Solvay process
Encyclopedia
The Solvay process, also referred to as the ammonia-soda process, is the major industrial process for the production of soda ash (sodium carbonate
). The ammonia-soda process was developed into its modern form by Ernest Solvay
during the 1860s. The ingredients for this process are readily available and inexpensive: salt brine (from inland sources or from the sea) and limestone
(from mines). The worldwide production of soda ash in 2005 has been estimated at 42 billion
kilograms (92 billion pounds), which is more than six kilograms per year for each person on earth. Solvay-based chemical plants now produce roughly three-fourths of this supply, with the remainder being mined from natural deposits.
 The Solvay process results in soda ash (predominantly sodium carbonate
The Solvay process results in soda ash (predominantly sodium carbonate
(Na2CO3)) from brine
(as a source of sodium chloride
(NaCl)) and from limestone
(as a source of calcium carbonate
(CaCO3)). The overall process is:
The actual implementation of this global, overall reaction is intricate.
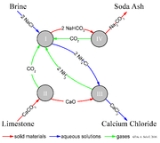
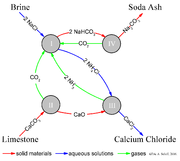
A simplified description can be given using the four different, interacting chemical reactions illustrated in the figure. In the first step in the process, carbon dioxide
(CO2) passes through a concentrated aqueous solution of sodium chloride (NaCl) and ammonia
(NH3).
In industrial practice, the reaction is carried out by passing concentrated brine through two towers. In the first, ammonia bubbles up through the brine and is absorbed by it. In the second, carbon dioxide bubbles up through the ammoniated brine, and sodium bicarbonate
(NaHCO3) precipitates out of the solution. Note that, in a basic
solution
, NaHCO3 is less water-soluble than sodium chloride. The ammonia (NH3) buffers
the solution at a basic pH
; without the ammonia, a hydrochloric acid
byproduct would render the solution acid
ic, and arrest the precipitation.
The necessary ammonia "catalyst" for reaction (I) is reclaimed in a later step, and relatively little ammonia is consumed. The carbon dioxide required for reaction (I) is produced by heating ("calcination
") of the limestone at 950 - 1100 °C. The calcium carbonate (CaCO3) in the limestone is partially converted to quicklime (calcium oxide (CaO)) and carbon dioxide:
The sodium bicarbonate (NaHCO3) that precipitates out in reaction (I) is filtered out from the hot ammonium chloride (NH4Cl) solution, and the solution is then reacted with the quicklime (calcium oxide (CaO)) left over from heating the limestone in step (II).
CaO makes a strong basic solution. The ammonia from reaction (III) is recycled back to the initial brine solution of reaction (I).
The sodium bicarbonate (NaHCO3) precipitate from reaction (I) is then converted to the final product, sodium carbonate (Na2CO3), by calcination
(160 - 230 C), producing water and carbon dioxide as byproducts:
The carbon dioxide from step (IV) is recovered for re-use in step (I). When properly designed and operated, a Solvay plant can reclaim almost all its ammonia, and consumes only small amounts of additional ammonia to make up for losses. The only major inputs to the Solvay process are salt, limestone and thermal energy, and its only major byproduct is calcium chloride
, which is sold as road salt.
In the modified Solvay process developed by Chinese chemist Hou Debang
in 1930s, the first few steps are the same as the Solvay process. However, the CaCl2 is supplanted by ammonium chloride
(NH4Cl). Instead of treating the remaining solution with lime, carbon dioxide and ammonia are pumped into the solution, then sodium chloride is added until the solution saturates at 40°C. Next, the solution is cooled to 10°C. Ammonium chloride precipitates and is removed by filtration, and the solution is recycled to produce more sodium carbonate. Hou's process eliminates the production of calcium chloride. The byproduct ammonium chloride can be refined, used as a fertilizer and may have greater commercial value than CaCl2, thus reducing the extent of waste beds.
Additional details of the industrial implementation of this process are available in the report prepared for the European Soda Ash Producer's Association.
