Photochemistry
Encyclopedia
Photochemistry, a sub-discipline of chemistry
, is the study of chemical reaction
s that proceed with the absorption of light by atoms or molecules.. Everyday examples include photosynthesis
, the degradation of plastics and the formation of vitamin D
with sunlight.
, a source of energy. The Grotthuss–Draper law (for chemists Theodor Grotthuss and John W. Draper), states that light must be absorbed by a chemical substance in order for a photochemical reaction to take place. For each photon of light absorbed by a chemical system, no more than one molecule is activated for a photochemical reaction, as defined by the quantum yield
.
Chemical reactions occur only when a molecule is provided the necessary "activation energy
". A simple example can be the combustion
of gasoline
(a hydrocarbon
) into carbon dioxide and water. In this reaction, the activation energy is provided in the form of heat or a spark. In case of photochemical reactions light provides the activation energy. Simplistically, light is one mechanism for providing the activation energy required for many reactions. If laser light is employed, it is possible to selectively excite a molecule so as to produce a desired electronic and vibrational state. Equally, the emission from a particular state may be selectively monitored, providing a measure of the population of that state. If the chemical system is at low pressure, this enables scientists to observe the energy distribution of the products of a chemical reaction before the differences in energy have been smeared out and averaged by repeated collisions.
The absorption of a photon of light by a reactant molecule may also permit a reaction to occur not just by bringing the molecule to the necessary activation energy, but also by changing the symmetry of the molecule's electronic configuration, enabling an otherwise inaccessible reaction path, as described by the Woodward-Hoffmann selection rules
. A 2+2 cycloaddition reaction is one example of a pericyclic reaction
that can be analyzed using these rules or by the related frontier molecular orbital
theory.
Photochemical reactions involve electronic reorganization initiated by electromagnetic radiation. The reactions are several orders of magnitude faster than thermal reactions; reactions as fast as 10−9 seconds and associated processes as fast as 10−15 seconds are often observed.
. Some of the most widely used sections, and their wavelengths, are the following:
, in which most plants use solar energy to convert carbon dioxide
and water into glucose
, disposing of oxygen
as a side-product. Humans rely on photochemistry for the formation of vitamin D. In fireflies
, an enzyme
in the abdomen catalyzes a reaction that results in bioluminescence
.
Photochemistry can also be highly destructive. Medicine bottles are often made with darkened glass to prevent the drugs from photodegradation. A pervasive reaction is the generation of singlet oxygen by photosensitized reactions of triplet oxygen. Typical photosensitizers include tetraphenylporphyrin
and methylene blue
. The resulting singlet oxygen is an aggressive oxidant, capable of converting C-H bonds into C-OH groups.In photodynamic therapy
, light is used to destroy tumors by the action of singlet oxygen.
Many polymerizations are started by photoinitiatiors, which decompose upon absorbing light to produce the free radicals for Radical polymerization
.
 In the area of photochemistry, a photochemical reaction is a chemical reaction
In the area of photochemistry, a photochemical reaction is a chemical reaction
that is induced by light. Photochemical reactions are valuable in organic
and inorganic chemistry
because they proceed differently than thermal reactions. Photochemical reactions are not only very useful but also can be a serious nuisance, as in the photodegradation of many materials, e.g. polyvinyl chloride
. A large-scale application of photochemistry is photoresist
technology, used in the production of microelectronic components. Vision
is initiated by a photochemical reaction of rhodopsin
.
s are more common in the laboratory. Low pressure mercury vapor lamps mainly emit at 254 nm. For polychromatic sources, wavelength ranges can be selected using filters. Alternatively, LED
s and Rayonet lamps emit monochromatically.
