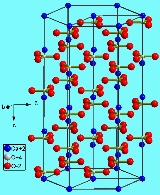
Calcium carbonate
Overview
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
with the formula
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
Ca
Calcium
Calcium is the chemical element with the symbol Ca and atomic number 20. It has an atomic mass of 40.078 amu. Calcium is a soft gray alkaline earth metal, and is the fifth-most-abundant element by mass in the Earth's crust...
C
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
O
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
3. It is a common substance found in rock
Rock (geology)
In geology, rock or stone is a naturally occurring solid aggregate of minerals and/or mineraloids.The Earth's outer solid layer, the lithosphere, is made of rock. In general rocks are of three types, namely, igneous, sedimentary, and metamorphic...
s in all parts of the world, and is the main component of shells of marine organisms, snail
Snail
Snail is a common name applied to most of the members of the molluscan class Gastropoda that have coiled shells in the adult stage. When the word is used in its most general sense, it includes sea snails, land snails and freshwater snails. The word snail without any qualifier is however more often...
s, coal ball
Coal ball
Coal balls, despite their name, are calcium-rich masses of permineralised life forms, generally having a round shape. Coal balls were formed roughly , during the Carboniferous Period...
s, pearls, and eggshell
Eggshell
An eggshell is the outer covering of a hard-shelled egg and of some forms of eggs with soft outer coats.- Insect eggs :Insects and other arthropods lay a variety of styles and shapes of eggs. Some have gelatinous or skin-like coverings, others have hard eggshells. Softer shells are mostly protein....
s. Calcium carbonate is the active ingredient in agricultural lime
Agricultural lime
Agricultural lime, also called aglime, agricultural limestone, garden lime or liming, is a soil additive made from pulverized limestone or chalk. The primary active component is calcium carbonate...
, and is usually the principal cause of hard water
Hard water
Hard water is water that has high mineral content . Hard water has high concentrations of Ca2+ and Mg2+ ions. Hard water is generally not harmful to one's health but can pose serious problems in industrial settings, where water hardness is monitored to avoid costly breakdowns in boilers, cooling...
. It is commonly used medicinally as a calcium
Calcium
Calcium is the chemical element with the symbol Ca and atomic number 20. It has an atomic mass of 40.078 amu. Calcium is a soft gray alkaline earth metal, and is the fifth-most-abundant element by mass in the Earth's crust...
supplement or as an antacid
Antacid
An antacid is a substance which neutralizes stomach acidity.-Mechanism of action:Antacids perform a neutralization reaction, increasing the pH to reduce acidity in the stomach. When gastric hydrochloric acid reaches the nerves in the gastrointestinal mucosa, they signal pain to the central nervous...
, but excessive consumption can be hazardous.
Calcium carbonate shares the typical properties of other carbonates.
Unanswered Questions