. The word "soda" (from the Middle Latin) originally referred to certain plants that grow in salt marshes; it was discovered that the ashes of these plants yielded the useful alkali "soda ash." The cultivation of such plants for production of soda ash reached a particularly high state of development in the 18th Century in Spain, where the plants are named barrilla; the English word is "barilla
." The ashes of kelp
also yield soda ash, and were the basis of an enormous 18th Century industry in Scotland. Alkali was also mined from dry lakebeds in Egypt.
By the late 18th century, however, these sources were insufficient to meet Europe's burgeoning demand for alkali for soap, textile, and glass industries. In 1791, the French physician Nicolas Leblanc developed a method to manufacture soda ash using salt, limestone
, sulfuric acid
, and coal
. Although the Leblanc process
came to dominate alkali production in the early 19th century, the expense of its inputs and its polluting byproducts (including hydrochloric acid
gas) made it apparent that it was far from an ideal solution.
It has been reported that in 1811 French physicist Augustin Jean Fresnel discovered that sodium bicarbonate precipitates when carbon dioxide is bubbled through ammonia-containing brine— which is the chemical reaction central to the Solvay process. The discovery wasn't published. As has been noted by Desmond Reilly, "The story of the evolution of the ammonium-soda process is an interesting example of the way in which a discovery can be made and then laid aside and not applied for a considerable time afterwards." Serious consideration of this reaction as the basis of an industrial process dates from the British patent issued in 1834 to H. G. Dyan and J. Henning. There were several attempts to reduce this reaction to industrial practice, with varying success.
In 1861, the Belgian
industrial chemist Ernest Solvay
turned his attention to the problem; he was apparently largely unaware of the extensive earlier work. His solution, an 80 feet (24.4 m)-tall gas absorption tower in which carbon dioxide bubbled up through a descending flow of brine, together with efficient recovery and recycling of the ammonia, proved effective, and by 1864, Solvay and his brother Alfred had acquired good financial backing and constructed a plant in the Belgian town of Charleroi
. The new process proved more economical and less polluting than the Leblanc method, and its use spread. In 1874, the Solvays expanded their facilities with a new, larger plant at Nancy, France.
In the same year, Ludwig Mond
visited Solvay in Belgium and acquired rights to use the new technology. He and John Brunner
formed the firm of Brunner, Mond & Co.
, and built a Solvay plant at Winnington
, near Northwich
, Cheshire
, England. The facility started up in 1874. Mond was instrumental in making the Solvay process a commercial success; he made several refinements between 1873 and 1880 that removed byproducts that could slow or halt the mass production of sodium carbonate through use of the process.
In 1884, the Solvay brothers licensed Americans William B. Cogswell and Rowland Hazard to produce soda ash in the US, and formed a joint venture (Solvay Process Company
) to build and operate a plant in Solvay, New York
.
 By the 1890s, Solvay process plants produced the majority of the world's soda ash.
By the 1890s, Solvay process plants produced the majority of the world's soda ash.
In 1938, large natural deposits of the mineral Trona
were discovered near the Green River
in Wyoming
. Sodium carbonate can be mined from this source less expensively than it can be produced by the Solvay process, and with the closing of the original Solvay, New York plant in 1986, there have been no Solvay-based plants operating in North America
. Throughout the rest of the world, however, the Solvay process remains the major source of soda ash.
(CaCl2) in aqueous solution. The process has other waste and byproducts as well. Not all of the limestone that is calcined is converted to quicklime and carbon dioxide (in reaction II); the residual calcium carbonate and other components of the limestone become wastes. In addition, the salt brine used by the process is usually purified to remove magnesium and calcium ions, typically to form carbonates; otherwise, these impurities would lead to scale in the various reaction vessels and towers. These carbonates are additional waste products.
In inland plants, such as that in Solvay, New York
, the byproducts have been deposited in "waste beds"; the weight of material deposited in these waste beds exceeded that of the soda ash produced by about 50%. These waste beds have led to water pollution, principally by calcium and chloride. The waste beds in Solvay, New York substantially increased the salinity in nearby Onondaga Lake
, which is among the most polluted lakes in the U.S.
and is a superfund
pollution site. As such waste beds age, they do begin to support plant communities which have been the subject of several scientific studies.
