra, for instance, have characteristics absorption bands that indicate if carbon-hydrogen or carbon-oxygen bonds are present.
An absorption spectrum can be quantitatively related to the amount of material present using the Beer-Lambert law
. Determining the absolute concentration of a compound requires knowledge of the compound's absorption coefficient. The absorption coefficient for some compounds is available from reference sources, and it can also be determined by measuring the spectrum of a calibration standard with a known concentration of the target.
. Remote spectral sensing is valuable in many situations. For example, measurements can be made in toxic or hazardous environments without placing an operator or instrument at risk. Also, sample material does not have to be brought into contact with the instrument—preventing possible cross contamination.
Remote spectral measurements present several challenges compared to laboratory measurements. The space in between the sample of interest and the instrument may also have spectral absorptions. These absorptions can mask or confound the absorption spectrum of the sample. These background interferences may also vary over time. The source of radiation in remote measurements is often an environmental source, such as sunlight or the thermal radiation from a warm object, and this makes it necessary to distinguish spectral absorption from changes in the source spectrum.
 Astronomical spectroscopy
Astronomical spectroscopy
is a particularly significant type of remote spectral sensing. In this case, the objects and samples of interest are so distant from earth that electromagnetic radiation is the only means available to measure them. Astronomical spectra contain both absorption and emission spectral information. Absorption spectroscopy has been particularly important for understanding interstellar clouds and determining that some of them contain molecules
. Absorption spectroscopy is also employed in the study of extrasolar planets. Detection of extrasolar planets by the transit method also measures their absorption spectrum and allows for the determination of the planet's atmospheric composition.
models, allow for the absorption spectra of atoms and molecules to be related to other physical properties such as electronic structure, atomic
or molecular mass
, and molecular geometry
. Therefore, measurements of the absorption spectrum are used to determine these other properties. Microwave spectroscopy, for example, allows for the determination of bond lengths and angles with high precision.
In addition, spectral measurements can be used to determine the accuracy of theoretical predictions. For example, the Lamb shift measured in the hydrogen
atomic absorption spectrum was not expected to exist at the time it was measured. Its discovery spurred and guided the development of quantum electrodynamics
, and measurements of the Lamb shift are now used to determine the fine-structure constant
.
and then re-measure the sample spectrum after placing the material of interest in between the source and detector. The two measured spectra can then be combined to determine the material's absorption spectrum. The sample spectrum alone is not sufficient to determine the absorption spectrum because it will be affected by the experimental conditions—the spectrum of the source, the absorption spectra of other materials in between the source and detector and the wavelength dependent characteristics of the detector. The reference spectrum will be affected in the same way, though, by these experimental conditions and therefore the combination yields the absorption spectrum of the material alone.
A wide variety of radiation sources are employed in order to cover the electromagnetic spectrum. For spectroscopy, it is generally desirable for a source to cover a broad swath of wavelengths in order to measure a broad region of the absorption spectrum. Some sources inherently emit a broad spectrum. Examples of these include globar
s or other black body
sources in the infrared, mercury lamps in the visible and ultraviolet and x-ray tube
s. One recently developed, novel source of broad spectrum radiation is synchotron radiation which covers all of these spectral regions. Other radiation sources generate a narrow spectrum but the emission wavelength can be tuned to cover a spectral range. Examples of these include klystron
s in the microwave region and laser
s across the infrared, visible and ultraviolet region (though not all lasers have tunable wavelengths).
The detector employed to measure the radiation power will also depend on the wavelength range of interest. Most detectors are sensitive to a fairly broad spectral range and the sensor selected will often depend more on the sensitivity and noise requirements of a given measurement. Examples of detectors common in spectroscopy include heterodyne receivers in the microwave, bolometer
s in the millimeter-wave and infrared, mercury cadmium telluride and other cooled semiconductor
detectors in the infrared, and photodiode
s and photomultiplier tubes
in the visible and ultraviolet.
If both the source and the detector cover a broad spectral region, then it is also necessary to introduce a means of resolving
the wavelength of the radiation in order to determine the spectrum. Often a spectrograph is used to spatially separate the wavelengths of radiation so that the power at each wavelength can be measured independently. It is also common to employ interferometry to determine resolve the spectrum—Fourier transform infrared spectroscopy is a widely used implementation of this technique.