9schlenkcropped.png) The emitted light must of course reach the targeted functional group
The emitted light must of course reach the targeted functional group
without being blocked by the reactor, medium, or other functional groups present. For many applications, quartz
is used for the reactors as well as to contain the lamp. Pyrex
absorbs at wavelengths shorter than 275 nm. The solvent is an important experimental parameter. Solvents are potential reactants and for this reason, chlorinated solvents are avoided because the C-Cl bond can lead to chlorination
of the substrate. Strongly absorbing solvents prevent photons from reaching the substrate. Hydrocarbon solvents absorb only at short wavelengths and are thus preferred for photochemical experiments requiring high energy photons. Solvents containing unsaturation absorb at longer wavelengths and can usefully filter out short wavelengths. For example, cyclohexane
and acetone
"cut off" (absorb strongly) at wavelengths shorter than 215 and 330 nm, respectively.
. The photon can be absorbed directly by the reactant or by a photosensitizers, which absorbs the photon and transfers the energy to the reactant. The opposite process is called quenching
when a photoexited state is deactivated by a chemical reagent.
Most photochemical transformations occur through a series of simple steps known as primary photochemical processes. One common example of these processes is the excited state proton transfer (ESPT).
are electrocyclic reaction
s, photoisomerization
and Norrish reaction
s.
Alkene
s undergo many important reactions that proceed via a photon-induced π to π* transition. The first electronic excited state of an alkene lack the π-bond, so that rotation about the C-C bond is rapid and the molecule engages in reactions not observed thermally. These reactions include cis-trans isomerization, cycloaddition to other (ground state) alkene to give cyclobutane
derivatives. The cis-trans isomerization of a (poly)alkene is involved in retinal
, a component of the machinery of vision
. The dimerization of alkenes is relevant to the photodamage of DNA
, where thymine dimers are observed upon illuminating DNA to UV radiation. Such dimers interfere with transcription
. The beneficial effects of sunlight are associated with the photochemically induced retro-cyclization (decyclization) reaction of ergosterol
to give vitamin D
. In the DeMayo reaction
, an alkene reacts with a 1,3-diketone reacts via its enol
to yield a 1,5-diketone. Still another common photochemical reaction is Zimmerman's Di-pi-methane rearrangement
.
In an industrial application, about 100,000 tonnes of benzyl chloride
are prepared annually by the gas-phase photochemical reaction of toluene
with chlorine
. The light is absorbed by chlorine molecule, the low energy of this transition being indicted by the yellowish color of the gas. The photon induces homolysis of the Cl-Cl bond, and the resulting chlorine radical converts toluene to the benzyl radical:
Mercaptans can be produced by photochemical addition of hydrogen sulfide
(H2S) to alpha olefins.
s that resist thermal substitution undergo decarbonylation upon irradiation with UV light. UV-irradiation of a THF
solution of molybdenum hexacarbonyl
gives the THF complex, which is synthetically useful:
In a related reaction, photolysis of iron pentacarbonyl
affords diiron nonacarbonyl
(see figure):
, arc lamp
, or flashbulb
. In the presence of air, Carbon Nanotubes will undergo combustion at high temperature (700-1500C). Under inert atmospheres, the nanotubes will break open and reorganize into larger "nanohorn" structures.
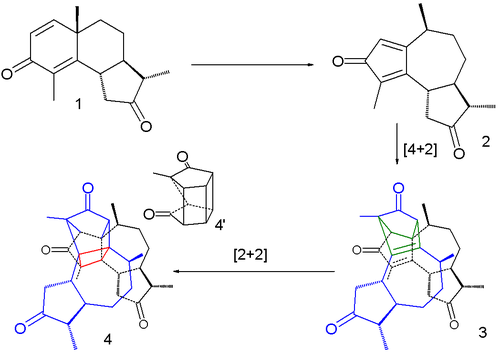
s of the compound α-santonin
when exposed to sunlight
turned yellow and burst. In a 2007 study the reaction was described as a succession of three steps taking place within a single crystal.
The first step is a rearrangement reaction
to a cyclopentadienone intermediate 2, the second one a dimerization in a Diels-Alder reaction
(3) and the third one a intramolecular
[2+2]cycloaddition
(4). The bursting effect is attributed to a large change in crystal volume on dimerization.