At seaside locations, such as those at Saurashtra, Gujarat, India, the CaCl2 solution may be discharged directly into the sea, apparently without substantial environmental harm. At Osborne, South Australia
, a settling pond is now used to remove 99% of the CaCl2 as the former discharge was silting up the shipping channel.
Variations in the Solvay process have been proposed to convert carbon dioxide emissions into sodium carbonates, but carbon sequestration by calcium or magnesium carbonates appears more promising.
Sodium carbonate
Sodium carbonate , Na2CO3 is a sodium salt of carbonic acid. It most commonly occurs as a crystalline heptahydrate, which readily effloresces to form a white powder, the monohydrate. Sodium carbonate is domestically well-known for its everyday use as a water softener. It can be extracted from the...
). The ammonia-soda process was developed into its modern form by Ernest Solvay
Ernest Solvay
Ernest Gaston Joseph Solvay was a Belgian chemist, industrialist and philanthropist.Born at Rebecq, he was prevented by acute pleurisy from going to university...
during the 1860s. The ingredients for this process are readily available and inexpensive: salt brine (from inland sources or from the sea) and limestone
Limestone
Limestone is a sedimentary rock composed largely of the minerals calcite and aragonite, which are different crystal forms of calcium carbonate . Many limestones are composed from skeletal fragments of marine organisms such as coral or foraminifera....
(from mines). The worldwide production of soda ash in 2005 has been estimated at 42 billion
1000000000 (number)
1,000,000,000 is the natural number following 999,999,999 and preceding 1,000,000,001.In scientific notation, it is written as 109....
kilograms (92 billion pounds), which is more than six kilograms per year for each person on earth. Solvay-based chemical plants now produce roughly three-fourths of this supply, with the remainder being mined from natural deposits.
Chemistry

Sodium carbonate
Sodium carbonate , Na2CO3 is a sodium salt of carbonic acid. It most commonly occurs as a crystalline heptahydrate, which readily effloresces to form a white powder, the monohydrate. Sodium carbonate is domestically well-known for its everyday use as a water softener. It can be extracted from the...
(Na2CO3)) from brine
Brine
Brine is water, saturated or nearly saturated with salt .Brine is used to preserve vegetables, fruit, fish, and meat, in a process known as brining . Brine is also commonly used to age Halloumi and Feta cheeses, or for pickling foodstuffs, as a means of preserving them...
(as a source of sodium chloride
Sodium chloride
Sodium chloride, also known as salt, common salt, table salt or halite, is an inorganic compound with the formula NaCl. Sodium chloride is the salt most responsible for the salinity of the ocean and of the extracellular fluid of many multicellular organisms...
(NaCl)) and from limestone
Limestone
Limestone is a sedimentary rock composed largely of the minerals calcite and aragonite, which are different crystal forms of calcium carbonate . Many limestones are composed from skeletal fragments of marine organisms such as coral or foraminifera....
(as a source of calcium carbonate
Calcium carbonate
Calcium carbonate is a chemical compound with the formula CaCO3. It is a common substance found in rocks in all parts of the world, and is the main component of shells of marine organisms, snails, coal balls, pearls, and eggshells. Calcium carbonate is the active ingredient in agricultural lime,...
(CaCO3)). The overall process is:
- 2 NaCl + CaCO3 → Na2CO3 + CaCl2
The actual implementation of this global, overall reaction is intricate.
A simplified description can be given using the four different, interacting chemical reactions illustrated in the figure. In the first step in the process, carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
(CO2) passes through a concentrated aqueous solution of sodium chloride (NaCl) and ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
(NH3).
- NaClSodium chlorideSodium chloride, also known as salt, common salt, table salt or halite, is an inorganic compound with the formula NaCl. Sodium chloride is the salt most responsible for the salinity of the ocean and of the extracellular fluid of many multicellular organisms...