Two other issues that must be considered in setting up an absorption spectroscopy experiment include the optics
used to direct the radiation and the means of holding or containing the sample material (called a cuvette
or cell). For most UV, visible, and NIR measurements the use of precision quartz cuvettes are necessary.. In both cases, it is important to select materials that have relatively little absorption of their own in the wavelength range of interest. The absorption of other materials could interfere with or mask the absorption from the sample. For instance, in several wavelength ranges it is necessary to measure the sample under vacuum
or in a rare gas environment because gases in the atmosphere have interfering absorption features.
Spectroscopy
Spectroscopy is the study of the interaction between matter and radiated energy. Historically, spectroscopy originated through the study of visible light dispersed according to its wavelength, e.g., by a prism. Later the concept was expanded greatly to comprise any interaction with radiative...
techniques that measure the absorption
Absorption (electromagnetic radiation)
In physics, absorption of electromagnetic radiation is the way by which the energy of a photon is taken up by matter, typically the electrons of an atom. Thus, the electromagnetic energy is transformed to other forms of energy for example, to heat. The absorption of light during wave propagation is...
of radiation
Electromagnetic radiation
Electromagnetic radiation is a form of energy that exhibits wave-like behavior as it travels through space...
, as a function of frequency
Frequency
Frequency is the number of occurrences of a repeating event per unit time. It is also referred to as temporal frequency.The period is the duration of one cycle in a repeating event, so the period is the reciprocal of the frequency...
or wavelength
Wavelength
In physics, the wavelength of a sinusoidal wave is the spatial period of the wave—the distance over which the wave's shape repeats.It is usually determined by considering the distance between consecutive corresponding points of the same phase, such as crests, troughs, or zero crossings, and is a...
, due to its interaction with a sample. The sample absorbs energy, i.e., photons, from the radiating field. The intensity of the absorption varies as a function of frequency, and this variation is the absorption spectrum. Absorption spectroscopy is performed across the electromagnetic spectrum
Electromagnetic spectrum
The electromagnetic spectrum is the range of all possible frequencies of electromagnetic radiation. The "electromagnetic spectrum" of an object is the characteristic distribution of electromagnetic radiation emitted or absorbed by that particular object....
.
Absorption spectroscopy is employed as an analytical chemistry tool to determine the presence of a particular substance in a sample and, in many cases, to quantify the amount of the substance present. Infrared
Infrared spectroscopy
Infrared spectroscopy is the spectroscopy that deals with the infrared region of the electromagnetic spectrum, that is light with a longer wavelength and lower frequency than visible light. It covers a range of techniques, mostly based on absorption spectroscopy. As with all spectroscopic...
and ultraviolet-visible spectroscopy
Ultraviolet-visible spectroscopy
Ultraviolet-visible spectroscopy or ultraviolet-visible spectrophotometry refers to absorption spectroscopy or reflectance spectroscopy in the ultraviolet-visible spectral region. This means it uses light in the visible and adjacent ranges...
are particularly common in analytical applications. Absorption spectroscopy is also employed in studies of molecular and atomic physics, astronomical spectroscopy and remote sensing.
There are a wide range of experimental approaches to measuring absorption spectra. The most common arrangement is to direct a generated beam of radiation at a sample and detect the intensity of the radiation that passes through it. The transmitted energy can be used to calculate the absorption. The source, sample arrangement and detection technique vary significantly depending on the frequency range and the purpose of the experiment.
Absorption spectrum

Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
ic and molecular
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
composition of the material. Radiation is more likely to be absorbed at frequencies that match the energy difference between two quantum mechanical states of the molecules. The absorption that occurs due to a transition between two states is referred to as an absorption line
Spectral line
A spectral line is a dark or bright line in an otherwise uniform and continuous spectrum, resulting from a deficiency or excess of photons in a narrow frequency range, compared with the nearby frequencies.- Types of line spectra :...
and a spectrum is typically composed of many lines.