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
, is the study of chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
s that proceed with the absorption of light by atoms or molecules.. Everyday examples include photosynthesis
Photosynthesis
Photosynthesis is a chemical process that converts carbon dioxide into organic compounds, especially sugars, using the energy from sunlight. Photosynthesis occurs in plants, algae, and many species of bacteria, but not in archaea. Photosynthetic organisms are called photoautotrophs, since they can...
, the degradation of plastics and the formation of vitamin D
Vitamin D
Vitamin D is a group of fat-soluble secosteroids. In humans, vitamin D is unique both because it functions as a prohormone and because the body can synthesize it when sun exposure is adequate ....
with sunlight.
Principles
Light is a type of electromagnetic radiationElectromagnetic radiation
Electromagnetic radiation is a form of energy that exhibits wave-like behavior as it travels through space...
, a source of energy. The Grotthuss–Draper law (for chemists Theodor Grotthuss and John W. Draper), states that light must be absorbed by a chemical substance in order for a photochemical reaction to take place. For each photon of light absorbed by a chemical system, no more than one molecule is activated for a photochemical reaction, as defined by the quantum yield
Quantum yield
The quantum yield of a radiation-induced process is the number of times that a defined event occurs per photon absorbed by the system. The "event" may represent a chemical reaction, for example the decomposition of a reactant molecule:...
.
Chemical reactions occur only when a molecule is provided the necessary "activation energy
Activation energy
In chemistry, activation energy is a term introduced in 1889 by the Swedish scientist Svante Arrhenius that is defined as the energy that must be overcome in order for a chemical reaction to occur. Activation energy may also be defined as the minimum energy required to start a chemical reaction...
". A simple example can be the combustion
Combustion
Combustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
of gasoline
Gasoline
Gasoline , or petrol , is a toxic, translucent, petroleum-derived liquid that is primarily used as a fuel in internal combustion engines. It consists mostly of organic compounds obtained by the fractional distillation of petroleum, enhanced with a variety of additives. Some gasolines also contain...
(a hydrocarbon
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
) into carbon dioxide and water. In this reaction, the activation energy is provided in the form of heat or a spark. In case of photochemical reactions light provides the activation energy. Simplistically, light is one mechanism for providing the activation energy required for many reactions. If laser light is employed, it is possible to selectively excite a molecule so as to produce a desired electronic and vibrational state. Equally, the emission from a particular state may be selectively monitored, providing a measure of the population of that state. If the chemical system is at low pressure, this enables scientists to observe the energy distribution of the products of a chemical reaction before the differences in energy have been smeared out and averaged by repeated collisions.
The absorption of a photon of light by a reactant molecule may also permit a reaction to occur not just by bringing the molecule to the necessary activation energy, but also by changing the symmetry of the molecule's electronic configuration, enabling an otherwise inaccessible reaction path, as described by the Woodward-Hoffmann selection rules
Woodward-Hoffmann rules
The Woodward–Hoffmann rules devised by Robert Burns Woodward and Roald Hoffmann are a set of rules in organic chemistry predicting the stereochemistry of pericyclic reactions based on orbital symmetry. These include electrocyclic reactions, cycloadditions , sigmatropic reactions, and group transfer...
. A 2+2 cycloaddition reaction is one example of a pericyclic reaction
Pericyclic reaction
In organic chemistry, a pericyclic reaction is a type of organic reaction wherein the transition state of the molecule has a cyclic geometry, and the reaction progresses in a concerted fashion. Pericyclic reactions are usually rearrangement reactions...
that can be analyzed using these rules or by the related frontier molecular orbital
Molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term "orbital" was first...
theory.
Photochemical reactions involve electronic reorganization initiated by electromagnetic radiation. The reactions are several orders of magnitude faster than thermal reactions; reactions as fast as 10−9 seconds and associated processes as fast as 10−15 seconds are often observed.
Spectral regions
Photochemists typically work in only a few sections of the electromagnetic spectrumElectromagnetic spectrum
The electromagnetic spectrum is the range of all possible frequencies of electromagnetic radiation. The "electromagnetic spectrum" of an object is the characteristic distribution of electromagnetic radiation emitted or absorbed by that particular object....