+ CO2Carbon dioxideCarbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
+ NH3AmmoniaAmmonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
+ H2OWaterWater is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
→ NaHCO3Sodium bicarbonateSodium bicarbonate or sodium hydrogen carbonate is the chemical compound with the formula Na HCO3. Sodium bicarbonate is a white solid that is crystalline but often appears as a fine powder. It has a slightly salty, alkaline taste resembling that of washing soda . The natural mineral form is...
+ NH4ClAmmonium chlorideAmmonium chloride NH4Cl is an inorganic compound with the formula NH4Cl. It is a white crystalline salt that is highly soluble in water. Solutions of ammonium chloride are mildly acidic. Sal ammoniac is a name of natural, mineralogical form of ammonium chloride...
(I)
In industrial practice, the reaction is carried out by passing concentrated brine through two towers. In the first, ammonia bubbles up through the brine and is absorbed by it. In the second, carbon dioxide bubbles up through the ammoniated brine, and sodium bicarbonate
Sodium bicarbonate
Sodium bicarbonate or sodium hydrogen carbonate is the chemical compound with the formula Na HCO3. Sodium bicarbonate is a white solid that is crystalline but often appears as a fine powder. It has a slightly salty, alkaline taste resembling that of washing soda . The natural mineral form is...
(NaHCO3) precipitates out of the solution. Note that, in a basic
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
solution
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
, NaHCO3 is less water-soluble than sodium chloride. The ammonia (NH3) buffers
Buffering agent
A buffering agent is a weak acid or base used to maintain the acidity of a solution at a chosen value. The function of a buffering agent is to prevent a rapid change in pH when acids or bases are added to the solution. Buffering agents have variable properties—some are more soluble than others;...
the solution at a basic pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
; without the ammonia, a hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
byproduct would render the solution acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
ic, and arrest the precipitation.
The necessary ammonia "catalyst" for reaction (I) is reclaimed in a later step, and relatively little ammonia is consumed. The carbon dioxide required for reaction (I) is produced by heating ("calcination
Calcination
Calcination is a thermal treatment process applied to ores and other solid materials to bring about a thermal decomposition, phase transition, or removal of a volatile fraction. The calcination process normally takes place at temperatures below the melting point of the product materials...
") of the limestone at 950 - 1100 °C. The calcium carbonate (CaCO3) in the limestone is partially converted to quicklime (calcium oxide (CaO)) and carbon dioxide:
- CaCO3Calcium carbonateCalcium carbonate is a chemical compound with the formula CaCO3. It is a common substance found in rocks in all parts of the world, and is the main component of shells of marine organisms, snails, coal balls, pearls, and eggshells. Calcium carbonate is the active ingredient in agricultural lime,...
→ CO2Carbon dioxideCarbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
+ CaOCalcium oxideCalcium oxide , commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline crystalline solid at room temperature....
(II)
The sodium bicarbonate (NaHCO3) that precipitates out in reaction (I) is filtered out from the hot ammonium chloride (NH4Cl) solution, and the solution is then reacted with the quicklime (calcium oxide (CaO)) left over from heating the limestone in step (II).
- 2 NH4ClAmmonium chlorideAmmonium chloride NH4Cl is an inorganic compound with the formula NH4Cl. It is a white crystalline salt that is highly soluble in water. Solutions of ammonium chloride are mildly acidic. Sal ammoniac is a name of natural, mineralogical form of ammonium chloride...
+ CaOCalcium oxideCalcium oxide , commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline crystalline solid at room temperature....
→ 2 NH3AmmoniaAmmonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
+ CaCl2Calcium chlorideCalcium chloride, CaCl2, is a salt of calcium and chlorine. It behaves as a typical ionic halide, and is solid at room temperature. Common applications include brine for refrigeration plants, ice and dust control on roads, and desiccation...
+ H2OWaterWater is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
(III)
CaO makes a strong basic solution. The ammonia from reaction (III) is recycled back to the initial brine solution of reaction (I).