The frequencies where absorption lines occur, as well as their relative intensities, primarily depend on the electronic and molecular structure
Molecular structure
The molecular structure of a substance is described by the combination of nuclei and electrons that comprise its constitute molecules. This includes the molecular geometry , the electronic properties of the...
of the molecule. The frequencies will also depend on the interactions between molecules in the sample, the crystal structure
Crystal
A crystal or crystalline solid is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. The scientific study of crystals and crystal formation is known as crystallography...
in solids, and on several environmental factors (e.g., temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
, pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
, electromagnetic field
Electric field
In physics, an electric field surrounds electrically charged particles and time-varying magnetic fields. The electric field depicts the force exerted on other electrically charged objects by the electrically charged particle the field is surrounding...
). The lines will also have a width
Spectral linewidth
The spectral linewidth characterizes the width of a spectral line, such as in the electromagnetic emission spectrum of an atom, or the frequency spectrum of an acoustic or electronic system...
and shape that are primarily determined by the spectral density
Spectral density
In statistical signal processing and physics, the spectral density, power spectral density , or energy spectral density , is a positive real function of a frequency variable associated with a stationary stochastic process, or a deterministic function of time, which has dimensions of power per hertz...
or the density of states
Density of states
In solid-state and condensed matter physics, the density of states of a system describes the number of states per interval of energy at each energy level that are available to be occupied by electrons. Unlike isolated systems, like atoms or molecules in gas phase, the density distributions are not...
of the system.
Basic theory
Absorption lines are typically classified by the nature of the quantum mechanical change induced in the molecule or atom. Rotational linesRotational spectroscopy
Rotational spectroscopy or microwave spectroscopy studies the absorption and emission of electromagnetic radiation by molecules associated with a corresponding change in the rotational quantum number of the molecule...
, for instance, occur when the rotational state of a molecule is changed. Rotational lines are typically found in the microwave spectral region. Vibrational lines correspond to changes in the vibrational state of the molecule and are typically found in the infrared region. Electronic lines correspond to a change in the electronic state of an atom or molecule and are typically found in the visible and ultraviolet region. X-ray absorptions are associated with the excitation of inner shell electrons in atoms. These changes can also be combined (e.g. rotation-vibration transitions
Rovibrational coupling
Rovibrational coupling is a coupled rotational and vibrational excitation of a molecule. It is different from rovibronic coupling, which involves a change in all of electronic, vibrational, and rotational states simultaneously....
), leading to new absorption lines at the combined energy of the two changes.
The energy associated with the quantum mechanical change primarily determines the frequency of the absorption line but the frequency can be shifted by several types of interactions. Electric and magnetic fields can cause a shift. Interactions with neighboring molecules can cause shifts. For instance, absorption lines of the gas phase molecule can shift significantly when that molecule is in a liquid or solid phase and interacting more strongly with neighboring molecules.
Absorption lines are often depicted as infinitesimally thin lines, i.e., delta function
Dirac delta function
The Dirac delta function, or δ function, is a generalized function depending on a real parameter such that it is zero for all values of the parameter except when the parameter is zero, and its integral over the parameter from −∞ to ∞ is equal to one. It was introduced by theoretical...
s, but observed lines always have a shape that is determined by the instrument used for the observation, the material absorbing the radiation and the physical environment of that material. It is common for lines to have the shape of a Gaussian or Lorentzian distribution. It is also common for a line to be characterized solely by its intensity and width
Spectral linewidth
The spectral linewidth characterizes the width of a spectral line, such as in the electromagnetic emission spectrum of an atom, or the frequency spectrum of an acoustic or electronic system...
instead of the entire shape being characterized.
The integrated intensity—obtained by integrating
Integral
Integration is an important concept in mathematics and, together with its inverse, differentiation, is one of the two main operations in calculus...
the area under the absorption line—is proportional to the amount of the absorbing substance present. The intensity is also related to the temperature of the substance and the quantum mechanical interaction between the radiation and the absorber. This interaction is quantified by the transition moment and depends on the particular lower state the transition starts from and the upper state it is connected to.