. Some of the most widely used sections, and their wavelengths, are the following:
- UltravioletUltravioletUltraviolet light is electromagnetic radiation with a wavelength shorter than that of visible light, but longer than X-rays, in the range 10 nm to 400 nm, and energies from 3 eV to 124 eV...
: 100–400 nm - Visible Light: 400–700 nm
- Near infrared: 700–2500 nm
Applications
Many important processes involve photochemistry. The premier example is photosynthesisPhotosynthesis
Photosynthesis is a chemical process that converts carbon dioxide into organic compounds, especially sugars, using the energy from sunlight. Photosynthesis occurs in plants, algae, and many species of bacteria, but not in archaea. Photosynthetic organisms are called photoautotrophs, since they can...
, in which most plants use solar energy to convert carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
and water into glucose
Glucose
Glucose is a simple sugar and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate...
, disposing of oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
as a side-product. Humans rely on photochemistry for the formation of vitamin D. In fireflies
Fireflies
Fireflies is a novel by Shiva Naipaul originally published in 1970. It was his first book, a comic novel set in Trinidad. In an essay in An Unfinished Journey, Naipaul described how in 1968 as a final year student at Oxford University studying Chinese, he had been moved to write down a sentence,...
, an enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
in the abdomen catalyzes a reaction that results in bioluminescence
Bioluminescence
Bioluminescence is the production and emission of light by a living organism. Its name is a hybrid word, originating from the Greek bios for "living" and the Latin lumen "light". Bioluminescence is a naturally occurring form of chemiluminescence where energy is released by a chemical reaction in...
.
Photochemistry can also be highly destructive. Medicine bottles are often made with darkened glass to prevent the drugs from photodegradation. A pervasive reaction is the generation of singlet oxygen by photosensitized reactions of triplet oxygen. Typical photosensitizers include tetraphenylporphyrin
Tetraphenylporphyrin
Tetraphenylporphyrin, abbreviated TPP or H2TPP, is a synthetic heterocyclic compound that resembles naturally occurring porphyrins. Porphyrins are dyes and cofactors found in hemoglobin and cytochromes and are related to chlorophyll and vitamin B12. The study of naturally occurring porphyrins is...
and methylene blue
Methylene blue
Methylene blue is a heterocyclic aromatic chemical compound with the molecular formula C16H18N3SCl. It has many uses in a range of different fields, such as biology and chemistry. At room temperature it appears as a solid, odorless, dark green powder, that yields a blue solution when dissolved in...
. The resulting singlet oxygen is an aggressive oxidant, capable of converting C-H bonds into C-OH groups.In photodynamic therapy
Photodynamic therapy
Photodynamic therapy is used clinically to treat a wide range of medical conditions, including malignant cancers, and is recognised as a treatment strategy which is both minimally invasive and minimally toxic...
, light is used to destroy tumors by the action of singlet oxygen.
Many polymerizations are started by photoinitiatiors, which decompose upon absorbing light to produce the free radicals for Radical polymerization
Radical polymerization
Free radical polymerization is a method of polymerization by which a polymer forms by the successive addition of free radical building blocks. Free radicals can be formed via a number of different mechanisms usually involving separate initiator molecules...
.

Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
that is induced by light. Photochemical reactions are valuable in organic
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
and inorganic chemistry
Inorganic chemistry
Inorganic chemistry is the branch of chemistry concerned with the properties and behavior of inorganic compounds. This field covers all chemical compounds except the myriad organic compounds , which are the subjects of organic chemistry...
because they proceed differently than thermal reactions. Photochemical reactions are not only very useful but also can be a serious nuisance, as in the photodegradation of many materials, e.g. polyvinyl chloride
Polyvinyl chloride
Polyvinyl chloride, commonly abbreviated PVC, is a thermoplastic polymer. It is a vinyl polymer constructed of repeating vinyl groups having one hydrogen replaced by chloride. Polyvinyl chloride is the third most widely produced plastic, after polyethylene and polypropylene. PVC is widely used in...
