
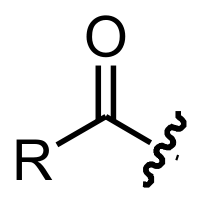
Acyl
Encyclopedia
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
derived by the removal of one or more hydroxyl
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
groups from an oxoacid
Oxoacid
An oxoacid is an acid that contains oxygen. To be more specific, it is an acid that:#contains oxygen#contains at least one other element#has at least one hydrogen atom bound to oxygen#forms an ion by the loss of one or more protons....
, including inorganic acids.
In organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
, the acyl group is usually derived from a carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
(IUPAC name: alkanoyl). Therefore, it has the formula RC
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
O
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
-, where R represents an alkyl group that is attached to the CO group with a single bond. Although the term is almost always applied to organic compounds, acyl groups can in principle be derived from other types of acids such as sulfonic acid
Sulfonic acid
Sulfonic acid usually refers to a member of the class of organosulfur compounds with the general formula RS2–OH, where R is an alkyl or aryl. The formal part of acid, HS2–OH, are formally derivatives of the "parent" inorganic compound with the formula HSO2.-Preparation:Sulfonic acid is...
s, phosphonic acids. In the most common arrangement, acyl groups are attached to a larger molecular fragment, in which case the carbon and oxygen atoms are linked by a double bond
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
.
Acyl compounds
Well-known acyl compounds are the acyl chlorides, such as acetyl chlorideAcetyl chloride
Acetyl chloride, CH3COCl, also known as ethanoyl chloride or acyl chloride, is an acid chloride derived from acetic acid. It belongs to the class of organic compounds called acyl halides. It is a colorless liquid. Acetyl chloride does not exist in nature, because contact with water would hydrolyze...
(CH3COCl) and benzoyl chloride (C6H5COCl). These compounds, which are treated as sources of acylium cations, are good reagent
Reagent
A reagent is a "substance or compound that is added to a system in order to bring about a chemical reaction, or added to see if a reaction occurs." Although the terms reactant and reagent are often used interchangeably, a reactant is less specifically a "substance that is consumed in the course of...
s for attaching acyl groups to various substrates. Amides (RC(O)NR2) and esters (RC(O)OR’) are classes of acyl compounds, as are ketones (RC(O)R) and aldehydes (RC(O)H).
Acylium cations, anions, and radicals
Acylium ions are cations of the formula RCO+. The oxygen and carbon are linked by a triple bond. Such species are common reactive intermediates, for example, in the Friedel-Crafts acylations also in many other organic reactionOrganic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
s such as the Hayashi rearrangement
Hayashi rearrangement
The Hayashi rearrangement is the chemical reaction of ortho-benzoylbenzoic acids catalyzed by sulfuric acid or phosphorus pentoxide.This reaction proceeds through electrophilic acylium ion attack with a spiro intermediate....
. Salts containing acylium ions can be generated by removal of the halide acyl halide
Acyl halide
An acyl halide is a chemical compound derived from an oxoacid by replacing a hydroxyl group with a halide group....
s:
- RC(O)Cl + SbCl5 → [RCO]SbCl6
The C-O distance in these cations is near 1.1 angstrom
Ångström
The angstrom or ångström, is a unit of length equal to 1/10,000,000,000 of a meter . Its symbol is the Swedish letter Å....
, even shorter than that in carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
. Acylium cations are characteristic fragments observed in EI-mass spectra of ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s.
Acyl anions and acyl radicals are very rare. Organolithium compounds with Li-C(O)R linkages are not well studied.
In biochemistry
In biochemistryBiochemistry
Biochemistry, sometimes called biological chemistry, is the study of chemical processes in living organisms, including, but not limited to, living matter. Biochemistry governs all living organisms and living processes...
there are many instances of acyl groups, in all major categories of biochemical molecules.
Acyl-CoA
Coenzyme A
Coenzyme A is a coenzyme, notable for its role in the synthesis and oxidation of fatty acids, and the oxidation of pyruvate in the citric acid cycle. All sequenced genomes encode enzymes that use coenzyme A as a substrate, and around 4% of cellular enzymes use it as a substrate...
s are acyl derivatives formed via fatty acid
Fatty acid
In chemistry, especially biochemistry, a fatty acid is a carboxylic acid with a long unbranched aliphatic tail , which is either saturated or unsaturated. Most naturally occurring fatty acids have a chain of an even number of carbon atoms, from 4 to 28. Fatty acids are usually derived from...
metabolism. Acetyl-CoA
Acetyl-CoA
Acetyl coenzyme A or acetyl-CoA is an important molecule in metabolism, used in many biochemical reactions. Its main function is to convey the carbon atoms within the acetyl group to the citric acid cycle to be oxidized for energy production. In chemical structure, acetyl-CoA is the thioester...
, the most common derivative, serves as an acyl donor in many biosynthetic transformations. Such acyl compounds are thiol esters.
Names of acyl groups of amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s are formed by the replacement of the ending -ine by the ending -yl. For example the acyl group of glycine
Glycine
Glycine is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its 'side chain', glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG cf. the genetic code.Glycine is a colourless, sweet-tasting crystalline solid...
is glycyl-, and of lysine
Lysine
Lysine is an α-amino acid with the chemical formula HO2CCH4NH2. It is an essential amino acid, which means that the human body cannot synthesize it. Its codons are AAA and AAG....
is lysyl-.
Names of acyl groups of ribonucleoside monophosphates such as AMP
Adenosine monophosphate
Adenosine monophosphate , also known as 5'-adenylic acid, is a nucleotide that is used as a monomer in RNA. It is an ester of phosphoric acid and the nucleoside adenosine. AMP consists of a phosphate group, the sugar ribose, and the nucleobase adenine...