The sodium bicarbonate (NaHCO3) precipitate from reaction (I) is then converted to the final product, sodium carbonate (Na2CO3), by calcination
Calcination
Calcination is a thermal treatment process applied to ores and other solid materials to bring about a thermal decomposition, phase transition, or removal of a volatile fraction. The calcination process normally takes place at temperatures below the melting point of the product materials...
(160 - 230 C), producing water and carbon dioxide as byproducts:
- 2 NaHCO3Sodium bicarbonateSodium bicarbonate or sodium hydrogen carbonate is the chemical compound with the formula Na HCO3. Sodium bicarbonate is a white solid that is crystalline but often appears as a fine powder. It has a slightly salty, alkaline taste resembling that of washing soda . The natural mineral form is...
→ Na2CO3Sodium carbonateSodium carbonate , Na2CO3 is a sodium salt of carbonic acid. It most commonly occurs as a crystalline heptahydrate, which readily effloresces to form a white powder, the monohydrate. Sodium carbonate is domestically well-known for its everyday use as a water softener. It can be extracted from the...
+ H2OWaterWater is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
+ CO2Carbon dioxideCarbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
(IV)
The carbon dioxide from step (IV) is recovered for re-use in step (I). When properly designed and operated, a Solvay plant can reclaim almost all its ammonia, and consumes only small amounts of additional ammonia to make up for losses. The only major inputs to the Solvay process are salt, limestone and thermal energy, and its only major byproduct is calcium chloride
Calcium chloride
Calcium chloride, CaCl2, is a salt of calcium and chlorine. It behaves as a typical ionic halide, and is solid at room temperature. Common applications include brine for refrigeration plants, ice and dust control on roads, and desiccation...
, which is sold as road salt.
In the modified Solvay process developed by Chinese chemist Hou Debang
Hou Debang
Hou Debang was a scientist and chemical engineer in China.Graduated from Tsinghua University in 1912, he was one of the scholars sent to the United States to study modern technologies...
in 1930s, the first few steps are the same as the Solvay process. However, the CaCl2 is supplanted by ammonium chloride
Ammonium chloride
Ammonium chloride NH4Cl is an inorganic compound with the formula NH4Cl. It is a white crystalline salt that is highly soluble in water. Solutions of ammonium chloride are mildly acidic. Sal ammoniac is a name of natural, mineralogical form of ammonium chloride...
(NH4Cl). Instead of treating the remaining solution with lime, carbon dioxide and ammonia are pumped into the solution, then sodium chloride is added until the solution saturates at 40°C. Next, the solution is cooled to 10°C. Ammonium chloride precipitates and is removed by filtration, and the solution is recycled to produce more sodium carbonate. Hou's process eliminates the production of calcium chloride. The byproduct ammonium chloride can be refined, used as a fertilizer and may have greater commercial value than CaCl2, thus reducing the extent of waste beds.
Additional details of the industrial implementation of this process are available in the report prepared for the European Soda Ash Producer's Association.
Uses of soda ash
Soda ash is used in many industrial processes, and its production is sometimes used as an indicator of economic health. The principal current uses include:- Glass making: More than half the worldwide production of soda ash is used to make glass. Bottle and window glass (Soda-lime glassSoda-lime glassSoda-lime glass, also called soda-lime-silica glass, is the most prevalent type of glass, used for windowpanes, and glass containers for beverages, food, and some commodity items...
) is made by melting a mixture of sodium carbonate, calcium carbonate and silica sand (silicon dioxideSilicon dioxideThe chemical compound silicon dioxide, also known as silica , is an oxide of silicon with the chemical formula '. It has been known for its hardness since antiquity...
(SiO2)). - Water treatment: Sodium carbonate is used to soften water (precipitates out Mg2+ and Ca2+ carbonates). This is used both industrially and domestically (in some washing powders).
- Making soaps and detergents: Often sodium carbonate is used as a cheaper alternative to lye (sodium hydroxide (NaOH)).
- Paper making: Sodium carbonate is used to make sodium bisulfiteSodium bisulfiteSodium bisulfite is a chemical compound with the chemical formula NaHSO3. Sodium bisulfite is a food additive with E number E222. This salt of bisulfite can be prepared by bubbling sulfur dioxide in a solution of sodium carbonate in water...