The width of absorption lines may be determined by the spectrometer
Spectrometer
A spectrometer is an instrument used to measure properties of light over a specific portion of the electromagnetic spectrum, typically used in spectroscopic analysis to identify materials. The variable measured is most often the light's intensity but could also, for instance, be the polarization...
used to record it. A spectrometer has an inherent limit on how narrow a line it can resolve
Spectral resolution
The spectral resolution of a spectrograph, or, more generally, of a frequency spectrum, is a measure of its ability to resolve features in the electromagnetic spectrum...
and so the observed width may be at this limit. If the width is larger than the resolution limit, then it is primarily determined by the environment of the absorber. A liquid or solid absorber, in which neighboring molecules strongly interact with one another, tends to have broader absorption lines than a gas. Increasing the temperature or pressure of the absorbing material will also tend to increase the line width. It is also common for several neighboring transitions to be close enough to one another that their lines overlap and the resulting overall line is therefore broader yet.
Relation to transmission spectrum
Absorption and transmission spectra represent equivalent information and one can be calculated from the other through a mathematical transformation. A transmission spectrum will have its maximum intensities at wavelengths where the absorption is weakest because more light is transmitted through the sample. An absorption spectrum will have its maximum intensities at wavelengths where the absorption is strongest.Relation to emission spectrum

Emission spectrum
The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted by the element's atoms or the compound's molecules when they are returned to a lower energy state....
will typically have a quite different intensity pattern from the absorption spectrum, though, so the two are not equivalent. The absorption spectrum can be calculated from the emission spectrum using appropriate theoretical models and additional information about the quantum mechanical states of the substance.
Relation to scattering and reflection spectra
The scattering and reflection spectra of a material are influenced by both its index of refraction and its absorption spectrum. In an optical context, the absorption spectrum is typically quantified by the extinction coefficient, and the extinction and index coefficients are quantitatively related through the Kramers-Kronig relationKramers-Kronig relation
The Kramers–Kronig relations are bidirectional mathematical relations, connecting the real and imaginary parts of any complex function that is analytic in the upper half-plane...
. Therefore, the absorption spectrum can be derived from a scattering or reflection spectrum. This typically requires simplifying assumptions or models, and so the derived absorption spectrum is an approximation.
Applications
Analytical chemistry
Absorption spectroscopy is useful in chemical analysis because of its specificity and its quantitative nature. The specificity of absorption spectra allows compounds to be distinguished from one another in a mixture, making absorption spectroscopy useful in wide variety of applications. For instance, Infrared gas analyzerInfrared gas analyzer
]An infrared gas analyzer measures trace gases by determining the absorption of an emitted infrared light source through a certain air sample. Trace gases found in the Earth's atmosphere get excited under specific wavelengths found in the infrared range. The concept behind the technology can be...
s can be used to identify the presence of pollutants in the air, distinguishing the pollutant from nitrogen, oxygen, water and other expected constituents.
The specificity also allows unknown samples to be identified by comparing a measured spectrum with a library of reference spectra. In many cases, it is possible to determine qualitative information about a sample even if it is not in a library. Infrared spectra, for instance, have characteristics absorption bands that indicate if carbon-hydrogen or carbon-oxygen bonds are present.
An absorption spectrum can be quantitatively related to the amount of material present using the Beer-Lambert law
Beer-Lambert law
In optics, the Beer–Lambert law, also known as Beer's law or the Lambert–Beer law or the Beer–Lambert–Bouguer law relates the absorption of light to the properties of the material through which the light is travelling.-Equations:The law states that there is a logarithmic dependence between the...
. Determining the absolute concentration of a compound requires knowledge of the compound's absorption coefficient. The absorption coefficient for some compounds is available from reference sources, and it can also be determined by measuring the spectrum of a calibration standard with a known concentration of the target.
Remote sensing
One of the unique advantages of spectroscopy as an analytical technique is that measurements can be made without bringing the instrument and sample into contact. Radiation that travels between a sample and an instrument will contain the spectral information, so the measurement can be made remotelyRemote sensing
Remote sensing is the acquisition of information about an object or phenomenon, without making physical contact with the object. In modern usage, the term generally refers to the use of aerial sensor technologies to detect and classify objects on Earth by means of propagated signals Remote sensing...
. Remote spectral sensing is valuable in many situations. For example, measurements can be made in toxic or hazardous environments without placing an operator or instrument at risk. Also, sample material does not have to be brought into contact with the instrument—preventing possible cross contamination.