. A large-scale application of photochemistry is photoresist
Photoresist
A photoresist is a light-sensitive material used in several industrial processes, such as photolithography and photoengraving to form a patterned coating on a surface.-Tone:Photoresists are classified into two groups: positive resists and negative resists....
technology, used in the production of microelectronic components. Vision
Visual perception
Visual perception is the ability to interpret information and surroundings from the effects of visible light reaching the eye. The resulting perception is also known as eyesight, sight, or vision...
is initiated by a photochemical reaction of rhodopsin
Rhodopsin
Rhodopsin, also known as visual purple, is a biological pigment of the retina that is responsible for both the formation of the photoreceptor cells and the first events in the perception of light. Rhodopsins belong to the G-protein coupled receptor family and are extremely sensitive to light,...
.
Experimental set-up
Photochemical reactions require a light source that emits wavelengths corresponding to an electronic transition in the reactant. In the early experiments (and in everyday life), sunlight was the light source, although it is polychromatic. Mercury-vapor lampMercury-vapor lamp
A mercury-vapor lamp is a gas discharge lamp that uses an electric arc through vaporized mercury to produce light. The arc discharge is generally confined to a small fused quartz arc tube mounted within a larger borosilicate glass bulb...
s are more common in the laboratory. Low pressure mercury vapor lamps mainly emit at 254 nm. For polychromatic sources, wavelength ranges can be selected using filters. Alternatively, LED
LEd
LEd is a TeX/LaTeX editing software working under Microsoft Windows. It is a freeware product....
s and Rayonet lamps emit monochromatically.
9schlenkcropped.png)
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
without being blocked by the reactor, medium, or other functional groups present. For many applications, quartz
Quartz
Quartz is the second-most-abundant mineral in the Earth's continental crust, after feldspar. It is made up of a continuous framework of SiO4 silicon–oxygen tetrahedra, with each oxygen being shared between two tetrahedra, giving an overall formula SiO2. There are many different varieties of quartz,...
is used for the reactors as well as to contain the lamp. Pyrex
Pyrex
Pyrex is a brand name for glassware, introduced by Corning Incorporated in 1915.Originally, Pyrex was made from borosilicate glass. In the 1940s the composition was changed for some products to tempered soda-lime glass, which is the most common form of glass used in glass bakeware in the US and has...
absorbs at wavelengths shorter than 275 nm. The solvent is an important experimental parameter. Solvents are potential reactants and for this reason, chlorinated solvents are avoided because the C-Cl bond can lead to chlorination
Chlorination
Chlorination is the process of adding the element chlorine to water as a method of water purification to make it fit for human consumption as drinking water...
of the substrate. Strongly absorbing solvents prevent photons from reaching the substrate. Hydrocarbon solvents absorb only at short wavelengths and are thus preferred for photochemical experiments requiring high energy photons. Solvents containing unsaturation absorb at longer wavelengths and can usefully filter out short wavelengths. For example, cyclohexane
Cyclohexane
Cyclohexane is a cycloalkane with the molecular formula C6H12. Cyclohexane is used as a nonpolar solvent for the chemical industry, and also as a raw material for the industrial production of adipic acid and caprolactam, both of which being intermediates used in the production of nylon...
and acetone
Acetone
Acetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
"cut off" (absorb strongly) at wavelengths shorter than 215 and 330 nm, respectively.
Excitation
Photoexcitation is the first step in a photochemical process where the reactant is elevated to a state of higher energy, an excited stateExcited state
Excitation is an elevation in energy level above an arbitrary baseline energy state. In physics there is a specific technical definition for energy level which is often associated with an atom being excited to an excited state....