(NaHSO3) for the "sulfite" method of separating ligninLigninLignin or lignen is a complex chemical compound most commonly derived from wood, and an integral part of the secondary cell walls of plants and some algae. The term was introduced in 1819 by de Candolle and is derived from the Latin word lignum, meaning wood...
from cellulose. - As a common alkali in many chemical factories because it is cheaper than NaOH and far safer to handle.
- Making sodium bicarbonateSodium bicarbonateSodium bicarbonate or sodium hydrogen carbonate is the chemical compound with the formula Na HCO3. Sodium bicarbonate is a white solid that is crystalline but often appears as a fine powder. It has a slightly salty, alkaline taste resembling that of washing soda . The natural mineral form is...
: NaHCO3 is used in baking soda and fire extinguishers. Although NaHCO3 is produced in the Solvay process, heating it to remove the ammonia it is contaminated with decomposes some NaHCO3, so it is actually cheaper to react the finished Na2CO3 product with CO2. - Removing sulfur dioxideSulfur dioxideSulfur dioxide is the chemical compound with the formula . It is released by volcanoes and in various industrial processes. Since coal and petroleum often contain sulfur compounds, their combustion generates sulfur dioxide unless the sulfur compounds are removed before burning the fuel...
(SO2) from flue gases in power stations. This is becoming more common, especially where stations have to meet stringent emission controls.
History
The name "soda ash" is based on the principal historical method of obtaining alkali, which was by using water to extract it from ashes. Wood fires yielded potash and the active ingredient potassium carbonatePotassium carbonate
Potassium carbonate is a white salt, soluble in water , which forms a strongly alkaline solution. It can be made as the product of potassium hydroxide's absorbent reaction with carbon dioxide. It is deliquescent, often appearing a damp or wet solid...
. The word "soda" (from the Middle Latin) originally referred to certain plants that grow in salt marshes; it was discovered that the ashes of these plants yielded the useful alkali "soda ash." The cultivation of such plants for production of soda ash reached a particularly high state of development in the 18th Century in Spain, where the plants are named barrilla; the English word is "barilla
Barilla
Barilla S.p.A. is a major Italian and European food company founded in 1877 in Parma, Italy by Pietro Barilla...
." The ashes of kelp
Kelp
Kelps are large seaweeds belonging to the brown algae in the order Laminariales. There are about 30 different genera....
also yield soda ash, and were the basis of an enormous 18th Century industry in Scotland. Alkali was also mined from dry lakebeds in Egypt.
By the late 18th century, however, these sources were insufficient to meet Europe's burgeoning demand for alkali for soap, textile, and glass industries. In 1791, the French physician Nicolas Leblanc developed a method to manufacture soda ash using salt, limestone
Limestone
Limestone is a sedimentary rock composed largely of the minerals calcite and aragonite, which are different crystal forms of calcium carbonate . Many limestones are composed from skeletal fragments of marine organisms such as coral or foraminifera....
, sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
, and coal
Coal
Coal is a combustible black or brownish-black sedimentary rock usually occurring in rock strata in layers or veins called coal beds or coal seams. The harder forms, such as anthracite coal, can be regarded as metamorphic rock because of later exposure to elevated temperature and pressure...
. Although the Leblanc process
Leblanc process
The Leblanc process was the industrial process for the production of soda ash used throughout the 19th century, named after its inventor, Nicolas Leblanc. It involved two stages: Production of sodium sulfate from sodium chloride, followed by reaction of the sodium sulfate with coal and calcium...
came to dominate alkali production in the early 19th century, the expense of its inputs and its polluting byproducts (including hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
gas) made it apparent that it was far from an ideal solution.
