Synthon
Encyclopedia
A synthon is a concept in retrosynthetic analysis
. It is defined as a structural unit within a molecule
which is related to a possible synthetic
operation. The term was coined by E.J. Corey. It is noted that the phrase does not feature very prominently in Corey's book, The Logic of Chemical Synthesis, as it is not included in the index.
In planning the synthesis of phenylacetic acid
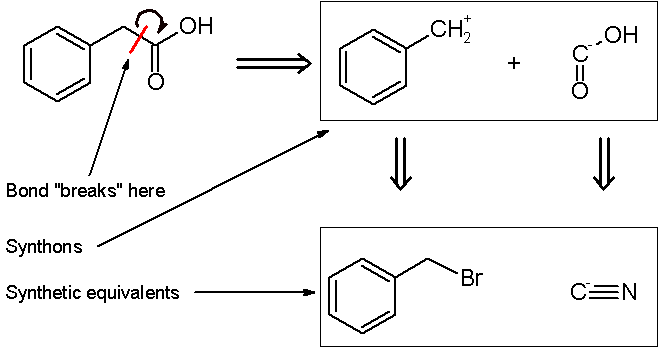
, two synthons are identified: a nucleophilic "-COOH" group, and an electrophilic "PhCH2+" group. Of course, both synthons do not exist per se; synthetic equivalents corresponding to the synthons are reacted to produce the desired product. In this case, the cyanide anion is the synthetic equivalent for the -COOH synthon, while benzyl bromide
is the synthetic equivalent for the benzyl synthon.
The synthesis of phenylacetic acid determined by retrosynthetic analysis is thus:
Retrosynthetic analysis
Retrosynthetic analysis is a technique for solving problems in the planning of organic syntheses. This is achieved by transforming a target molecule into simpler precursor structures without assumptions regarding starting materials. Each precursor material is examined using the same method. This...
. It is defined as a structural unit within a molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
which is related to a possible synthetic
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
operation. The term was coined by E.J. Corey. It is noted that the phrase does not feature very prominently in Corey's book, The Logic of Chemical Synthesis, as it is not included in the index.
Example
In planning the synthesis of phenylacetic acid
Phenylacetic acid
Phenylacetic acid is an organic compound containing a phenyl functional group and a carboxylic acid functional group. It is a white solid with a disagreeable odor...
, two synthons are identified: a nucleophilic "-COOH" group, and an electrophilic "PhCH2+" group. Of course, both synthons do not exist per se; synthetic equivalents corresponding to the synthons are reacted to produce the desired product. In this case, the cyanide anion is the synthetic equivalent for the -COOH synthon, while benzyl bromide
Benzyl bromide
Benzyl bromide, or α-bromotoluene, is an organic compound consisting of a benzene ring substituted with a bromomethyl group. It can be prepared by the bromination of toluene at room temperature in air, using manganese oxide as a heterogeneous catalyst...
is the synthetic equivalent for the benzyl synthon.
The synthesis of phenylacetic acid determined by retrosynthetic analysis is thus:
- PhCH2Br + NaCN → PhCH2CN + NaBr
- PhCH2CN + 2 H2O → PhCH2COOH + NH3
- C2 synthons - acetyleneAcetyleneAcetylene is the chemical compound with the formula C2H2. It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in pure form and thus is usually handled as a solution.As an alkyne, acetylene is unsaturated because...
, acetaldehydeAcetaldehydeAcetaldehyde is an organic chemical compound with the formula CH3CHO or MeCHO. It is one of the most important aldehydes, occurring widely in nature and being produced on a large scale industrially. Acetaldehyde occurs naturally in coffee, bread, and ripe fruit, and is produced by plants as part... - -C2H4OH synthon - ethylene oxideEthylene oxideEthylene oxide, also called oxirane, is the organic compound with the formula . It is a cyclic ether. This means that it is composed of two alkyl groups attached to an oxygen atom in a cyclic shape . This colorless flammable gas with a faintly sweet odor is the simplest epoxide, a three-membered...
- carbocationCarbocationA carbocation is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability . Therefore carbocations are often reactive,...
synthons - alkyl halides - carbanionCarbanionA carbanion is an anion in which carbon has an unshared pair of electrons and bears a negative charge usually with three substituents for a total of eight valence electrons. The carbanion exists in a trigonal pyramidal geometry. Formally a carbanion is the conjugate base of a carbon acid.where B...
synthons - Grignard reagents, organolithiums, substituted acetylides