. The photon can be absorbed directly by the reactant or by a photosensitizers, which absorbs the photon and transfers the energy to the reactant. The opposite process is called quenching
Quenching (fluorescence)
Quenching refers to any process which decreases the fluorescence intensity of a given substance. A variety of processes can result in quenching, such as excited state reactions, energy transfer, complex-formation and collisional quenching. As a consequence, quenching is often heavily dependent on...
when a photoexited state is deactivated by a chemical reagent.
Most photochemical transformations occur through a series of simple steps known as primary photochemical processes. One common example of these processes is the excited state proton transfer (ESPT).
Organic photochemistry
Examples of photochemical organic reactionsOrganic Reactions
Organic Reactions is a secondary reference which synthesizes the organic chemistry literature around particular chemical transformations. Each chapter of Organic Reactions is devoted to a particular organic chemical reaction, and chapters provide exhaustive coverage of literature work in the form...
are electrocyclic reaction
Electrocyclic reaction
In organic chemistry, an electrocyclic reaction is a type of pericyclic rearrangement reaction where the net result is one pi bond being converted into one sigma bond or vice-versa...
s, photoisomerization
Mechanistic organic photochemistry
Mechanistic organic photochemistry is that aspect of organic photochemistry which seeks to explain the mechanisms of organic photochemical reactions. The absorption of ultraviolet light by organic molecules very often leads to reactions. In the earliest days sunlight was employed while in more...
and Norrish reaction
Norrish reaction
The Norrish reaction in organic chemistry describes the photochemical reactions taking place with ketones and aldehydes. This type of reaction is subdivided in Norrish type I reactions and Norrish type II reactions...
s.
Alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s undergo many important reactions that proceed via a photon-induced π to π* transition. The first electronic excited state of an alkene lack the π-bond, so that rotation about the C-C bond is rapid and the molecule engages in reactions not observed thermally. These reactions include cis-trans isomerization, cycloaddition to other (ground state) alkene to give cyclobutane
Cyclobutane
Cyclobutane is an organic compound with the formula 4. Cyclobutane is a colourless gas and commercially available as a liquefied gas. Derivatives of cyclobutane are called cyclobutanes...
derivatives. The cis-trans isomerization of a (poly)alkene is involved in retinal
Retinal
Retinal, also called retinaldehyde or vitamin A aldehyde, is one of the many forms of vitamin A . Retinal is a polyene chromophore, and bound to proteins called opsins, is the chemical basis of animal vision...
, a component of the machinery of vision
Visual perception
Visual perception is the ability to interpret information and surroundings from the effects of visible light reaching the eye. The resulting perception is also known as eyesight, sight, or vision...
. The dimerization of alkenes is relevant to the photodamage of DNA
DNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
, where thymine dimers are observed upon illuminating DNA to UV radiation. Such dimers interfere with transcription
Transcription (genetics)
Transcription is the process of creating a complementary RNA copy of a sequence of DNA. Both RNA and DNA are nucleic acids, which use base pairs of nucleotides as a complementary language that can be converted back and forth from DNA to RNA by the action of the correct enzymes...
. The beneficial effects of sunlight are associated with the photochemically induced retro-cyclization (decyclization) reaction of ergosterol
Ergosterol
Ergosterol is a sterol found in fungi, and named for ergot, a common name for the members of the fungal genus Claviceps from which ergosterol was first isolated. Ergosterol does not occur in plant or animal cells...
to give vitamin D
Vitamin D
Vitamin D is a group of fat-soluble secosteroids. In humans, vitamin D is unique both because it functions as a prohormone and because the body can synthesize it when sun exposure is adequate ....
. In the DeMayo reaction
DeMayo reaction
The DeMayo reaction is a photochemical reaction in which the enol of a 1,3-diketone reacts with an alkene and the resulting cyclobutane ring undergoes a retro-aldol reaction to yield a 1,5-diketone :...
