
Diarylethene
Encyclopedia
In chemistry
, diarylethene is the general name of a class of compounds
that have aromatic groups
bonded to each end of a carbon
-carbon
double bond
. The simplest example is stilbene, which has two geometric isomers, E and Z.
Under the influence of light, these compounds can generally perform two kinds of reversible isomer
izations:
Thermal isomerization is also possible. In E-Z isomerization, the thermal equilibrium
lies well towards the trans-form because of its lower energy (~15 kJ mol−1 in stilbene). The activation energy for thermal E-Z isomerization is 150-190 kJ mol−1 for stilbene, meaning that temperatures above 200°C are required to isomerize stilbene at a reasonable rate, but most derivatives have lower energy barriers (e.g. 65 kJ mol−1 for 4-aminostilbene). The activation energy of the electrocyclization is 73 kJ mol−1 for stilbene.
Both processes are often applied in molecular switch
es and for photochromism
(reversible state changes from exposure to light).
After the 6π electrocyclization of the Z
form to the "close-ring" form, most unsubstituted diarylethenes are prone to oxidation, leading to a re-aromatization of the π-system. The most common example is E-stilbene, which upon irradiation undergoes an E
to Z
isomerization, which can be followed by a 6π electrocyclization. Reaction of the product of this reaction with molecular oxygen
affords phenanthrene
, and it has been suggested by some studies that dehydrogenation may even occur spontaneously. The dihydrophenanthrene intermediate has never been isolated, but it has been detected spectroscopically in pump-probe experiments by virtue of its long wavelength optical absorption band. Although both the E-Z isomerization and the 6π electrocyclization are reversible
processes, this oxidation renders the entire sequence irreversible.
to the carbon-carbon double bond
by groups that can not be removed during the oxidation. Following the Woodward-Hoffmann rules
, the photochemical 6π cyclization takes place in a conrotatory fashion, leading to products with an anti configuration of the methyl substitutents. As both methyl groups are attached to a stereogenic center, two enantiomers (R,R and S,S) are formed, normally as a racemic
mixture. This approach also has the advantage that the thermal (disrotatory
) ring closure can not take place because of steric hindrance between the substitution groups.
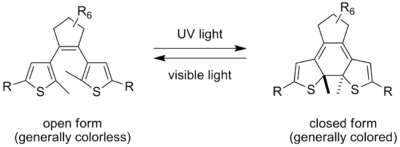 Ortho-substitution of the aromatic units results in a stabilization against oxidation, but the closed-ring form still has a low thermodynamic stability in most cases (e.g. 2,3-dimesityl-2-butene has a half-life of 90 seconds at 20°C). This problem can be addressed by lowering the aromaticity of the system. The most commonly used example are the dithienylethenes, i.e. alkene
Ortho-substitution of the aromatic units results in a stabilization against oxidation, but the closed-ring form still has a low thermodynamic stability in most cases (e.g. 2,3-dimesityl-2-butene has a half-life of 90 seconds at 20°C). This problem can be addressed by lowering the aromaticity of the system. The most commonly used example are the dithienylethenes, i.e. alkene
s with a thiophene
ring on either side. The 2-position of the thiophene
s is substituted with a methyl group, preventing oxidation of the ring closed form. Often the two free α-positions on the double bond are connected in a 5 or 6-membered ring in order to lock the double bond into the cis-form. This makes the dithienylethene undergo only open-closed ring isomerization, unconfused by E-Z isomerization.
The dithienylethenes are also of interest for the fact that their isomerization requires very little change of shape. This means that their isomerization in a solid matrix can take place much more quickly than with most other photochromic molecules. In the case of some analogs, photochromic behavior can even be carried out in single crystals without disrupting the crystal structure.
, due to the extended conjugation
along the molecular backbone. Therefore many diarylethenes have photochromic behavior both in solution
and in solid state
. Moreover, these two isomers differ from one another not only in their absorption spectra but also in various physical and chemical properties, such as their refractive indices
, dielectric constant
s, and oxidation-reduction
potentials. These properties can be readily controlled by reversible isomerization between the open- and closed-ring states using photoirradiation, and thus they have been suggested for use in optical data storage and 3D optical data storage
in particular. The closed form has a conjugated path from one end of the molecule to the other, whereas the open form has not. This allows for the electronic communication between functional groups attached to the far ends of the diarylethene to be switched on and off using UV and visible light.
