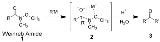
Weinreb ketone synthesis
Encyclopedia
The Weinreb ketone synthesis is a chemical reaction used in organic chemistry
to make carbon–carbon bonds. It was discovered in 1981 by Steven M. Weinreb
and Steven Nahm as a method to synthesize ketones. The original reaction involved two subsequent nucleophilic acyl substitution
s: the conversion of an acid chloride into an N,O-dimethylhydroxyamide, known as a Weinreb amide, and subsequent treatment of this species with an organometallic reagent such as a Grignard reagent
or organolithium reagent
. Nahm and Weinreb also reported the synthesis of aldehyde
s by reduction
of the amide
with an excess of lithium aluminum hydride
(See amide reduction
).
The major advantage of this method over addition of organometallic reagents to more typical acyl compounds is that it avoids the common problem of over-addition. For these latter reactions, two equivalents
of the incoming group add to form an alcohol
rather than a ketone or aldehyde. This occurs even if the equivalents of nucleophile are closely controlled.
The Weinreb amide has since been adopted into regular use by organic chemists as a dependable method for the synthesis of ketones. These functional group
s are present in a large number of natural product
s and can be reliably reacted to form new carbon–carbon bonds or converted into other functional groups. This method has been used in a number of syntheses, including Macrosphelides A and B, Amphidinolide J, and Spirofungins A and B. (See Scope below)
to explain the selectivity shown in reactions of the Weinreb amide. Their suggestion was that the tetrahedral intermediate (A below) formed as a result of nucleophilic acyl substitution
by the organometallic reagent is stabilized by chelation
from the methoxy
group as shown. This intermediate is stable only at low temperatures, requiring a low-temperature quench.
This chelation is in contrast to the mechanism for formation of the over-addition product wherein collapse of the tetrahedral intermediate allows a second addition. The mechanistic conjecture on the part of Weinreb was immediately accepted by the academic community, but it was not until 2006 that it was confirmed by spectroscopic and kinetic analyses.
compounds. The vast majority of these procedures utilize the commercially available salt N,O-dimethylhydroxylamine hydrochloride
[MeO(Me)NH•HCl], which is typically easier to handle than the free amine.
Treatment of an ester
or lactone
with AlMe3 or AlMe2Cl affords the corresponding Weinreb amide in good yields. Alternatively, non-nucleophilic Grignard reagents such as isopropyl magnesium chloride can be used to activate the amine before addition of the ester.
A variety of peptide coupling reagents can also be used to prepare Weinreb amides from carboxylic acids. Various carbodiimide
-, hydroxybenzotriazole
-, and triphenylphosphine
- based couplings have been reported specifically for this purpose.
Finally, an aminocarbonylation reaction reported by Stephen Buchwald allows conversion of aryl
halides directly into aryl Weinreb amides.
s, various lactams and lactones, sulfonates, sulfinates, and phosphonate esters. A wide variety of nucleophiles can be used in conjunction with the amide. Lithiates and Grignard reagents are most commonly employed; examples involving aliphatic, vinyl
, aryl
, and alkynyl
carbon nucleophile
s have been reported. However, with highly basic or sterically hindered nucleophiles, elimination of the methoxide moiety to release formaldehyde can occur as a significant side reaction.
Nonetheless, the Weinreb amide figures prominently into many syntheses, serving as an important coupling partner for various fragments. Shown below are key steps involving Weinreb amides in the synthesis of several natural products, including members of the immunosuppressant
family of Macrosphelides, and the antibiotic
family of Spirofungins.
that converts to the corresponding ketone or aldehyde upon hydrolytic workup.
Additionally, a one-pot magnesium-halogen exchange with subsequent arylation has been developed, showcasing the stability of the Weinreb amide and providing an operationally simple method for the synthesis of aryl ketones.
More unusual reagents with multiple Weinreb amide functional groups have been synthesized, serving as CO2 and α-diketone synthon
s.
Finally, Stephen G. Davies
of Oxford
has designed a chiral auxiliary
that combines the functionality of the Weinreb amide with that of the Myers’ pseudoephedrine
auxiliary, allowing diastereoselective enolate alkylation followed by facile cleavage to the corresponding enantioenriched aldehyde or ketone.