(5'-adenylic acid), GMP
Guanosine monophosphate
Guanosine monophosphate, also known as 5'-guanidylic acid or guanylic acid and abbreviated GMP, is a nucleotide that is used as a monomer in RNA. It is an ester of phosphoric acid with the nucleoside guanosine. GMP consists of the phosphate group, the pentose sugar ribose, and the nucleobase...
(5'-guanylic acid), CMP
Cytidine monophosphate
Cytidine monophosphate, also known as 5'-cytidylic acid or simply cytidylate, and abbreviated CMP, is a nucleotide that is used as a monomer in RNA. It is an ester of phosphoric acid with the nucleoside cytidine...
(5'-cytidylic acid), and UMP
Uridine monophosphate
Uridine monophosphate, also known as 5'-uridylic acid and abbreviated UMP, is a nucleotide that is used as a monomer in RNA. It is an ester of phosphoric acid with the nucleoside uridine...
(5'-uridylic acid) are adenylyl-, guanylyl-, cytidylyl-, and uridylyl- respectively.
In phospholipid
Phospholipid
Phospholipids are a class of lipids that are a major component of all cell membranes as they can form lipid bilayers. Most phospholipids contain a diglyceride, a phosphate group, and a simple organic molecule such as choline; one exception to this rule is sphingomyelin, which is derived from...
s, the acyl group of phosphatidic acid is called phosphatidyl-.
Finally, many saccharides are acylated.
In organometallic chemistry and catalysis
Acyl ligandLigand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
s are intermediates in many carbonylation
Carbonylation
Carbonylation refers to reactions that introduce carbon monoxide into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemistry.-Organic chemistry:...
reactions, which are important in some catalytic reactions. Metal acyls arise usually via insertion of carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
into metal-alkyl bonds. Metal acyls also arise from the reactions involving acyl chlorides with low-valence metal complexes or by the reaction of organolithium compound with metal carbonyls. Metal acyls are often described by two resonance structures, one of which emphasizes the basicity of the oxygen center. O-Alkylation of metal acyls gives Fischer carbenes. complexes.
Nomenclature
The names of acyl groups are derived typically from the corresponding acid by substituting the acid ending -ic with the ending -yl as shown in the table below. Note that methyl, ethylEthyl group
In chemistry, an ethyl group is an alkyl substituent derived from ethane . It has the formula -C2H5 and is very often abbreviated -Et.Ethylation is the formation of a compound by introduction of the ethyl functional group, C2H5....
, propyl
Propyl
In organic chemistry, propyl is a three-carbon alkyl substituent with chemical formula -C3H7. It is the substituent form of the alkane propane...
, butyl
Butyl
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula -C4H9, derived from either of the two isomers of butane....
, etc. that end in -yl are not acyl but alkyl groups derived from alkane
Alkane
Alkanes are chemical compounds that consist only of hydrogen and carbon atoms and are bonded exclusively by single bonds without any cycles...
s. IUPAC nomenclature is recommended but rarely used.
| Acyl group name (R-CO-) |
Corresponding carboxylic acid name (R-CO-OH) |
||
|---|---|---|---|
| common | systematic | common | systematic |
| formyl | methanoyl | formic acid Formic acid Formic acid is the simplest carboxylic acid. Its chemical formula is HCOOH or HCO2H. It is an important intermediate in chemical synthesis and occurs naturally, most notably in the venom of bee and ant stings. In fact, its name comes from the Latin word for ant, formica, referring to its early... |
methanoic acid |
| acetyl Acetyl In organic chemistry, acetyl is a functional group, the acyl with chemical formula COCH3. It is sometimes represented by the symbol Ac . The acetyl group contains a methyl group single-bonded to a carbonyl... |
ethanoyl | acetic acid Acetic acid Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell... |
ethanoic acid |
| propionyl | propanoyl | propionic acid Propionic acid Propanoic acid is a naturally occurring carboxylic acid with chemical formula CH3CH2COOH. It is a clear liquid with a pungent odor... |
propanoic acid |
| benzoyl Benzoyl In organic chemistry, benzoyl is the acyl of benzoic acid, with structure C6H5CO-. It should not be confused with benzyl, which is the radical or ion formed from the removal of one of the methyl hydrogens of toluene... |
benzoic acid Benzoic acid Benzoic acid , C7H6O2 , is a colorless crystalline solid and the simplest aromatic carboxylic acid. The name derived from gum benzoin, which was for a long time the only source for benzoic acid. Its salts are used as a food preservative and benzoic acid is an important precursor for the synthesis... |
||
| acrylyl | propenoyl | acrylic acid Acrylic acid Acrylic acid is an organic compound with the formula CH2=CHCO2H. It is the simplest unsaturated carboxylic acid, consisting of a vinyl group connected directly to a carboxylic acid terminus. This colorless liquid has a characteristic acrid or tart smell. It is miscible with water, alcohols,... |
propenoic acid |
Acyl species
In acyloxy groups the acyl group is bonded to oxygen: R-C(=O)-O-R' where R-C(=O) is the acyl group.Acylium ions are cations of the type R-C+=O and play an important role as intermediates in organic reaction
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
s for example the Hayashi rearrangement
Hayashi rearrangement
The Hayashi rearrangement is the chemical reaction of ortho-benzoylbenzoic acids catalyzed by sulfuric acid or phosphorus pentoxide.This reaction proceeds through electrophilic acylium ion attack with a spiro intermediate....
.