Remote spectral measurements present several challenges compared to laboratory measurements. The space in between the sample of interest and the instrument may also have spectral absorptions. These absorptions can mask or confound the absorption spectrum of the sample. These background interferences may also vary over time. The source of radiation in remote measurements is often an environmental source, such as sunlight or the thermal radiation from a warm object, and this makes it necessary to distinguish spectral absorption from changes in the source spectrum.
Astronomy

Astronomical spectroscopy
Astronomical spectroscopy is the technique of spectroscopy used in astronomy. The object of study is the spectrum of electromagnetic radiation, including visible light, which radiates from stars and other celestial objects...
is a particularly significant type of remote spectral sensing. In this case, the objects and samples of interest are so distant from earth that electromagnetic radiation is the only means available to measure them. Astronomical spectra contain both absorption and emission spectral information. Absorption spectroscopy has been particularly important for understanding interstellar clouds and determining that some of them contain molecules
Molecular cloud
A molecular cloud, sometimes called a stellar nursery if star formation is occurring within, is a type of interstellar cloud whose density and size permits the formation of molecules, most commonly molecular hydrogen ....
. Absorption spectroscopy is also employed in the study of extrasolar planets. Detection of extrasolar planets by the transit method also measures their absorption spectrum and allows for the determination of the planet's atmospheric composition.
Atomic and molecular physics
Theoretical models, principally quantum mechanicalQuantum mechanics
Quantum mechanics, also known as quantum physics or quantum theory, is a branch of physics providing a mathematical description of much of the dual particle-like and wave-like behavior and interactions of energy and matter. It departs from classical mechanics primarily at the atomic and subatomic...
models, allow for the absorption spectra of atoms and molecules to be related to other physical properties such as electronic structure, atomic
Atomic mass
The atomic mass is the mass of a specific isotope, most often expressed in unified atomic mass units. The atomic mass is the total mass of protons, neutrons and electrons in a single atom....
or molecular mass
Molecular mass
The molecular mass of a substance is the mass of one molecule of that substance, in unified atomic mass unit u...
, and molecular geometry
Molecular geometry
Molecular geometry or molecular structure is the three-dimensional arrangement of the atoms that constitute a molecule. It determines several properties of a substance including its reactivity, polarity, phase of matter, color, magnetism, and biological activity.- Molecular geometry determination...
. Therefore, measurements of the absorption spectrum are used to determine these other properties. Microwave spectroscopy, for example, allows for the determination of bond lengths and angles with high precision.
In addition, spectral measurements can be used to determine the accuracy of theoretical predictions. For example, the Lamb shift measured in the hydrogen
Hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively-charged proton and a single negatively-charged electron bound to the nucleus by the Coulomb force...
atomic absorption spectrum was not expected to exist at the time it was measured. Its discovery spurred and guided the development of quantum electrodynamics
Quantum electrodynamics
Quantum electrodynamics is the relativistic quantum field theory of electrodynamics. In essence, it describes how light and matter interact and is the first theory where full agreement between quantum mechanics and special relativity is achieved...
, and measurements of the Lamb shift are now used to determine the fine-structure constant
Fine-structure constant
In physics, the fine-structure constant is a fundamental physical constant, namely the coupling constant characterizing the strength of the electromagnetic interaction. Being a dimensionless quantity, it has constant numerical value in all systems of units...
.
Basic approach
The most straight-forward approach to absorption spectroscopy is to generate radiation with a source, measure a reference spectrum of that radiation with a detectorPhotodetector
Photosensors or photodetectors are sensors of light or other electromagnetic energy. There are several varieties:*Active pixel sensors are image sensors consisting of an integrated circuit that contains an array of pixel sensors, each pixel containing a both a light sensor and an active amplifier...
and then re-measure the sample spectrum after placing the material of interest in between the source and detector. The two measured spectra can then be combined to determine the material's absorption spectrum. The sample spectrum alone is not sufficient to determine the absorption spectrum because it will be affected by the experimental conditions—the spectrum of the source, the absorption spectra of other materials in between the source and detector and the wavelength dependent characteristics of the detector. The reference spectrum will be affected in the same way, though, by these experimental conditions and therefore the combination yields the absorption spectrum of the material alone.