It has been reported that in 1811 French physicist Augustin Jean Fresnel discovered that sodium bicarbonate precipitates when carbon dioxide is bubbled through ammonia-containing brine— which is the chemical reaction central to the Solvay process. The discovery wasn't published. As has been noted by Desmond Reilly, "The story of the evolution of the ammonium-soda process is an interesting example of the way in which a discovery can be made and then laid aside and not applied for a considerable time afterwards." Serious consideration of this reaction as the basis of an industrial process dates from the British patent issued in 1834 to H. G. Dyan and J. Henning. There were several attempts to reduce this reaction to industrial practice, with varying success.
In 1861, the Belgian
Belgium
Belgium , officially the Kingdom of Belgium, is a federal state in Western Europe. It is a founding member of the European Union and hosts the EU's headquarters, and those of several other major international organisations such as NATO.Belgium is also a member of, or affiliated to, many...
industrial chemist Ernest Solvay
Ernest Solvay
Ernest Gaston Joseph Solvay was a Belgian chemist, industrialist and philanthropist.Born at Rebecq, he was prevented by acute pleurisy from going to university...
turned his attention to the problem; he was apparently largely unaware of the extensive earlier work. His solution, an 80 feet (24.4 m)-tall gas absorption tower in which carbon dioxide bubbled up through a descending flow of brine, together with efficient recovery and recycling of the ammonia, proved effective, and by 1864, Solvay and his brother Alfred had acquired good financial backing and constructed a plant in the Belgian town of Charleroi
Charleroi
Charleroi is a city and a municipality of Wallonia, located in the province of Hainaut, Belgium. , the total population of Charleroi was 201,593. The metropolitan area, including the outer commuter zone, covers an area of and had a total population of 522,522 as of 1 January 2008, ranking it as...
. The new process proved more economical and less polluting than the Leblanc method, and its use spread. In 1874, the Solvays expanded their facilities with a new, larger plant at Nancy, France.
In the same year, Ludwig Mond
Ludwig Mond
Dr Ludwig Mond , was a German-born chemist and industrialist who took British nationality.-Education and career:...
visited Solvay in Belgium and acquired rights to use the new technology. He and John Brunner
John Tomlinson Brunner
Sir John Tomlinson Brunner, 1st Baronet, DL was a British chemical industrialist and Liberal Party politician. At Hutchinson's alkali works in Widnes he rose to the position of general manager...
formed the firm of Brunner, Mond & Co.
Brunner Mond
Tata Chemicals Europe is a UK-based chemicals company that is a subsidiary of Tata Chemicals Limited, itself a part of the India-based Tata Group...
, and built a Solvay plant at Winnington
Winnington
Winnington is a small, mainly residential area of the town of Northwich in Cheshire, England.-Industry:Winnington is the home to Brunner Mond UK chemical works, where soda ash is created. Polythene, the material used in many plastic items , was first made at the chemical works by R.O. Gibson and...
, near Northwich
Northwich
Northwich is a town and civil parish in the unitary authority of Cheshire West and Chester and the ceremonial county of Cheshire, England. It lies in the heart of the Cheshire Plain, at the confluence of the rivers Weaver and Dane...
, Cheshire
Cheshire
Cheshire is a ceremonial county in North West England. Cheshire's county town is the city of Chester, although its largest town is Warrington. Other major towns include Widnes, Congleton, Crewe, Ellesmere Port, Runcorn, Macclesfield, Winsford, Northwich, and Wilmslow...
, England. The facility started up in 1874. Mond was instrumental in making the Solvay process a commercial success; he made several refinements between 1873 and 1880 that removed byproducts that could slow or halt the mass production of sodium carbonate through use of the process.
In 1884, the Solvay brothers licensed Americans William B. Cogswell and Rowland Hazard to produce soda ash in the US, and formed a joint venture (Solvay Process Company
Solvay Process Company
The Solvay Process Company was a pioneer chemical industry of the United States in the manufacture of soda ash and a major employer in Central New York...
) to build and operate a plant in Solvay, New York
Solvay, New York
Solvay is a village located in Onondaga County, New York, and a suburb of the city of Syracuse. According to the 2000 census, the village had a total population of 6,845...
.