, an alkene reacts with a 1,3-diketone reacts via its enol
Enol
Enols are alkenes with a hydroxyl group affixed to one of the carbon atoms composing the double bond. Alkenes with a hydroxyl group on both sides of the double bond are called enediols. Deprotonated anions of enols are called enolates...
to yield a 1,5-diketone. Still another common photochemical reaction is Zimmerman's Di-pi-methane rearrangement
Di-pi-methane rearrangement
The di-pi-methane rearrangement is a photochemical reaction of a molecular entity comprising two π-systems, separated by a saturated carbon atom , to form an ene- substituted cyclopropane...
.
In an industrial application, about 100,000 tonnes of benzyl chloride
Benzyl chloride
Benzyl chloride, or α-chlorotoluene, is an organic compound with the formula C6H5CH2Cl. This colourless liquid is a reactive organochlorine compound that is a widely used chemical building block.-Preparation:...
are prepared annually by the gas-phase photochemical reaction of toluene
Toluene
Toluene, formerly known as toluol, is a clear, water-insoluble liquid with the typical smell of paint thinners. It is a mono-substituted benzene derivative, i.e., one in which a single hydrogen atom from the benzene molecule has been replaced by a univalent group, in this case CH3.It is an aromatic...
with chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
. The light is absorbed by chlorine molecule, the low energy of this transition being indicted by the yellowish color of the gas. The photon induces homolysis of the Cl-Cl bond, and the resulting chlorine radical converts toluene to the benzyl radical:
- Cl2 + hν → 2 Cl·
- C6H5CH3 + Cl· → C6H5CH2· + HCl
- C6H5CH2· + Cl· → C6H5CH2Cl
Mercaptans can be produced by photochemical addition of hydrogen sulfide
Hydrogen sulfide
Hydrogen sulfide is the chemical compound with the formula . It is a colorless, very poisonous, flammable gas with the characteristic foul odor of expired eggs perceptible at concentrations as low as 0.00047 parts per million...
(H2S) to alpha olefins.
Inorganic and organometallic photochemistry
Coordination complexes and organometallic compounds are also photoreactive. These reactions can entail cis-trans isomerization. More commonly photoreactions result in dissociation of ligands, since the photon excites an electron on the metal to an orbital that is antibonding with respect to the ligands. Thus, metal carbonylMetal carbonyl
Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. These complexes may be homoleptic, that is containing only CO ligands, such as nickel carbonyl , but more commonly metal carbonyls contain a mix of ligands, such as Re3Cl...
s that resist thermal substitution undergo decarbonylation upon irradiation with UV light. UV-irradiation of a THF
ThF
Follicular B helper T cells , are antigen-experienced CD4+ T cells found in the B cell follicles of secondary lymphoid organs such as lymph nodes, spleens and Peyer's patches, and are identified by their constitutive expression of the B cell follicle homing receptor CXCR5...
solution of molybdenum hexacarbonyl
Molybdenum hexacarbonyl
Molybdenum hexacarbonyl is the chemical compound with the formula Mo6. This colorless solid, like its chromium and tungsten analogues, is noteworthy as a volatile, air-stable derivative of a metal in its zero oxidation state.-Structure and properties:Mo6 adopts an octahedral geometry consisting...
gives the THF complex, which is synthetically useful:
- Mo(CO)6 + THF → Mo(CO)5(THF) + CO
In a related reaction, photolysis of iron pentacarbonyl
Iron pentacarbonyl
Iron pentacarbonyl, also known as iron carbonyl, is the compound with formula 5. Under standard conditions Fe5 is a free-flowing, straw-colored liquid with a pungent odour. This compound is a common precursor to diverse iron compounds, including many that are useful in organic synthesis. Fe5 is...
affords diiron nonacarbonyl
Diiron nonacarbonyl
Diiron nonacarbonyl is an inorganic compound with the formula Fe29. This metal carbonyl is an important reagent in organometallic chemistry and of occasional use in organic synthesis. It is a more reactive source of Fe than Fe5 and less dangerous to handle because it is nonvolatile...
(see figure):
- 2 Fe(CO)5 → Fe2(CO)9 + CO
Carbon Nanotubes
Certain species of Carbon Nanotubes also undergo photochemical reactions when exposed to intense pulsed light from a laserLaser
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of photons. The term "laser" originated as an acronym for Light Amplification by Stimulated Emission of Radiation...