A wide variety of radiation sources are employed in order to cover the electromagnetic spectrum. For spectroscopy, it is generally desirable for a source to cover a broad swath of wavelengths in order to measure a broad region of the absorption spectrum. Some sources inherently emit a broad spectrum. Examples of these include globar
Globar
A Globar is a silicon carbide rod of 5 to 10 mm width and 20 to 50 mm length which is electrically heated up to . When combined with a downstream variable interference filter, it emits radiation from 4 to 15 micrometres wavelength...
s or other black body
Black body
A black body is an idealized physical body that absorbs all incident electromagnetic radiation. Because of this perfect absorptivity at all wavelengths, a black body is also the best possible emitter of thermal radiation, which it radiates incandescently in a characteristic, continuous spectrum...
sources in the infrared, mercury lamps in the visible and ultraviolet and x-ray tube
X-ray tube
An X-ray tube is a vacuum tube that produces X-rays. They are used in X-ray machines. X-rays are part of the electromagnetic spectrum, an ionizing radiation with wavelengths shorter than ultraviolet light...
s. One recently developed, novel source of broad spectrum radiation is synchotron radiation which covers all of these spectral regions. Other radiation sources generate a narrow spectrum but the emission wavelength can be tuned to cover a spectral range. Examples of these include klystron
Klystron
A klystron is a specialized linear-beam vacuum tube . Klystrons are used as amplifiers at microwave and radio frequencies to produce both low-power reference signals for superheterodyne radar receivers and to produce high-power carrier waves for communications and the driving force for modern...
s in the microwave region and laser
Laser
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of photons. The term "laser" originated as an acronym for Light Amplification by Stimulated Emission of Radiation...
s across the infrared, visible and ultraviolet region (though not all lasers have tunable wavelengths).
The detector employed to measure the radiation power will also depend on the wavelength range of interest. Most detectors are sensitive to a fairly broad spectral range and the sensor selected will often depend more on the sensitivity and noise requirements of a given measurement. Examples of detectors common in spectroscopy include heterodyne receivers in the microwave, bolometer
Bolometer
A bolometer is a device for measuring the power of incident electromagnetic radiation via the heating of a material with a temperature-dependent electrical resistance. It was invented in 1878 by the American astronomer Samuel Pierpont Langley...
s in the millimeter-wave and infrared, mercury cadmium telluride and other cooled semiconductor
Semiconductor
A semiconductor is a material with electrical conductivity due to electron flow intermediate in magnitude between that of a conductor and an insulator. This means a conductivity roughly in the range of 103 to 10−8 siemens per centimeter...
detectors in the infrared, and photodiode
Photodiode
A photodiode is a type of photodetector capable of converting light into either current or voltage, depending upon the mode of operation.The common, traditional solar cell used to generateelectric solar power is a large area photodiode....
s and photomultiplier tubes
Photomultiplier
Photomultiplier tubes , members of the class of vacuum tubes, and more specifically phototubes, are extremely sensitive detectors of light in the ultraviolet, visible, and near-infrared ranges of the electromagnetic spectrum...
in the visible and ultraviolet.
If both the source and the detector cover a broad spectral region, then it is also necessary to introduce a means of resolving
Spectral resolution
The spectral resolution of a spectrograph, or, more generally, of a frequency spectrum, is a measure of its ability to resolve features in the electromagnetic spectrum...
the wavelength of the radiation in order to determine the spectrum. Often a spectrograph is used to spatially separate the wavelengths of radiation so that the power at each wavelength can be measured independently. It is also common to employ interferometry to determine resolve the spectrum—Fourier transform infrared spectroscopy is a widely used implementation of this technique.
Two other issues that must be considered in setting up an absorption spectroscopy experiment include the optics
Optics
Optics is the branch of physics which involves the behavior and properties of light, including its interactions with matter and the construction of instruments that use or detect it. Optics usually describes the behavior of visible, ultraviolet, and infrared light...
used to direct the radiation and the means of holding or containing the sample material (called a cuvette
Cuvette
A cuvette is a small tube of circular or square cross section, sealed at one end, made of plastic, glass, or fused quartz and designed to hold samples for spectroscopic experiments. The best cuvettes are as clear as possible, without impurities that might affect a spectroscopic reading...
or cell). For most UV, visible, and NIR measurements the use of precision quartz cuvettes are necessary.. In both cases, it is important to select materials that have relatively little absorption of their own in the wavelength range of interest. The absorption of other materials could interfere with or mask the absorption from the sample. For instance, in several wavelength ranges it is necessary to measure the sample under vacuum
Vacuum
In everyday usage, vacuum is a volume of space that is essentially empty of matter, such that its gaseous pressure is much less than atmospheric pressure. The word comes from the Latin term for "empty". A perfect vacuum would be one with no particles in it at all, which is impossible to achieve in...
or in a rare gas environment because gases in the atmosphere have interfering absorption features.