In 1938, large natural deposits of the mineral Trona
Trona
Trona ; Na3•2H2O is an evaporite mineral. It is mined as the primary source of sodium carbonate in the United States, where it has replaced the Solvay process used in most of the rest of the world for sodium carbonate production.- Etymology :The word "trona" comes to English by way of either...
were discovered near the Green River
Green River (Utah)
The Green River, located in the western United States, is the chief tributary of the Colorado River. The watershed of the river, known as the Green River Basin, covers parts of Wyoming, Utah, and Colorado. The Green River is long, beginning in the Wind River Mountains of Wyoming and flowing...
in Wyoming
Wyoming
Wyoming is a state in the mountain region of the Western United States. The western two thirds of the state is covered mostly with the mountain ranges and rangelands in the foothills of the Eastern Rocky Mountains, while the eastern third of the state is high elevation prairie known as the High...
. Sodium carbonate can be mined from this source less expensively than it can be produced by the Solvay process, and with the closing of the original Solvay, New York plant in 1986, there have been no Solvay-based plants operating in North America
North America
North America is a continent wholly within the Northern Hemisphere and almost wholly within the Western Hemisphere. It is also considered a northern subcontinent of the Americas...
. Throughout the rest of the world, however, the Solvay process remains the major source of soda ash.
Byproducts and wastes
The principal byproduct of the Solvay process is calcium chlorideCalcium chloride
Calcium chloride, CaCl2, is a salt of calcium and chlorine. It behaves as a typical ionic halide, and is solid at room temperature. Common applications include brine for refrigeration plants, ice and dust control on roads, and desiccation...
(CaCl2) in aqueous solution. The process has other waste and byproducts as well. Not all of the limestone that is calcined is converted to quicklime and carbon dioxide (in reaction II); the residual calcium carbonate and other components of the limestone become wastes. In addition, the salt brine used by the process is usually purified to remove magnesium and calcium ions, typically to form carbonates; otherwise, these impurities would lead to scale in the various reaction vessels and towers. These carbonates are additional waste products.
In inland plants, such as that in Solvay, New York
Solvay, New York
Solvay is a village located in Onondaga County, New York, and a suburb of the city of Syracuse. According to the 2000 census, the village had a total population of 6,845...
, the byproducts have been deposited in "waste beds"; the weight of material deposited in these waste beds exceeded that of the soda ash produced by about 50%. These waste beds have led to water pollution, principally by calcium and chloride. The waste beds in Solvay, New York substantially increased the salinity in nearby Onondaga Lake
Onondaga Lake
Onondaga Lake is a lake in Central New York located northwest of Syracuse, New York. The southeastern end of the lake and the southwestern shore abut industrial areas and expressways; the northeastern shore and northwestern end border a series of parks and museums. Although it is near the Finger...
, which is among the most polluted lakes in the U.S.
and is a superfund
Superfund
Superfund is the common name for the Comprehensive Environmental Response, Compensation, and Liability Act of 1980 , a United States federal law designed to clean up sites contaminated with hazardous substances...
pollution site. As such waste beds age, they do begin to support plant communities which have been the subject of several scientific studies.
At seaside locations, such as those at Saurashtra, Gujarat, India, the CaCl2 solution may be discharged directly into the sea, apparently without substantial environmental harm. At Osborne, South Australia
Osborne, South Australia
Osborne is a north-western suburb of Adelaide, South Australia, Australia 19 km from the CBD, in the City of Port Adelaide Enfield. It is on the LeFevre Peninsula, adjacent to Outer Harbor, Taperoo and North Haven...
, a settling pond is now used to remove 99% of the CaCl2 as the former discharge was silting up the shipping channel.
Carbon sequestration and the Solvay process
Variations in the Solvay process have been proposed for carbon sequestration. One idea is to react carbon dioxide, produced perhaps by the combustion of coal, to form solid carbonates (such as sodium bicarbonate) that could be permanently stored, thus avoiding carbon dioxide emission into the atmosphere.Variations in the Solvay process have been proposed to convert carbon dioxide emissions into sodium carbonates, but carbon sequestration by calcium or magnesium carbonates appears more promising.