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
to make carbon–carbon bonds. It was discovered in 1981 by Steven M. Weinreb
Steven M. Weinreb
Steven M. Weinreb is an American chemist and is a professor of chemistry at Pennsylvania State University in United States. Together with Steven Nahm, he discovered the Weinreb ketone synthesis, which allows for mono-addition of an organometallic reagent such as a Grignard reagent or organolithium...
and Steven Nahm as a method to synthesize ketones. The original reaction involved two subsequent nucleophilic acyl substitution
Nucleophilic acyl substitution
Nucleophilic acyl substitution describes the substitution reaction involving nucleophiles and acyl compounds. Acyl compounds are carboxylic acid derivatives including esters, amides and acid halides...
s: the conversion of an acid chloride into an N,O-dimethylhydroxyamide, known as a Weinreb amide, and subsequent treatment of this species with an organometallic reagent such as a Grignard reagent
Grignard reaction
The Grignard reaction is an organometallic chemical reaction in which alkyl- or aryl-magnesium halides add to a carbonyl group in an aldehyde or ketone. This reaction is an important tool for the formation of carbon–carbon bonds...
or organolithium reagent
Organolithium reagent
An organolithium reagent is an organometallic compound with a direct bond between a carbon and a lithium atom. As the electropositive nature of lithium puts most of the charge density of the bond on the carbon atom, effectively creating a carbanion, organolithium compounds are extremely powerful...
. Nahm and Weinreb also reported the synthesis of aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s by reduction
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
of the amide
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
with an excess of lithium aluminum hydride
Lithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH or known as LithAl, is an inorganic compound with the chemical formula LiAlH4. It was discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters,...
(See amide reduction
Amide reduction
Amide reduction in chemistry is the organic reduction of amides . The main reaction product in this functional group interconversion is an amine...
).
The major advantage of this method over addition of organometallic reagents to more typical acyl compounds is that it avoids the common problem of over-addition. For these latter reactions, two equivalents
Equivalent (chemistry)
The equivalent , sometimes termed the molar equivalent, is a unit of amount of substance used in chemistry and the biological sciences.The equivalent is formally defined as the amount of a substance which will either:...
of the incoming group add to form an alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
rather than a ketone or aldehyde. This occurs even if the equivalents of nucleophile are closely controlled.
The Weinreb amide has since been adopted into regular use by organic chemists as a dependable method for the synthesis of ketones. These functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
s are present in a large number of natural product
Natural product
A natural product is a chemical compound or substance produced by a living organism - found in nature that usually has a pharmacological or biological activity for use in pharmaceutical drug discovery and drug design...
s and can be reliably reacted to form new carbon–carbon bonds or converted into other functional groups. This method has been used in a number of syntheses, including Macrosphelides A and B, Amphidinolide J, and Spirofungins A and B. (See Scope below)
Mechanism
Weinreb and Nahm originally proposed the following reaction mechanismReaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
to explain the selectivity shown in reactions of the Weinreb amide. Their suggestion was that the tetrahedral intermediate (A below) formed as a result of nucleophilic acyl substitution
Nucleophilic acyl substitution
Nucleophilic acyl substitution describes the substitution reaction involving nucleophiles and acyl compounds. Acyl compounds are carboxylic acid derivatives including esters, amides and acid halides...
by the organometallic reagent is stabilized by chelation
Chelation
Chelation is the formation or presence of two or more separate coordinate bonds between apolydentate ligand and a single central atom....
from the methoxy
Methoxy
In chemistry , methoxy refers to the functional group consisting of a methyl group bound to oxygen. This alkoxy group has the formula O–CH3.The word is used in organic nomenclature usually to describe an ether...
group as shown. This intermediate is stable only at low temperatures, requiring a low-temperature quench.
This chelation is in contrast to the mechanism for formation of the over-addition product wherein collapse of the tetrahedral intermediate allows a second addition. The mechanistic conjecture on the part of Weinreb was immediately accepted by the academic community, but it was not until 2006 that it was confirmed by spectroscopic and kinetic analyses.
Preparation
In addition to the original procedure shown above (which may have compatibility issues for sensitive substrates), Weinreb amides can be synthesized from a variety of acylAcyl
An acyl group is a functional group derived by the removal of one or more hydroxyl groups from an oxoacid, including inorganic acids.In organic chemistry, the acyl group is usually derived from a carboxylic acid . Therefore, it has the formula RCO-, where R represents an alkyl group that is...
compounds. The vast majority of these procedures utilize the commercially available salt N,O-dimethylhydroxylamine hydrochloride
N,O-dimethylhydroxylamine
N,O-Dimethylhydroxylamine is used in amide coupling reactions to form Weinreb amides for use in the Weinreb ketone synthesis.It was identified as a microbial degradation product of the herbicide linuron formed in the presence of extracts of Bacillus sphaericus ATCC 12123 by characterization of its...
[MeO(Me)NH•HCl], which is typically easier to handle than the free amine.
Treatment of an ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
or lactone
Lactone
In chemistry, a lactone is a cyclic ester which can be seen as the condensation product of an alcohol group -OH and a carboxylic acid group -COOH in the same molecule...
with AlMe3 or AlMe2Cl affords the corresponding Weinreb amide in good yields. Alternatively, non-nucleophilic Grignard reagents such as isopropyl magnesium chloride can be used to activate the amine before addition of the ester.