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
, diarylethene is the general name of a class of compounds
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
that have aromatic groups
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
bonded to each end of a carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
-carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
double bond
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
. The simplest example is stilbene, which has two geometric isomers, E and Z.
Under the influence of light, these compounds can generally perform two kinds of reversible isomer
Isomer
In chemistry, isomers are compounds with the same molecular formula but different structural formulas. Isomers do not necessarily share similar properties, unless they also have the same functional groups. There are many different classes of isomers, like stereoisomers, enantiomers, geometrical...
izations:
- EAlkeneIn organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
to ZAlkeneIn organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
isomerizations, most common for stilbenes (and azobenzeneAzobenzeneAzobenzene is a chemical compound composed of two phenyl rings linked by a N=N double bond. It is the best known example of an azo compound. The term 'azobenzene' or simply 'azo' is often used to refer to a wide class of molecules that share the core azobenzene structure, with different chemical...
s). This process goes through an excited stateExcited stateExcitation is an elevation in energy level above an arbitrary baseline energy state. In physics there is a specific technical definition for energy level which is often associated with an atom being excited to an excited state....
energy minimum where the aromatic rings lie at 90° to each other. This conformation drops to the ground stateGround stateThe ground state of a quantum mechanical system is its lowest-energy state; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state...
and generally relaxes to trans and cis forms in a 1:1 ratio, thus the quantum yieldQuantum yieldThe quantum yield of a radiation-induced process is the number of times that a defined event occurs per photon absorbed by the system. The "event" may represent a chemical reaction, for example the decomposition of a reactant molecule:...
for E-Z isomerization is very rarely greater than 0.5. - 6π electrocyclizations of the ZAlkeneIn organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
form, leading to an additional bond between the two arylArylIn the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
functionalities and a disruption of the aromatic character of these groups. The quantum yield of this reaction is generally less than 0.1, and in most diarylethenes the close-ring form is thermally unstable, reverting to the cis-form in a matter of seconds or minutes under ambient conditions.
Thermal isomerization is also possible. In E-Z isomerization, the thermal equilibrium
Thermal equilibrium
Thermal equilibrium is a theoretical physical concept, used especially in theoretical texts, that means that all temperatures of interest are unchanging in time and uniform in space...
lies well towards the trans-form because of its lower energy (~15 kJ mol−1 in stilbene). The activation energy for thermal E-Z isomerization is 150-190 kJ mol−1 for stilbene, meaning that temperatures above 200°C are required to isomerize stilbene at a reasonable rate, but most derivatives have lower energy barriers (e.g. 65 kJ mol−1 for 4-aminostilbene). The activation energy of the electrocyclization is 73 kJ mol−1 for stilbene.
Both processes are often applied in molecular switch
Molecular switch
A molecular switch is a molecule that can be reversibly shifted between two or more stable states. The molecules may be shifted between the states in response to changes in e.g. pH, light, temperature, an electrical current, microenvironment, or the presence of a ligand. In some cases, a...
es and for photochromism
Photochromism
Photochromism is the reversible transformation of a chemical species between two forms by the absorption of electromagnetic radiation, where the two forms have different absorption spectra. Trivially, this can be described as a reversible change of colour upon exposure to light...
(reversible state changes from exposure to light).
After the 6π electrocyclization of the Z
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
form to the "close-ring" form, most unsubstituted diarylethenes are prone to oxidation, leading to a re-aromatization of the π-system. The most common example is E-stilbene, which upon irradiation undergoes an E
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
to Z
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
isomerization, which can be followed by a 6π electrocyclization. Reaction of the product of this reaction with molecular oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
affords phenanthrene
Phenanthrene
Phenanthrene is a polycyclic aromatic hydrocarbon composed of three fused benzene rings. The name phenanthrene is a composite of phenyl and anthracene. In its pure form, it is found in cigarette smoke and is a known irritant, photosensitizing skin to light...
, and it has been suggested by some studies that dehydrogenation may even occur spontaneously. The dihydrophenanthrene intermediate has never been isolated, but it has been detected spectroscopically in pump-probe experiments by virtue of its long wavelength optical absorption band. Although both the E-Z isomerization and the 6π electrocyclization are reversible
Reversible reaction
A reversible reaction is a chemical reaction that results in an equilibrium mixture of reactants and products. For a reaction involving two reactants and two products this can be expressed symbolically as...
processes, this oxidation renders the entire sequence irreversible.