Specific approaches
- Cavity Ring Down Spectroscopy (CRDS)Cavity ring down spectroscopyCavity ring-down spectroscopy is a highly sensitive optical spectroscopic technique that enables measurement of absolute optical extinction by samples that scatter and absorb light. It has been widely used to study gaseous samples which absorb light at specific wavelengths, and in turn to...
- Mössbauer spectroscopy
- Photoemission spectroscopyPhotoemission spectroscopyPhotoemission spectroscopy , also known as photoelectron spectroscopy, refers to energy measurement of electrons emitted from solids, gases or liquids by the photoelectric effect, in order to determine the binding energies of electrons in a substance...
- Reflectance spectroscopy
- Laser Absorption Spectrometry (LAS)Laser absorption spectrometryLaser absorption spectrometry refers to techniques that use lasers to assess the concentration or amount of a species in gas phase by absorption spectrometry ....
- Tunable Diode Laser Absorption Spectroscopy (TDLAS)TDLASTunable diode laser absorption spectroscopy is a technique for measuring the concentration of certain species such as methane, water vapor and many more, in a gaseous mixture using tunable diode lasers and laser absorption spectrometry...
- X-ray absorption fine structure (XAFS)X-ray absorption fine structureX-ray absorption fine structure is a specific structure observed in X-ray absorption spectroscopy . By analyzing the XAFS, information can be acquired on the local structure and on the unoccupied electronic states.-Spectra:...
- X-ray Absorption Near Edge Structure (XANES)XANESX-ray Absorption Near Edge Structure , also known as Near edge X-ray absorption fine structure is a type of absorption spectroscopy. NEXAFS also at times used the abbreviation EXAFS....
- Astronomical spectroscopyAstronomical spectroscopyAstronomical spectroscopy is the technique of spectroscopy used in astronomy. The object of study is the spectrum of electromagnetic radiation, including visible light, which radiates from stars and other celestial objects...
See also
- Absorption (optics)
- DensitometryDensitometryDensitometry is the quantitative measurement of optical density in light-sensitive materials, such as photographic paper or film, due to exposure to light...
- Fraunhofer linesFraunhofer linesIn physics and optics, the Fraunhofer lines are a set of spectral lines named for the German physicist Joseph von Fraunhofer . The lines were originally observed as dark features in the optical spectrum of the Sun....
- HITRANHITRANHITRAN - HITRAN is a compilation of spectroscopic parameters that a variety of computer codes use to predict and simulate the transmission and emission of light in the atmosphere. The original version was compiled by the Air Force Cambridge Research Laboratories...
- Infrared gas analyzerInfrared gas analyzer]An infrared gas analyzer measures trace gases by determining the absorption of an emitted infrared light source through a certain air sample. Trace gases found in the Earth's atmosphere get excited under specific wavelengths found in the infrared range. The concept behind the technology can be...
- Lyman-alpha forestLyman-alpha forestIn astronomical spectroscopy, the Lyman-alpha forest is the sum of absorption lines arising from the Lyman-alpha transition of the neutral hydrogen in the spectra of distant galaxies and quasars....
- Optical density
- Photoemission spectroscopyPhotoemission spectroscopyPhotoemission spectroscopy , also known as photoelectron spectroscopy, refers to energy measurement of electrons emitted from solids, gases or liquids by the photoelectric effect, in order to determine the binding energies of electrons in a substance...
- X-ray absorption spectroscopy
- Transparent materials
- Water absorptionWater absorptionDuring the transmission of electromagnetic radiation through a medium containing water molecules, portions of the electromagnetic spectrum are absorbed by water molecules...
- White cell (spectroscopy)White cell (spectroscopy)A White cell is a type of long path gas phase spectroscopy cell that is commonly used to measure low-concentration components in gases or liquids. In 1942 John U...