A variety of peptide coupling reagents can also be used to prepare Weinreb amides from carboxylic acids. Various carbodiimide
Carbodiimide
A carbodiimide or a methanediimine is a functional group consisting of the formula RN=C=NR. Carbodiimides hydrolyze to form ureas, which makes them uncommon in nature.-Carbodiimide formation:...
-, hydroxybenzotriazole
Hydroxybenzotriazole
Hydroxybenzotriazole is an organic compound that is a derivative of benzotriazole. It is mainly used to suppress racemization and improve the efficiency of peptide synthesis. It is a white crystalline powder...
-, and triphenylphosphine
Triphenylphosphine
Triphenylphosphine is a common organophosphorus compound with the formula P3 - often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature...
- based couplings have been reported specifically for this purpose.
Finally, an aminocarbonylation reaction reported by Stephen Buchwald allows conversion of aryl
Aryl
In the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
halides directly into aryl Weinreb amides.
Scope
The standard conditions for the Weinreb ketone synthesis are known to tolerate a wide variety of functional groups elsewhere in the molecule, including alpha-halogen substitution, N-protected amino acids, α-β unsaturation, silyl etherSilyl ether
Silyl ethers are a group of chemical compounds which contain a silicon atom covalently bonded to an alkoxy group. The general structure is R1R2R3Si−O−R4 where R4 is an alkyl group or an aryl group. Silyl ethers are usually used as protecting groups for alcohols in organic synthesis...
s, various lactams and lactones, sulfonates, sulfinates, and phosphonate esters. A wide variety of nucleophiles can be used in conjunction with the amide. Lithiates and Grignard reagents are most commonly employed; examples involving aliphatic, vinyl
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
, aryl
Aryl
In the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
, and alkynyl
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
carbon nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
s have been reported. However, with highly basic or sterically hindered nucleophiles, elimination of the methoxide moiety to release formaldehyde can occur as a significant side reaction.
Nonetheless, the Weinreb amide figures prominently into many syntheses, serving as an important coupling partner for various fragments. Shown below are key steps involving Weinreb amides in the synthesis of several natural products, including members of the immunosuppressant
Immunosuppressant
An immunosuppressant is any substance that performs immunosuppression of the immune system. They may be either exogenous, as immunosuppressive drugs, or endogenous, as ,e. g., testosterone...
family of Macrosphelides, and the antibiotic
Antibiotic
An antibacterial is a compound or substance that kills or slows down the growth of bacteria.The term is often used synonymously with the term antibiotic; today, however, with increased knowledge of the causative agents of various infectious diseases, antibiotic has come to denote a broader range of...
family of Spirofungins.
Variations
Reaction of Weinreb amides with Wittig reagents has been performed to avoid the sometimes harsh conditions required for addition of hydride reagents or organometallic compounds. This yields an N-methyl-N-methoxy-enamineEnamine
An enamine is an unsaturated compound derived by the reaction of an aldehyde or ketone with a secondary amine followed by loss of H2O.The word "enamine" is derived from the affix en-, used as the suffix of alkene, and the root amine. This can be compared with enol, which is a functional group...
that converts to the corresponding ketone or aldehyde upon hydrolytic workup.
Additionally, a one-pot magnesium-halogen exchange with subsequent arylation has been developed, showcasing the stability of the Weinreb amide and providing an operationally simple method for the synthesis of aryl ketones.
More unusual reagents with multiple Weinreb amide functional groups have been synthesized, serving as CO2 and α-diketone synthon
Synthon
A synthon is a concept in retrosynthetic analysis. It is defined as a structural unit within a molecule which is related to a possible synthetic operation. The term was coined by E.J. Corey...
s.
Finally, Stephen G. Davies
Stephen G. Davies
Stephen Graham "Steve" Davies is a British chemist and the Waynflete Professor of Chemistry at the University of Oxford.- Career :...
of Oxford
Oxford
The city of Oxford is the county town of Oxfordshire, England. The city, made prominent by its medieval university, has a population of just under 165,000, with 153,900 living within the district boundary. It lies about 50 miles north-west of London. The rivers Cherwell and Thames run through...
has designed a chiral auxiliary
Chiral auxiliary
A chiral auxiliary is a chemical compound or unit that is temporarily incorporated into an organic synthesis so that it can be carried out asymmetrically with the selective formation of one of two enantiomers...
that combines the functionality of the Weinreb amide with that of the Myers’ pseudoephedrine
Pseudoephedrine
Pseudoephedrine is a sympathomimetic drug of the phenethylamine and amphetamine chemical classes. It is used as a nasal/sinus decongestant and stimulant, or as a wakefulness-promoting agent....
auxiliary, allowing diastereoselective enolate alkylation followed by facile cleavage to the corresponding enantioenriched aldehyde or ketone.