, arc lamp
Arc lamp
"Arc lamp" or "arc light" is the general term for a class of lamps that produce light by an electric arc . The lamp consists of two electrodes, first made from carbon but typically made today of tungsten, which are separated by a gas...
, or flashbulb
Flashbulb
Flashbulb may refer to:*Flash photography*The Flashbulb, one of the pseudonyms of electronica musician Benn Jordan*"Flashbulb", a bonus track by Nirvana...
. In the presence of air, Carbon Nanotubes will undergo combustion at high temperature (700-1500C). Under inert atmospheres, the nanotubes will break open and reorganize into larger "nanohorn" structures.
History
Although bleaching has long been practiced, the first photochemical reaction was described by Trommsdorf in 1834. He observed that crystalCrystal
A crystal or crystalline solid is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. The scientific study of crystals and crystal formation is known as crystallography...
s of the compound α-santonin
Santonin
Santonin is a drug which was widely used in the past as an anthelminthic, a drug that expels parasitic worms from the body, by either killing or stunning them. Santonin was formerly listed in U.S...
when exposed to sunlight
Sunlight
Sunlight, in the broad sense, is the total frequency spectrum of electromagnetic radiation given off by the Sun. On Earth, sunlight is filtered through the Earth's atmosphere, and solar radiation is obvious as daylight when the Sun is above the horizon.When the direct solar radiation is not blocked...
turned yellow and burst. In a 2007 study the reaction was described as a succession of three steps taking place within a single crystal.
The first step is a rearrangement reaction
Rearrangement reaction
A rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule...
to a cyclopentadienone intermediate 2, the second one a dimerization in a Diels-Alder reaction
Diels-Alder reaction
The Diels–Alder reaction is an organic chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon...
(3) and the third one a intramolecular
Intramolecular
Intramolecular in chemistry describes a process or characteristic limited within the structure of a single molecule, a property or phenomenon limited to the extent of a single molecule.- Examples :...
[2+2]cycloaddition
Cycloaddition
A cycloaddition is a pericyclic chemical reaction, in which "two or more unsaturated molecules combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity." The resulting reaction is a cyclization reaction.Cycloadditions are usually described by the...
(4). The bursting effect is attributed to a large change in crystal volume on dimerization.
See also
- Journal of Photochemistry and PhotobiologyJournal of Photochemistry and PhotobiologyJournal of Photochemistry and Photobiology is a series of peer-reviewed scientific journals covering the fields of photochemistry and photobiology, published by Elsevier...
- Photoelectrochemical cellPhotoelectrochemical cellPhotoelectrochemical cells or PECs are solar cells which generate electrical energy from light, including visible light. Some photoelectrochemical cells simply produce electrical energy, while others produce hydrogen in a process similar to the electrolysis of water.-Photogeneration cell:In this...
- Photochemical and Photobiological SciencesPhotochemical and Photobiological SciencesPhotochemical & Photobiological Sciences is a peer-reviewed scientific journal publishing original research and review articles from all areas of photochemistry and photobiology...
- Trends in Photochemistry & Photobiology
- Photochemistry and PhotobiologyPhotochemistry and PhotobiologyPhotochemistry and Photobiology is a peer-reviewed scientific journal that publishes original research papers, rapid communications, research notes, technical notes, invited articles, and reviews covering photochemistry and photobiology...
- Photochemical Logic GatesPhotochemical Logic GatesA photochemical logic gate is based on the photochemical intersystem crossing and molecular electronic transition between photochemically active molecules, leading to logic gates that can be produced.-The OR gate electron–photon transfer chain:...
- PhotosynthesisPhotosynthesisPhotosynthesis is a chemical process that converts carbon dioxide into organic compounds, especially sugars, using the energy from sunlight. Photosynthesis occurs in plants, algae, and many species of bacteria, but not in archaea. Photosynthetic organisms are called photoautotrophs, since they can...