Stabilization of the closed-ring form to oxidation
One solution to the problem of oxidation is to replace the hydrogens orthoArene substitution patterns
Arene substitution patterns are part of organic chemistry IUPAC nomenclature and pinpoint the position of substituents other than hydrogen in relation to each other on an aromatic hydrocarbon.- Ortho, meta, and para substitution :...
to the carbon-carbon double bond
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
by groups that can not be removed during the oxidation. Following the Woodward-Hoffmann rules
Woodward-Hoffmann rules
The Woodward–Hoffmann rules devised by Robert Burns Woodward and Roald Hoffmann are a set of rules in organic chemistry predicting the stereochemistry of pericyclic reactions based on orbital symmetry. These include electrocyclic reactions, cycloadditions , sigmatropic reactions, and group transfer...
, the photochemical 6π cyclization takes place in a conrotatory fashion, leading to products with an anti configuration of the methyl substitutents. As both methyl groups are attached to a stereogenic center, two enantiomers (R,R and S,S) are formed, normally as a racemic
Racemic
In chemistry, a racemic mixture, or racemate , is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule. The first known racemic mixture was "racemic acid", which Louis Pasteur found to be a mixture of the two enantiomeric isomers of tartaric acid.- Nomenclature :A...
mixture. This approach also has the advantage that the thermal (disrotatory
Disrotatory
In a conrotatory mode of an electrocyclic reaction the substituents located at the termini of a conjugated double bond system move in the same direction during ring opening or ring closure...
) ring closure can not take place because of steric hindrance between the substitution groups.
Dithienylethenes

Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s with a thiophene
Thiophene
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a flat five-membered ring, it is aromatic as indicated by its extensive substitution reactions. Related to thiophene are benzothiophene and dibenzothiophene, containing the thiophene ring fused with one and two benzene...
ring on either side. The 2-position of the thiophene
Thiophene
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a flat five-membered ring, it is aromatic as indicated by its extensive substitution reactions. Related to thiophene are benzothiophene and dibenzothiophene, containing the thiophene ring fused with one and two benzene...
s is substituted with a methyl group, preventing oxidation of the ring closed form. Often the two free α-positions on the double bond are connected in a 5 or 6-membered ring in order to lock the double bond into the cis-form. This makes the dithienylethene undergo only open-closed ring isomerization, unconfused by E-Z isomerization.
The dithienylethenes are also of interest for the fact that their isomerization requires very little change of shape. This means that their isomerization in a solid matrix can take place much more quickly than with most other photochromic molecules. In the case of some analogs, photochromic behavior can even be carried out in single crystals without disrupting the crystal structure.
Applications
Typically, the open-ring isomers are colorless compounds, whereas the closed-ring isomers have colors dependent on their chemical structureChemical structure
A chemical structure includes molecular geometry, electronic structure and crystal structure of molecules. Molecular geometry refers to the spatial arrangement of atoms in a molecule and the chemical bonds that hold the atoms together. Molecular geometry can range from the very simple, such as...
, due to the extended conjugation
Conjugated system
In chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds, which in general may lower the overall energy of the molecule and increase stability. Lone pairs, radicals or carbenium ions may be part of the...
along the molecular backbone. Therefore many diarylethenes have photochromic behavior both in solution
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
and in solid state
Solid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
. Moreover, these two isomers differ from one another not only in their absorption spectra but also in various physical and chemical properties, such as their refractive indices
Refractive index
In optics the refractive index or index of refraction of a substance or medium is a measure of the speed of light in that medium. It is expressed as a ratio of the speed of light in vacuum relative to that in the considered medium....
, dielectric constant
Dielectric constant
The relative permittivity of a material under given conditions reflects the extent to which it concentrates electrostatic lines of flux. In technical terms, it is the ratio of the amount of electrical energy stored in a material by an applied voltage, relative to that stored in a vacuum...
s, and oxidation-reduction
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
potentials. These properties can be readily controlled by reversible isomerization between the open- and closed-ring states using photoirradiation, and thus they have been suggested for use in optical data storage and 3D optical data storage
3D optical data storage
3D optical data storage is the term given to any form of optical data storage in which information can be recorded and/or read with three dimensional resolution ....
in particular. The closed form has a conjugated path from one end of the molecule to the other, whereas the open form has not. This allows for the electronic communication between functional groups attached to the far ends of the diarylethene to be switched on and off using UV and visible light.

