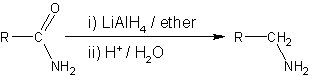Amide reduction
Encyclopedia
Amide reduction in chemistry
is the organic reduction of amides . The main reaction product in this functional group
interconversion is an amine
. Reagents are lithium aluminium hydride
, diborane
and catalytic hydrogenation
(requires high temperatures and pressures).
N,N-disubstituted amides can be reduced to aldehydes by using an excess of the amide:
With further reduction the alcohol
is obtained.
Some amides can be reduced to aldehydes in the Sonn-Müller method.
with silyl hydrides and a suitable catalyst based on Rh, Ru, Pt, Pd, Ir, Os, Re, Mn, Mo, In, or Ti.
Iron catalysis by triiron dodecacarbonyl
in combination with polymethylhydrosiloxane has been reported .
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
is the organic reduction of amides . The main reaction product in this functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
interconversion is an amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
. Reagents are lithium aluminium hydride
Lithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH or known as LithAl, is an inorganic compound with the chemical formula LiAlH4. It was discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters,...
, diborane
Diborane
Diborane is the chemical compound consisting of boron and hydrogen with the formula B2H6. It is a colorless gas at room temperature with a repulsively sweet odor. Diborane mixes well with air, easily forming explosive mixtures. Diborane will ignite spontaneously in moist air at room temperature...
and catalytic hydrogenation
Hydrogenation
Hydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
(requires high temperatures and pressures).
N,N-disubstituted amides can be reduced to aldehydes by using an excess of the amide:
- R(CO)NRR' + LiAlH4 → RCHO + HNRR'
With further reduction the alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
is obtained.
Some amides can be reduced to aldehydes in the Sonn-Müller method.
Hydrosilylation
A well known method for amide reduction is hydrosilylationHydrosilylation
Hydrosilylation, also called catalytic hydrosilation, describes the addition of Si-H bonds across unsaturated bonds. Ordinarily the reaction is conducted catalytically and usually the substrates are unsaturated organic compounds. Alkenes and alkynes give alkyl and vinyl silanes; aldehydes and...
with silyl hydrides and a suitable catalyst based on Rh, Ru, Pt, Pd, Ir, Os, Re, Mn, Mo, In, or Ti.
Iron catalysis by triiron dodecacarbonyl
Triiron dodecacarbonyl
Triiron dodecarbonyl is the chemical compound with the formula Fe312. It was one of the first metal carbonyl clusters synthesized. It is a more reactive source of iron than is iron pentacarbonyl.-General properties:...
in combination with polymethylhydrosiloxane has been reported .